Persistently High Levels of Coagulation Factor XI as a Risk Factor for Venous Thrombosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. Laboratory Studies
2.3. Statistical Analysis
3. Results
3.1. T1—At Least 1 Month after Discontinuation of Anticoagulant Treatment
3.2. T2—At Least Three Months after T1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geng, Y.; Verhamme, I.M.; Smith, S.B.; Sun, M.-F.; Matafonov, A.; Cheng, Q.; Smith, S.A.; Morrissey, J.H.; Gailani, D. The dimeric structure of factor XI and zymogen activation. Blood 2013, 121, 3962–3969. [Google Scholar] [CrossRef]
- Mohammed, B.M.; Matafonov, A.; Ivanov, I.; Sun, M.F.; Cheng, Q.; Dickeson, S.K.; Li, C.; Sun, D.; Verhamme, I.M.; Emsley, J.; et al. An update on factor XI structure and function. Thromb. Res. 2018, 161, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Puy, C.; Rigg, R.A.; McCarty, O.J. The haemostatic role of factor XI. Thromb. Res. 2016, 141 (Suppl. S2), S8–S11. [Google Scholar] [CrossRef]
- Kravtsov, D.V.; Matafonov, A.; Tucker, E.I.; Sun, M.-F.; Walsh, P.N.; Gruber, A.; Gailani, D. Factor XI contributes to thrombin generation in the absence of factor XII. Blood 2009, 114, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Puy, C.; Tucker, E.I.; Matafonov, A.; Cheng, Q.; Zientek, K.D.; Gailani, D.; Gruber, A.; McCarty, O.J. Activated factor XI increases the procoagulant activity of the extrinsic pathway by inactivating tissue factor pathway inhibitor. Blood. 2015, 26, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, M.D.; Connors, J.M. Factor XI deficiency. Hematol. Oncol. Clin. N. Am. 2021, 35, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Meijers, J.C.M.; Tekelenburg, W.L.H.; Bouma, B.N.; Bertina, R.M.; Rosendaal, F.R. High levels of coagulation factor XI as a risk factor for venous thrombosis. N. Engl. J. Med. 2000, 342, 696–701. [Google Scholar] [CrossRef]
- Cushman, M.; O’Meara, E.S.; Folsom, A.R.; Heckbert, S.R. Coagulation factors IX through XIII and the risk of future venous thrombosis: The longitudinal investigation of thromboembolism etiology. Blood 2009, 114, 2878–2883. [Google Scholar] [CrossRef]
- Folsom, A.R.; Tang, W.; Roetker, N.S.; Heckbert, S.R.; Cushman, M.; Pankow, J.S. Prospective study of circulating factor XI and incident venous thromboembolism: The longitudinal investigation of thromboembolism etiology (LITE). Am. J. Hematol. 2015, 90, 1047–1051. [Google Scholar] [CrossRef]
- Libourel, E.J.; Bank, I.; Meinardi, J.R.; Baljé -Volkers, C.P.; Hamulyak, K.; Middeldorp, S.; Koopman, M.M.W.; van Pampus, E.C.M.; Prins, M.H.; Büller, H.R.; et al. Co-segregation of thrombophilic disorders in factor V Leiden carriers; the contributions of factor VIII, factor XI, thrombin actvatable fibrinolysis inhibitor and lipoprotein(a) to the absolute risk of venous thromboembolism. Haematologica 2002, 87, 1068–1073. [Google Scholar]
- Nagy, M.; Robles, A.P.; Visser, M.; Koeck, T.; Ten Cate, V.; Ten Cate-Hoek, A.J.; Schwers, S.; Heitmeier, S.; Ten Cate, H.; Wild, P.S.; et al. Predictive value for increased activated factor XI activity in acute venous thromboembolism. J. Thromb. Haemost. 2023, 21, 1610–1622. [Google Scholar] [CrossRef] [PubMed]
- Merlo, C.; Wuillemin, W.A.; Redondo, M.; Furlan, M.; Sulzer, I.; Kremer-Hovinga, J.; Binder, B.R.; Lammle, B. Elevated levels of plasma prekallikrein, high molecular weight kininogen and factor XI in coronary heart disease. Atherosclerosis 2002, 161, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Doggen, C.J.; Rosendaal, F.R.; Meijers, J.C. Levels of intrinsic coagulation factors and the risk of myocardial infarction among men: Opposite and synergistic effects of factors XI and XII. Blood 2006, 108, 4045–4051. [Google Scholar] [CrossRef] [PubMed]
- Berliner, J.I.; Rybicki, A.C.; Kaplan, R.C.; Monrad, E.S.; Freeman, R.; Billett, H.H. Elevated levels of factor XI are associated with cardiovascular disease in women. Thromb. Res. 2002, 107, 55–60. [Google Scholar] [CrossRef]
- Suri, M.F.; Yamagishi, K.; Aleksic, N.; Hannan, P.J.; Folsom, A.R. Novel haemostatic factor levels and risk of ischaemic stroke: The Atherosclerosis Risk in Communities (ARIC) Study. Cerebrovasc. Dis. 2010, 29, 497–502. [Google Scholar] [CrossRef]
- Yang, D.T.; Flanders, M.M.; Kim, H.; Rodgers, G.M. Elevated factor XI activity levels are associated with an increased odds ratio for cerebrovascular events. Am. J. Clin. Pathol. 2006, 126, 411–415. [Google Scholar] [CrossRef]
- Ząbczyk, M.T.; Hanarz, M.; Malinowski, K.P.; Pociask, E.; Butenas, S.; Gajos, G.; Undas, A. Active FXI Can Independently Predict Ischaemic Stroke in Anticoagulated Atrial Fibrillation Patients: A Cohort Study. Thromb. Haemost. 2022, 122, 1397–1406. [Google Scholar] [CrossRef]
- Spiezia, L.; Campello, E.; Simion, C.; Poretto, A.; Dalla Valle, F.; Simioni, P. Risk factors for post-thrombotic syndrome in patients with a first proximal deep venous thrombosis treated with direct oral anticoagulants. Angiology 2022, 73, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.; Anderson, D. The diagnosis and treatment of venous thromboembolism. Hematol. Am. Soc. Hematol. Educ. Program 2013, 2013, 457–463. [Google Scholar] [CrossRef][Green Version]
- Spiezia, L.; Campello, E.; Bon, M.; Tison, T.; Milan, M.; Simioni, P.; Prandoni, P. ABO blood groups and the risk of venous thrombosis in patients with inherited thrombophilia. Blood. Transfus. 2013, 11, 250–253. [Google Scholar]
- Bulato, C.; Radu, C.M.; Campello, E.; Gavasso, S.; Spiezia, L.; Tormene, D.; Simioni, P. New prothrombin mutation (Arg596Trp, prothrombin padua 2) associated with venous thromboembolism. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Campello, E.; Spiezia, L.; Radu, C.M.; Bulato, C.; Gavasso, S.; Tormene, D.; Woodhams, B.; Dalla Valle, F.; Simioni, P. Circulating microparticles and the risk of thrombosis in inherited deficiencies of antithrombin, protein C and protein S. Thromb. Haemost. 2016, 115, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Sanson, B.J.; Simioni, P.; Tormene, D.; Moia, M.; Friederich, P.W.; Huisman, M.V.; Prandoni, P.; Bura, A.; Rejto, L.; Wells, P.; et al. The incidence of venous thromboembolism in asymptomatic carriers of a deficiency of antithrombin, protein C, or protein S: A prospective cohort study. Blood 1999, 94, 3702–3706. [Google Scholar] [PubMed]
- Montagnana, M.; Lippi, G.; Danese, E. An Overview of Thrombophilia and Associated Laboratory Testing. Methods. Mol. Biol. 2017, 1646, 113–135. [Google Scholar]
- Bouma, B.N.; Mosnier, L.O.; Meijers, J.C.; Griffin, J.H. Factor XI dependent and independent activation of thrombin activatable fibrinolysis inhibitor (TAFI) in plasma associated with clot formation. Thromb. Haemost. 1999, 82, 1703–1708. [Google Scholar]
- Li, Y.; Bezemer, I.D.; Rowland, C.M.; Tong, C.H.; Arellano, A.R.; Catanese, J.J.; Devlin, J.J.; Reitsma, P.H.; Bare, L.A.; Rosendaal, F.R. Genetic variants associated with deep vein thrombosis: The F11 locus. J. Thromb. Haemost. 2009, 7, 1802–1808. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, K.; Zou, J.; Ma, H.; Yang, H.; Zhang, X.; Jiao, Y. Associations between polymorphisms in coagulation-related genes and venous thromboembolism: A meta-analysis with trial sequential analysis. Medicine 2017, 96, e7233. [Google Scholar] [CrossRef]
- Manco, L.; Silva, C.; Fidalgo, T.; Martinho, P.; Sarmento, A.B.; Ribeiro, M.L. Venous thromboembolism risk associated with ABO, F11 and FGG loci. Blood Coagul. Fibrinolysis 2018, 29, 528–532. [Google Scholar] [CrossRef]
- Bruzelius, M.; Ljungqvist, M.; Bottai, M.; Bergendal, A.; Strawbridge, R.J.; Holmström, M.; Silveira, A.; Kieler, H.; Hamsten, A.; Lärfars, G.; et al. F11 is associated with recurrent VTE in women. A prospective cohort study. Thromb. Haemost. 2016, 115, 406–414. [Google Scholar] [CrossRef]
- Gailani, D.; Bane, C.E.; Gruber, A. Factor XI and contact activation as targets for antithrombotic therapy. J. Thromb. Haemost. 2015, 13, 1383–1395. [Google Scholar] [CrossRef]
- Zhang, H.; Löwenberg, E.C.; Crosby, J.R.; MacLeod, A.R.; Zhao, C.; Gao, D.; Black, C.; Revenko, A.S.; Meijers, J.C.; Stroes, E.S.; et al. Inhibition of the intrinsic coagulation pathway factor XI by antisense oligonucleotides: A novel antithrombotic strategy with lowered bleeding risk. Blood 2010, 116, 4684–4692. [Google Scholar] [CrossRef] [PubMed]
- Campello, E.; Simioni, P.; Prandoni, P.; Ferri, N. Clinical Pharmacology of Factor XI Inhibitors: New Therapeutic Approaches for Prevention of Venous and Arterial Thrombotic Disorders. J. Clin. Med. 2022, 11, 6314. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.; Piccini, J.P.; Alexander, J.H.; Granger, C.B.; Patel, M.R. Clinical Evaluation of Factor XIa Inhibitor Drugs: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2023, 81, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Laborante, R.; Ortega-Paz, L.; Franchi, F.; Rollini, F.; D’Amario, D.; Capodanno, D.; Tremoli, E.; Gibson, C.M.; Mehran, R.; et al. Factor XI Inhibitors in Early Clinical Trials: A Meta-analysis. Thromb. Haemost. 2023, 123, 576–584. [Google Scholar] [CrossRef]

| Patients (n = 200) | Controls (n = 200) | |
|---|---|---|
| Age (yr) | ||
| Mean | 57.4 | 55.6 |
| Range | 26–87 | 25–89 |
| Sex (M/F) | 114/86 | 114/86 |
| Body Mass Index (Kg/m2) | ||
| Mean | 27 | 24 |
| Obese, BMI ≥ 30 (no.) | 56 | 34 |
| Thrombophilia | ||
| FVIII, >250% | 5 | 3 |
| Fibrinogen, >600 mg/dL | 9 | 5 |
| Protein C, <60%, (no.) | 8 | 4 |
| Protein S, <60% (no.) | 7 | 6 |
| Antithrombin, <70% (no.) | 4 | 2 |
| Factor V Leiden (no.) † | 14 | 6 |
| Prothrombin G20210A (no.) † | 11 | 7 |
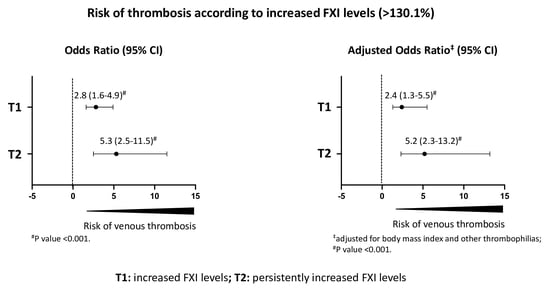
| Factor XI * | Patients | Controls | Odds Ratio (95% CI) | Adjusted Odds Ratio ‡ (95% CI) |
|---|---|---|---|---|
| Study population | ||||
| ≤90th percentile | 153 | 180 | 1.0 | 1.0 |
| >90th percentile | 47 | 20 | 2.8 (1.6–4.9) # | 2.4 (1.3–5.5) # |
| Patients with persistently increased FXI levels | ||||
| ≤90th percentile | 27 | 180 | 1.0 | 1.0 |
| >90th percentile | 16 | 20 | 5.3 (2.5–11.5) # | 5.2 (2.3–13.2) # |
| FXI > 90th Percentile (n = 16) | FXI ≤ 90th Percentile (n = 27) | |
|---|---|---|
| Age (y) | ||
| Mean | 58.1 | 57.4 |
| Range | 31–87 | 26–85 |
| Sex (M/F) | 7/9 | 15/12 |
| Body Mass Index (Kg/m2) | 4 | |
| Mean | 26 | 23 |
| Obese, BMI ≥ 30 (n) | 26 | 3 |
| Thrombophilia | ||
| FVIII, >250% | 1 | 1 |
| Fibrinogen, >600 mg/dL | 1 | 1 |
| Protein C, <60%, (n) | 0 | 2 |
| Protein S, <60% (n) | 0 | 1 |
| Antithrombin, <70% (n) | 0 | 0 |
| Factor V Leiden (n) | 1 | 4 |
| Prothrombin G20210A (n) | 2 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiezia, L.; Forestan, C.; Campello, E.; Simion, C.; Simioni, P. Persistently High Levels of Coagulation Factor XI as a Risk Factor for Venous Thrombosis. J. Clin. Med. 2023, 12, 4890. https://doi.org/10.3390/jcm12154890
Spiezia L, Forestan C, Campello E, Simion C, Simioni P. Persistently High Levels of Coagulation Factor XI as a Risk Factor for Venous Thrombosis. Journal of Clinical Medicine. 2023; 12(15):4890. https://doi.org/10.3390/jcm12154890
Chicago/Turabian StyleSpiezia, Luca, Chiara Forestan, Elena Campello, Chiara Simion, and Paolo Simioni. 2023. "Persistently High Levels of Coagulation Factor XI as a Risk Factor for Venous Thrombosis" Journal of Clinical Medicine 12, no. 15: 4890. https://doi.org/10.3390/jcm12154890
APA StyleSpiezia, L., Forestan, C., Campello, E., Simion, C., & Simioni, P. (2023). Persistently High Levels of Coagulation Factor XI as a Risk Factor for Venous Thrombosis. Journal of Clinical Medicine, 12(15), 4890. https://doi.org/10.3390/jcm12154890