Abstract
Osteoarthritis (OA) is the most frequent worldwide cause of adult population disabilities. The study evaluated the effects of a 21-day individual rehabilitation exercise training program focused on improving patients’ functional capacity. The study analyzed the changes in irisin, chemerin, and BDNF serum levels in 36 OA patients subjected to an individually-adjusted rehabilitation program 90 days after surgical hip or knee replacement. The changes in irisin, chemerin, and BDNF serum levels were measured using enzyme-linked immunosorbent assay (ELISA) kits. A 21-day individual rehabilitation exercise training program significantly increased irisin and BDNF, and decreased chemerin serum levels. The presented study indicates that individually-adjusted exercise training is an important modulator influencing serum levels of anti- and pro-inflammatory factors, leading to positive clinical outcomes in osteoarthritis therapy. Selected factors are considered potential markers of various pathophysiological conditions. The presented study brings new details to the discussion.
1. Introduction
Osteoarthritis (OA) is the most prevalent degenerative joint disease affecting the adult population. It is also one of the most important causes of adult disabilities worldwide. Osteoarthritis prevalence is associated with different factors, with metabolic syndrome being one of them []. Metabolic syndrome in OA patients influences biomechanics, dysregulates chondrocyte metabolism, and interplays between metabolic regulation and immune response, leading to further clinical complications [].
Irisin is one of the recently discovered myokines identified as a marker of muscle weakness and atrophy []. It is mainly expressed and secreted by skeletal muscles as a product of the fibronectin type III domain containing 5 (FNDC5) cleavage []. Irisin levels depend on PPAR-γ (peroxisome proliferator-activated receptor gamma) coactivator-1-α (PGC1α), which is expressed after physical exertion. Increased PGC1α levels upregulate the expression of FNDC5, from which irisin is eventually derived [,]. Therefore, post-exercise irisin production exerts positive effects on the metabolism, and may play a beneficial role in treatment of obesity and obesity-related diseases, type 2 diabetes mellitus (T2DM), or non-alcoholic fatty liver disease (NAFLD) [], especially since decreased irisin levels accompany obesity, type 2 T2D, and other diseases like chronic renal failure and prolonged hypothyroidism []. Some studies show that irisin is also produced by adipose tissue, and acts as adipokine [,,]. As irisin levels decrease with age, irisin seems to be associated with a wide range of aging-related diseases [,,,].
Chemerin is an adipokine secreted by adipose, endothelial, synovial cells, and chondrocytes, and might show chemotherapeutic activity through the chemerin receptor 23 (Chem23) and increased TNF, IL1-β, IL-6, MMP-1, and MMP-8 expression []. It was reported that chemerin is associated with obesity, disease severity, inflammation, and cartilage destruction in patients with knee OA. The study showed that it was linked to obesity, BMI, joint inflammation, and cartilage degradation, independent of mechanical factors [].
Brain-derived neurotrophic factor (BDNF) is a protein found in the brain and, peripherally, in the blood []. Peripherally, it is expressed by skeletal [], adipose [], and endothelial cells [], and stored in a form bound to platelets in the blood, liver, and spleen []. As a highly conserved neurotrophic protein, it regulates synapses, affecting various brain regions structurally and functionally. It promotes neuron survival, neurite growth (the process by which developing neurons form new processes), and synapse formation [,,], ensuring neuroplasticity, learning, and memory. It also plays a role in the hypothalamic signaling pathway: it controls body weight, decreases food intake, and lowers blood glucose levels, thus controlling energy homeostasis [,]. About 70–80% of circulating plasma BDNF originates from the brain, both during exercise and recovery [].
Each of the above-mentioned factors seems to be related to physical activity, age-related diseases, or energy homeostasis. Different types of physical activity increase the release of myokines, including irisin, which, aside from other functions, stimulates the metabolism of energy-related signaling and memory formation-related signaling like BDNF [].
New therapies of degenerative disorders, like rheumatoid arthritis (RA), include nanomedical management []. However, we hypothesized that physical therapy, which usually provides good results for OA patients [], in the form of moderate, controlled exercise training after hip or knee replacement in OA patients with chronic pain would improve the profile of irisin, chemerin, and brain-derived neurotrophic factor (BDNF). The study aimed to determine the efficacy of individual rehabilitation exercise training in improving the functional capacity of patients who underwent hip or knee replacement surgery, and the accompanying changes in irisin, chemerin, and BDNF levels after a 21-day individually-adjusted exercise rehabilitation program.
2. Materials and Methods
2.1. Ethical Statement and Permissions
The study followed the Declaration of Helsinki guidelines and was approved by the Ethics Committee of the Medical University of Silesia in Katowice (N° KNW/002/KB1/106/17; 3 October 2017). Every participant of the study received the study protocol description, was informed about its benefits and possible risks, and returned the written informed consent before the study started.
2.2. Study Group
The participants were recruited over 2017–2018 from the outpatient clinic and the Department of Rehabilitation at the 3rd Specialist Hospital in Rybnik. The clinical interview carried out during the recruitment process excluded patients with inflammatory disorders, infections, renal or hepatic insufficiency, active coronary artery disease, diabetes, heart failure, hormonal replacement therapy, or supplementation with antioxidants taken up to 3 months before the study. Eventually, 41 patients after total hip (n = 29) or knee (n = 12) replacement, aged 61.0 ± 8.1 years, 22 men and 19 women, were included in the study (Table 1). On the initial rehabilitation day, the patients were 89.6 ± 9.7 days after the joint replacement surgery. On the first day they arrived at the outpatient clinic, the resting electrocardiogram (ECG) and blood pressure measurement were recorded, and the body mass and height measurements were taken. Later on, five patients were excluded from the irisin, chemerin, and BDNF analyses, due to health conditions that occurred during the study.

Table 1.
The inclusion and exclusion criteria for osteoarthritis (OA) patients after hip or knee replacement surgery enrolled to a 21-day individual rehabilitation exercise training.
2.3. Individual Rehabilitation Exercise Training
All patients underwent a 21-day individual rehabilitation exercise training program. The daily rehabilitation sessions started between 8:00 and 8:45 a.m. The individual rehabilitation exercise training mainly consisted of physiotherapy, and living activities training focused on improving the patients’ walking functionality: lengthening stride, increasing pace, walking backward and on uneven surfaces, climbing stairs, and Nordic walking. Additionally, the rehabilitation program included patients’ nutritional education. The individual rehabilitation sessions comprised 30–45 min of aerobic walking, 20–30 min of strength training, 30–45 min of rotor/bicycle training, and a 15 min cool-down phase. The patients were instructed to continue the learned activities at home, to keep their physical fitness and biochemical parameters at the beneficial level []. The choice of exercises (different strength and balance exercises) and training modalities (number and sets of repetitions as well as the duration of resting time) were individually adjusted to each patient, then monitored in the rehabilitation by the responsible physiotherapist.
2.4. Samples Collection
Blood samples were collected before the initial and after the final (after the patient’s HR returned to the resting value) rehabilitation sessions. A blood sample (5 mL) from the ulnar vein was collected to the standard blood tubes with a clot activator (S-Monovette, SARSTEDT). The samples for serum analysis were centrifuged at 4000 rpm for 10 min at 4 °C and then subsequently frozen and stored at −80 °C until further analyses could be performed.
2.5. Irisin, Chemerin, and Brain-Derived Neurotrophic Factor (BDNF) Assessment
Irisin and chemerin concentrations were assessed using an enzyme-linked immunoabsorbent assay (ELISA) kit (cat. no. RAG018R and RD191136200R, respectively, BioVendor, Brno, Czech Republic). BDNF concentration was assessed using an enzyme-linked immunoabsorbent assay (ELISA) kit (cat. no. SEA011Hu, Cloud Clone Corp., Katy, TX, USA). The serum samples and all reagents were prepared and processed as per the manufacturers’ guidelines. The color change in the samples was measured spectrophotometrically using the microplate reader (BioTek Synergy HTX Multimode Reader, BioTek® Instruments, Inc., Winooski, VT, USA). The results were calculated as per the manufacturers’ guidelines, using dedicated Gen 5 Microplate Data Collection and Analysis Software ver. 3.14.03 (BioTek® Instruments, Inc., Winooski, VT, USA). The results for irisin were read against an 8-point calibration curve ranging from 0.001–5 μg irisin/mL. Intra-assay precision was CV < 7%, inter-assay precision was CV < 10%, and the lower limit of detection was 1 ng irisin/mL. The results for chemerin were read against a 6-point calibration curve ranging from 0.25–8 ng chemerin/mL. Intra-assay precision was CV = 6%, inter-assay precision was CV = 7.6%, and the lower limit of detection was 1 ng irisn/mL.
The results for BDNF were read against a 7-point calibration curve ranging from 0.156–10 ng BDNF/mL. Intra-assay precision was CV < 10%, inter-assay precision was CV < 12%, and the lower limit of detection was 0.061 ng BDNF/mL.
2.6. Statistical Analysis
The analysis was performed, and graphs were created, using Statistica ver. 13.0 (TIBCO Software Inc., Palo Alto, CA, USA). Data distribution was assessed using the Shapiro–Wilk test and quantile–quantile plots. The mean values with standard deviation (SD) were calculated for normally distributed data, and the median and lower-upper quartile (Me (Q1–Q3)) for non-normal distributed data. The non-normal distributed data were log-transformed. The t-test for related samples was used to compare the parameters before and after rehabilitation. All tests were two-tailed. Statistical significance was set at p < 0.05.
3. Results
A 21-day individual rehabilitation exercise training program significantly changed irisin levels in patients after hip or knee replacement (Table 2). Irisin levels increased after the rehabilitation (p < 0.05) by about 0.06 ± 0.16 µg/mL (95%CI: 0.01–0.12) (Figure 1).

Table 2.
Irisin, chemerin, and brain-derived neurotrophic factor (BDNF) concentrations in the serum of patients after hip or knee replacement surgery, before and after a 21-day individual rehabilitation exercise training program.
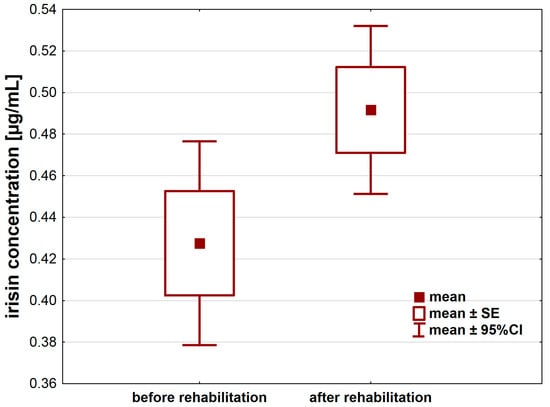
Figure 1.
Irisin concentration [µg/mL] in the serum of patients after hip or knee replacement surgery enrolled in a 21-day individual rehabilitation exercise training program.
Chemerin levels also significantly differed before and after a 21-day individual rehabilitation exercise training program (p < 0.001). The rehabilitation decreased chemerin concentration by about 60.9 (33.1–128.5) ng/mL (Figure 2, Table 2).
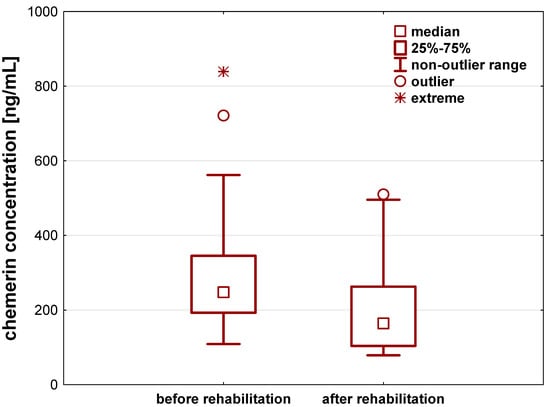
Figure 2.
Chemerin concentration [ng/mL] in the serum of patients after hip or knee replacement surgery enrolled to a 21-day individual rehabilitation exercise training program.
In case of BDNF, we observed its statistically significant increase in the patients serum after a 21-day individual rehabilitation exercise training program, compared to its concentration before the rehabilitation started to (p < 0.001). In the course of 21 days, BDNF levels increased by about 1.86 ± 1.96 ng/mL (95%CI: 1.22–2.51) (Figure 3, Table 2).
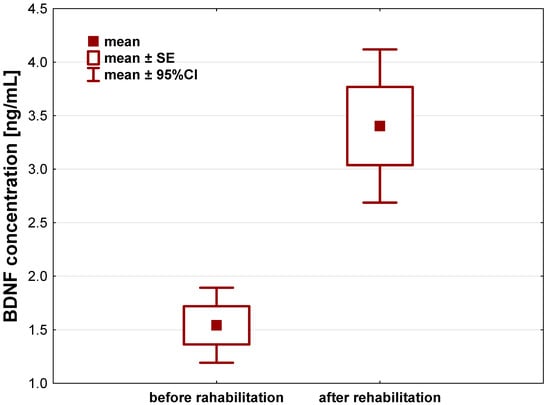
Figure 3.
Brain-derived neurotrophic factor (BDNF) concentration [ng/mL] in the serum of patients after hip or knee replacement surgery enrolled to a 21-day individual rehabilitation exercise training program.
A pairwise comparison of pre-treatment irisin, chemerin, and BDNF serum concentrations results was made, and no correlations were found. A pairwise comparison of post-treatment results for the same factors showed a weak negative correlation for irisin and BDNF (rho = −0.351, p < 0.05), and for chemerin and BDNF (rho = −0.416, p < 0.01) serum concentrations.
4. Discussion
Hip or knee arthroplasty constitutes a significant percentage of orthopedic surgeries. Arthroplasty improves patients’ motor capacity and their quality of life [,]. A hip arthroplasty procedure is recognized as one of the most common and most significant operations improving patients’ quality of life []. According to OECD data, access to arthroplasty treatment improved by about 7% from 2000 to 2009. Günsche et al. calculated the age-standardized incidence rates for total hip or knee replacements based on OECD data []. The authors found that the age-standardized incidence rates for total hip replacement is positively related to incidence and length of stay of coxarthrosis, age-standardized incidence rates for total knee replacement, health expenditures, number of nurses, and social insurance. On the other hand, diabetes prevalence, gross domestic product, and the number of doctor consultations negatively influence the age-standardized incidence rates. In contrast, the total knee replacement rate is positively influenced by health expenditures and the incidence rate of gonarthrosis, and negatively by the number of primary practitioners []. In Poland, the number of arthroplasty surgeries increased by 20% in 2017 []. Unfortunately, data on osteoarthritis (OA) prevalence, the frequency of its clinical phenotypes, and the physical disabilities it generates, or on the OA’s economic impact on the health system in Poland are non-existent.
In the presented study, we observed that a 21-day individual rehabilitation exercise training program led to a significant increase in irisin and brain-derived neurotrophic factor (BDNF), and a decrease in chemerin serum concentration in patients after hip or knee replacement surgery. Exercise is an effective non-pharmacological intervention that improves physical capacity, body functions, and health. Since the correlation between the beneficial effects of exercise and exercise intensity or duration shows the dose–response relationship, it seems that individually-adjusted rehabilitation exercise training is crucial for patients after hip or knee replacement surgery. In vitro and in vivo studies demonstrated that irisin influences bone cells [,,,].
Irisin’s effect on bone cells was demonstrated in several in vitro and in vivo studies [,,,]. Irisin stimulates osteoblasts’ differentiation, their activity, and increases osteocytes’ viability. Simultaneously, irisin affects the osteoclasts in two ways: indirectly, through the increased expression of osteoprotegerin (OPG) in osteoblasts, and directly, as a counter-regulatory hormone increasing osteoclast progenitors differentiation and promoting bone resorption [,,,]. Colaianni et al. [] have demonstrated that irisin affects all stages of osteoblast differentiation: the early stage, by increasing the number of ALP+ colonies, and the late stage, by enhancing the mineralized nodules formation []. In adults, irisin levels are affected by age, gender, obesity, and muscle mass []. Apart from improving the bone strength, irisin has also more broad effects, like increasing energy expenditure and improving cognition [,,]. Anastasilakis et al. [] demonstrated that the basic irisin level did not depend on the degree of physical activity, but it increased after 20 min of intense muscle exercise []. Kurdiova et al. [] also reported that irisin levels are related to the usual degree of physical activity and to muscular strength, contractility, and volume []. According to Anastasilakis et al. [] and Loffler et al. [], acute and strenuous exercise increase irisin blood concentration, but they do not change after long-term exercise (6 weeks/1 year).
In the past decade, scientific reports have focused on the responses of irisin to various exercise patterns and types of physical activity. Sprint-type exercises led to an acute increase in the peripheral concentration of irisin in Greyhound dogs [] and in humans [,]. Some studies showed that high-volume resistance exercises engaging all muscle groups led to an increase in the irisin concentration 1 h after exercise [,,], whereas irisin concentration remained unchanged when the exercise engaged only one muscle group []. Similarly, the chronic whole-body vibration exercise also increased irisin concentration [].
However, the meta-analysis [] of three randomized controlled trials showed that chronic resistance exercise training has a moderate and significant effect on circulating irisin and decreases it compared with the control, and endurance exercise training has only a similar but not significant trend. Similar analysis [] of nine non-randomized studies revealed that regular exercise training was associated with a small and non-significant overall effect and decreased irisin levels compared with the baseline. On the other hand, Gaudio et al. [] reported that physical activity positively increased serum irisin levels and bone turnover markers in competitive footballers, when compared to similar subjects with a predominantly sedentary lifestyle []. However, in pathophysiological conditions, reduced circulating irisin levels were reported in patients with chronic kidney disease or T2DM, preeclamptic women during gestation, and osteoporotic patients [].
Here, we report that a 21-day individual rehabilitation exercise training program increased irisin serum concentrations. The assumption of the applied individual rehabilitation exercise training was to ensure that each of the patients, for 21 consecutive days, completed the individually-adjusted exercise sessions that consisted of 30–45 min of aerobic walking, 20–30 min of strength training, and 30–45 min of rotor/bicycle training. The physiotherapy and the living activity training focused on improving the patient’s walking functionality, and engaged all body muscle groups. The obtained results agree with the studies that reported increased levels of irisin after high-volume resistance exercises engaging all muscle groups [,,]. Due to its numerous biological roles, irisin is a prospective therapeutic target, and it offers a new potential foundation for physical therapy []. Nevertheless, further studies are needed to determine clinical significance of irisin as the potential marker of successful rehabilitation exercise training of OA patients.
Chemerin presence is related to inflammatory processes such as psoriasis, obesity, metabolic syndrome, hypertension, angina, and cancer []. High chemerin concentrations induce MMP-2, MMP-3, MMP-13, and IL8, which accompany joint cartilage degradation []. However, studies on chemerin in OA patients are infrequent, and give diverse results. Valcamonica et al. [] observed high chemerin levels in patients with knee OA. In this study, chemerin levels were related to C-reactive protein, IL-6, and TNF-α levels. The authors suggested that chemerin comprises an inflammatory component []. In their previous study, Valcamonica et al. [] reported contradictory results, showing no significant difference in serum chemerin levels in 11 OA patients, 8 psoriatic arthritis, and 18 rheumatoid arthritis patients. On the contrary, Ma et al. [] observed higher chemerin levels at the synovial fluid and membrane level in patients with knee OA than in the control group. Similarly, Huang et al. also reported increased chemerin levels in the serum and synovial fluid of patients with knee OA []. Bozaoglu et al. [] related serum chemerin levels to metabolic syndrome components because, in glucose tolerant subjects, plasma chemerin levels were significantly associated with BMI, circulating triglycerides, and blood pressure. We noted a significant decrease in chemerin levels in patients after knee or hip replacement surgery, who underwent the 21-day individual rehabilitation exercise training program. Our findings are similar to the results by Stefanov et al. [], who reported that a 6-month exercise program combining endurance and resistance exercises led to a statistically significant reduction in serum chemerin concentration in middle-aged, overweight or obese, non-diabetic individuals [].
Physical exercise (muscle contraction) is an important modulator influencing the production of cytokines such as BDNF []. Systematic reviews [,] and meta-analyses [,] conclude that an acute bout of physical activity transiently increases BDNF peripheral levels. Additionally, chronic physical activity increases BDNF response to an acute bout of physical activity []. On the other side of the spectrum, physical inactivity determines the activation of systemic inflammatory pathways in chronic diseases []. Low BDNF levels are associated with aging and several diseases: neurologic [], psychiatric [], frailty syndrome [], and impaired cognitive function []. Elevated BDNF concentrations that follow intervention (like physical therapy) [] and physical activity [] suggest that BDNF may be an important regulatory factor in the elderly population. The results presented here demonstrate increased BDNF serum concentrations after 21 days of regular and moderate exercise. Our previous study showed that the 21-day general alternative rehabilitation exercise training also improved the clinical parameters, such as blood morphology, dyslipidemia, BMI, oxidative stress markers, and the patient’s overall fitness measured with the six-minute walking test (6MWT) of elderly patients after hip or knee replacement surgery due to OA []. Since the studies on BDNF plasma concentrations and inflammatory diseases are scarce, it may also be possible that initially low BDNF serum levels are not related to OA, but are instead associated with chronic inflammatory conditions related to aging, as suggested by Gomes et al. [] and Vasto et al. []. Nevertheless, even in light of this possibility, the presented study brings novel data to this discussion.
We are aware of two limitations of the presented study. The first one is related to the number of patients included in the study. It was mostly due to successive loss of patients or withdrawals. For some patients, it was difficult to comply with the rehabilitation program schedule due to personal reasons, or problems with mobility or transport. The second limitation is related to the study design, which does not include a control group consisting of healthy individuals.
5. Conclusions
A 21-day individual rehabilitation exercise training program led to a significant increase in irisin and brain-derived neurotrophic factor (BDNF), and a decrease in chemerin serum concentration in patients after hip or knee replacement surgery. Selected markers are widely studied in the context of various diseases, and considered potential new markers of these pathophysiological conditions. Irisin and BDNF seem best choice for that use, however, more research in this area is necessary.
Author Contributions
Conceptualization, D.S., B.S.-P. and M.I.; methodology, D.S. and B.S.-P.; software, D.S. and B.S.-P.; validation, D.S., B.S.-P. and J.Z.-F.; formal analysis, D.S., B.S.-P., M.I., J.Z.-F. and K.M.; investigation, D.S., B.S.-P., M.K., B.B. and J.P.; resources, D.S., B.S.-P. and J.J.; data curation, D.S., B.S.-P., M.I., E.C., D.N. and M.K.; writing—original draft preparation, D.S., B.S.-P., M.I., J.P., K.M., B.B., D.N., J.J. and E.C.; writing—review and editing, D.S., B.S.-P., J.P., K.M., B.B., D.N., J.J., E.C., J.Z.-F. and M.K.; visualization, D.N., M.K., K.M. and E.C.; supervision, D.S. and B.S.-P.; project administration, D.S., B.S.-P. and J.P.; funding acquisition, D.S., B.S.-P. and M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC were funded by Medical University of Silesia grant number N° KNW/002/KB1/106/17; 3 October 2017.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Medical University of Silesia in Katowice (N° KNW/002/KB1/106/17; 3 October 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cajas Santana, L.J.; Rondón Herrera, F.; Rojas, A.P.; Martínez Lozano, D.J.; Prieto, N.; Bohorquez Castañeda, M. Serum chemerin in a cohort of Colombian patients with primary osteoarthritis. Reumatol. Clin. (Engl. Ed.) 2021, 17, 530–535. [Google Scholar] [CrossRef]
- Batushansky, A.; Zhu, S.; Komaravolu, R.K.; South, S.; Mehta-D’souza, P.; Griffin, T.M. Fundamentals of OA. An initiative of osteoarthritis and cartilage. obesity and metabolic factors in OA. Osteoarthr. Cartil. 2022, 30, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Kim, T.H.; Nguyen, T.T.; Park, K.-S.; Kim, N.; Kong, I.D. Circulating irisin levels as a predictive biomarker for sarcopenia: A cross-sectional community-based study. Geriatr. Gerontol. Int. 2017, 17, 2266–2273. [Google Scholar] [CrossRef] [PubMed]
- Munoz, I.Y.M.; Romero, E.D.S.C.; Garcia, J.J.G. Irisin a novel metabolic biomarker: Present knowledge and future directions. Int. J. Endocrinol. 2018, 2018, 7816806. [Google Scholar] [CrossRef]
- Ma, E.B.; Sahar, N.E.; Jeong, M.; Huh, J.Y. Irisin exerts inhibitory effect on adipogenesis through regulation of Wnt signaling. Front. Physiol. 2019, 10, 1085. [Google Scholar] [CrossRef] [PubMed]
- Arhire, L.I.; Mihalache, L.; Covasa, M. Irisin: A hope in understanding and managing obesity and metabolic syndrome. Front. Endocrinol. 2019, 10, 524. [Google Scholar] [CrossRef]
- Korta, P.; Pochec, E.; Mazur-Biały, A. Irisin as a multifunctional protein: Implications for health and certain diseases. Medicina 2019, 55, 485. [Google Scholar] [CrossRef]
- Kukla, M.; Menżyk, T.; Dembiński, M.; Winiarski, M.; Garlicki, A.; Bociąga-Jasik, M.; Skonieczna, M.; Hudy, D.; Maziarz, B.; Kuśnierz-Cabala, B.; et al. Fetuin-A deficiency but not pentraxin 3, FGF-21, or irisin, predisposes to more serious COVID-19 course. Biomolecules 2021, 11, 1422. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Liang, J.; Kirberger, M.; Chen, N. Irisin, an exercise-induced bioactive peptide beneficial for health promotion during aging process. Ageing Res. Rev. 2022, 80, 101680. [Google Scholar] [CrossRef]
- Madhu, L.N.; Somayaji, Y.; Shetty, A.K. Promise of irisin to attenuate cognitive dysfunction in aging and Alzheimer’s disease. Ageing Res. Rev. 2022, 78, 101637. [Google Scholar] [CrossRef]
- Colaianni, G.; Cinti, S.; Colucci, S.; Grano, M. Irisin and musculoskeletal health. Ann. N. Y. Acad. Sci. 2017, 1402, 5–9. [Google Scholar] [CrossRef]
- Eisinger, K.; Bauer, S.; Schäffler, A.; Walter, R.; Neumann, E.; Buechler, C.; Müller-Ladner, U.; Frommer, K.W. Chemerin induces CCL2 and TLR4 in synovial fi broblasts of patients with rheumatoid arthritis and osteoarthritis. Exp. Mol. Pathol. 2012, 92, 90–96. [Google Scholar] [CrossRef]
- Abou-Raya, S.; Abou-Raya, A.; Khadrawi, T. Chemerin and knee osteoarthritis: Effects on inflammation and cartilage destruction. Ann. Rheum. Dis. 2013, 72, A104. [Google Scholar] [CrossRef]
- Binder, D.K.; Scharfman, H.E. Brain-derived neurotrophic factor. Growth Factors 2004, 22, 123. [Google Scholar] [CrossRef]
- Matthews, V.B.; Åström, M.-B.; Chan, M.H.S.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Åkerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Nakagomi, A.; Okada, S.; Yokoyama, M.; Yoshida, Y.; Shimizu, I.; Miki, T.; Kobayashi, Y.; Minamino, T. Role of the central nervous system and adipose tissue BDNF/TrkB axes in metabolic regulation. NPJ Aging Mech. Dis. 2015, 1, 15009. [Google Scholar] [CrossRef]
- Helan, M.; Aravamudan, B.; Hartman, W.R.; Thompson, M.A.; Johnson, B.D.; Pabelick, C.M.; Prakash, Y. BDNF secretion by human pulmonary artery endothelial cells in response to hypoxia. J. Mol. Cell. Cardiol. 2014, 68, 89–97. [Google Scholar] [CrossRef]
- Yang, B.; Ren, Q.; Zhang, J.; Chen, Q.; Hashimoto, K. Altered expression of BDNF, BDNF pro-peptide and their precursor proBDNF in brain and liver tissues from psychiatric disorders: Rethinking the brain–liver axis. Transl. Psychiatry 2017, 7, e1128. [Google Scholar] [CrossRef] [PubMed]
- Babiarz, M.; Laskowski, R.; Grzywacz, T. Effects of strength training on BDNF in healthy young adults. Int. J. Environ. Res. Public Health 2022, 19, 13795. [Google Scholar] [CrossRef]
- Goekint, M.; De Pauw, K.; Roelands, B.; Njemini, R.; Bautmans, I.; Mets, T.; Meeusen, R. Strength training does not influence serum brain-derived neurotrophic factor. Eur. J. Appl. Physiol. 2010, 110, 285–293. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Chao, M.V. Downstream consequences of exercise through the action of BDNF. Brain Plast. 2015, 1, 143–148. [Google Scholar] [CrossRef]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Fargali, S.; Sadahiro, M.; Jiang, C.; Frick, A.L.; Indall, T.; Cogliani, V.; Welagen, J.; Lin, W.J.; Salton, S.R. Role of neurotrophins in the development and function of neural circuits that regulate energy homeostasis. J. Mol. Neurosci. 2012, 48, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Papp, C.; Pak, K.; Erdei, T.; Juhasz, B.; Seres, I.; Szentpéteri, A.; Kardos, L.; Szilasi, M.; Gesztelyi, R.; Zsuga, J. Alteration of the irisin-brain-derived neurotrophic factor axis contributes to disturbance of mood in COPD patients. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 2023–2033. [Google Scholar] [CrossRef]
- Radu, A.F.; Bungau, S.G. Nanomedical approaches in the realm of rheumatoid arthritis. Ageing Res. Rev. 2023, 87, 101927. [Google Scholar] [CrossRef] [PubMed]
- Radu, A.F.; Bungau, S.G.; Tit, D.M.; Behl, T.; Uivaraseanu, B.; Marcu, M.F. Highlighting the benefits of rehabilitation treatments in hip osteoarthritis. Medicina 2022, 58, 494. [Google Scholar] [CrossRef]
- Li, Y.; Su, Y.; Chen, S.; Zhang, Y.; Zhang, Z.; Liu, C.; Lu, M.; Liu, F.; Li, S.; He, Z.; et al. The effects of resistance exercise in patients with knee osteoarthritis: A systematic review and meta-analysis. Clin. Rehab. 2016, 30, 947–959. [Google Scholar] [CrossRef]
- Singh, J.A. Epidemiology of knee and hip arthroplasty: A systematic review. Open Orthop. J. 2011, 5, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Meessen, J.M.T.A.; Peter, W.F.; Wolterbeek, R.; ·Cannegiete, S.C.; Tilbury, C.; Bénard, M.R.; van der Linden, H.M.J.; Onstenk, R.; Tordoir, R.; Vehmeijer, S.B.; et al. Patients who underwent total hip or knee arthroplasty are more physically active than the general Dutch population. J. Rheumatol. Int. 2017, 37, 219–227. [Google Scholar] [CrossRef]
- McPherson, K.; Gon, G.; Scott, M. International Variations in a Selected Number of Surgical Procedures; OECD Health Working Papers, No. 61; OECD Publishing: Paris, France, 2013. [Google Scholar]
- Günsche, J.L.; Pilz, V.; Hanstein, T.; Skripitz, R. The variation of arthroplasty procedures in the OECD Countries: Analysis of possible influencing factors by linear regression. Orthop. Rev. 2020, 12, 8526. [Google Scholar] [CrossRef]
- Gajda, M.; Pac, A.; Gryglewska, B.; Gajda, P.; Różańska, A.; Wójkowska-Mach, J. Patients undergoing hip or knee arthroplasty in poland based on national data—Challenge for healthcare in aging society. Healthcare 2021, 9, 924. [Google Scholar] [CrossRef]
- Colucci, S.; Colaianni, G.; Brunetti, G.; Ferranti, F.; Mascetti, G.; Mori, G.; Grano, M. Irisin prevents microgravity-induced impairment of osteoblast differentiation in vitro during the space flight CRS-14 mission. FASEB J. 2020, 34, 10096–10106. [Google Scholar] [CrossRef]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G.; et al. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.A.; Novick, S.J.; et al. Irisin mediates effects on bone and fat via αv integrin receptors. Cell 2018, 175, 1756–1768. [Google Scholar] [CrossRef]
- Estell, E.G.; Le, P.T.; Vegting, Y.; Kim, H.; Wrann, C.; Bouxsein, M.L.; Nagano, K.; Baron, R.; Spiegelman, B.M.; Rosen, C.J. Irisin directly stimulates osteoclastogenesis and bone resorption in vitro and in vivo. Elife 2020, 9, e58172. [Google Scholar] [CrossRef] [PubMed]
- Zerlotin, R.; Oranger, A.; Pignataro, P.; Dicarlo, M.; Maselli, F.; Mori, G.; Colucci, S.C.; Grano, M.; Colaianni, G. Irisin and secondary osteoporosis in humans. Int. J. Mol. Sci. 2022, 23, 690. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Mongelli, T.; Cuscito, C.; Pignataro, P.; Lippo, L.; Spiro, G.; Notarnicola, A.; Severi, I.; Passeri, G.; Mori, G.; et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci. Rep. 2017, 7, 2811. [Google Scholar] [CrossRef] [PubMed]
- Löffler, D.; Müller, U.; Scheuermann, K.; Friebe, D.; Gesing, J.; Bielitz, J.; Erbs, S.; Landgraf, K.; Wagner, I.V.; Kiess, W.; et al. Serum irisin levels are regulated by acute strenuous exercise. J. Clin. Endocrinol. Metab. 2015, 100, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Valverde, P.; Zhu, X.; Murray, D.; Wu, Y.; Yu, L.; Jiang, H.; Dard, M.M.; Huang, J.; Xu, Z.; et al. Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Res. 2017, 5, 16056. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Saridakis, Z.G.; Kynigopoulos, G.; Skouvaklidou, E.C.; Molyvas, D.; Vasiloglou, M.F.; Apostolou, A.; Karagiozoglou-Lampoudi, T.; Siopi, A.; et al. Circulating irisin in healthy, young individuals: Day-night rhythm, effects of food intake and exercise, and associations with gender, physical activity, diet, and body composition. J. Clin. Endocrinol. Metab. 2014, 99, 3247–3255. [Google Scholar] [CrossRef]
- Kurdiova, T.; Balaz, M.; Vician, M.; Maderova, D.; Vlcek, M.; Valkovic, L.; Srbecky, M.; Imrich, R.; Kyselovicova, O.; Belan, V.; et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: In vivo and in vitro studies. J. Physiol. 2014, 592 Pt 5, 1091–1107. [Google Scholar] [CrossRef]
- Bell, M.A.; Levine, C.; Downey, R.L.; Griffitts, C.; Mann, S.; Frye, C.W.; Wakshlag, J.J. Influence of endurance and sprinting exercise on plasma adiponectin, leptin and irisin concentrations in racing Greyhounds and sled dogs. Aust. Vet. J. 2016, 94, 154–159. [Google Scholar] [CrossRef]
- Huh, J.Y.; Siopi, A.; Mougios, V.; Park, K.H.; Mantzoros, C.S. Irisin in response to exercise in humans with and without metabolic syndrome. J. Clin. Endocrinol. Metab. 2015, 100, E453–E457. [Google Scholar] [CrossRef]
- Huh, J.Y.; Mougios, V.; Kabasakalis, A.; Fatouros, I.; Siopi, A.; Douroudos, I.I.; Filippaios, A.; Panagiotou, G.; Park, K.H.; Mantzoros, C.S. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J. Clin. Endocrinol. Metab. 2014, 99, E2154–E2161. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Ando, D.; Takamatsu, K.; Goto, K. Resistance exercise induces a greater irisin response than endurance exercise. Metabolism 2015, 64, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, H.; Slettaløkken, G.; Vegge, G.; Hollan, I.; Whist, J.E.; Strand, T.; Rønnestad, B.R.; Ellefsen, S. Irisin in blood increases transiently after single sessions of intense endurance exercise and heavy strength training. PLoS ONE 2015, 10, e0121367. [Google Scholar] [CrossRef] [PubMed]
- Pekkala, S.; Wiklund, P.K.; Hulmi, J.J.; Ahtiainen, J.P.; Horttanainen, M.; Pöllänen, E.; Mäkelä, K.A.; Kainulainen, H.; Häkkinen, K.; Nyman, K.; et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J. Physiol. 2013, 591, 5393–5400. [Google Scholar] [CrossRef]
- Huh, J.Y.; Mougios, V.; Skraparlis, A.; Kabasakalis, A.; Mantzoros, C.S. Irisin in response to acute and chronic whole-body vibration exercise in humans. Metabolism 2014, 63, 918–921. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, X.; Sun, Z.; Schumann, U.; Zügel, M.; Steinacker, J.M. Chronic exercise training and circulating irisin in adults: A meta-analysis. Sports Med. 2015, 45, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Gaudio, A.; Rapisarda, R.; Xourafa, A.; Zanoli, L.; Manfrè, V.; Catalano, A.; Signorelli, S.S.; Castellino, P. Effects of competitive physical activity on serum irisin levels and bone turnover markers. J. Endocrinol. Investig. 2021, 44, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Irisin as an exercise-stimulated hormone binding crosstalk between organs. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 316–321. [Google Scholar]
- Bao, J.F.; She, Q.Y.; Hu, P.P.; Jia, N.; Li, A. Irisin, a fascinating field in our times. Trends Endocrinol. Metab. 2022, 33, 601–613. [Google Scholar] [CrossRef]
- Acewicz, M.; Kasacka, I. Chemerin activity in selected pathological states of human body—A systematic review. Adv. Med. Sci. 2021, 66, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Du, G.; Li, L.; Liang, H.; Zhang, B. Association of chemerin levels in synovial fluid with the severity of knee osteoarthritis. Biomarkers 2012, 17, 16–20. [Google Scholar] [CrossRef]
- Valcamonica, E.; Chighizola, C.; Comi, D.; De Lucia, O.; Pisoni, L.; Murgo, A.; Salvi, V.; Sozzani, S.; Meroni, P.L. Levels of chemerin and interleukin 8 in the synovial fluid of patients with inflammatory arthritides and osteoarthritis. Clin. Exp. Rheumatol. 2014, 32, 243–250. [Google Scholar] [PubMed]
- Ma, J.; Niu, D.; Wan, N.; Qin, Y.; Guo, C. Elevated chemerin levels in synovial fluid and synovial membrane from patients with knee osteoarthritis. Int. J. Clin. Exp. Pathol. 2015, 8, 13393–13398. [Google Scholar] [PubMed]
- Huang, T.; Larsen, K.; Ried-Larsen, M.; Møller, N.; Andersen, L.B. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review Scand. J. Med. Sci. Sports 2014, 24, 1–10. [Google Scholar] [CrossRef]
- Bozaoglu, K.; Bolton, K.; McMillan, J.; Zimmet, P.; Jowett, J.; Collier, G.; Walder, K.; Segal, D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 2007, 148, 4687–4694. [Google Scholar] [CrossRef]
- Stefanov, T.; Blüher, M.; Vekova, A.; Bonova, I.; Tzvetkov, S.; Kurktschiev, D.; Temelkova-Kurktschiev, T. Circulating chemerin decreases in response to a combined strength and endurance training. Endocrine 2014, 45, 382–391. [Google Scholar] [CrossRef]
- Pedersen, B.K. Exercise-induced myokines and their role in chronic diseases. Brain Behav. Immun. 2011, 25, 811–816. [Google Scholar] [CrossRef]
- Knaepen, K.; Goekint, M.; Heyman, E.M.; Meeusen, R. Neuroplasticity—Exercise-induced response of peripheral brain-derived neurotrophic factor. Sports Med. 2010, 40, 765–801. [Google Scholar] [CrossRef]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Lanctôt, K.L. The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: A meta-analysis. Eur. J. Neurosci. 2017, 46, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Schabitz, W.R.; Steigleder, T.; Cooper-Kuhn, C.M.; Schwab, S.; Sommer, C.; Schneider, A.; Kuhn, H.G. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke 2007, 38, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, G.; Lira, C.M.; Johansson, J.; Wisen, A.; Wohlfart, B.; Ekman, R.; Westrin, A. The acute response of plasma brain-derived neurotrophic factor as a result of exercise in major depressive disorder. Psychiatry Res. 2009, 169, 244–248. [Google Scholar] [CrossRef]
- Coelho, F.M.; Pereira, D.S.; Lustosa, L.P.; Silva, J.P.; Dias, J.M.; Dias, R.C.; Queiroz, B.Z.; Teixeira, A.L.; Teixeira, M.M.; Pereira, L.S. Physical therapy intervention (PTI) increases plasma brain-derived neurotrophic factor (BDNF) levels in non-frail and pre-frail elderly women. Arch. Gerontol. Geriatr. 2012, 54, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Komulainen, P.; Pedersen, M.; Hanninen, T.; Bruunsgaard, H.; Lakka, T.A.; Kivipelto, M.; Hassinen, M.; Rauramaa, T.H.; Pedersen, B.K.; Rauramaa, R. BDNF is a novel marker of cognitive function in ageing women: The DR’s EXTRA Study. Neurobiol. Learn. Mem. 2008, 90, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Correia, P.R.; Scorza, F.A.; Gomes da Silva, S.; Pansani, A.; Toscano-Silva, M.; de Almeida, A.C.; Arida, R.M. Increased basal plasma brain-derived neurotrophic factor levels in sprint runners. Neurosci. Bull. 2011, 27, 325–329. [Google Scholar] [CrossRef]
- Skrzep-Poloczek, B.; Poloczek, J.; Chełmecka, E.; Kazura, W.; Dulska, A.; Idzik, M.; Jochem, J.; Stygar, D. General, 21-day postoperative rehabilitation program has beneficial effect on oxidative stress markers in patients after total hip or knee replacement. Oxid. Med. Cell. Longev. 2020, 2020, 4598437. [Google Scholar] [CrossRef]
- Gomes, W.F.; Lacerda, A.C.R.; Mendonça, V.A.; Arrieiro, A.N.; Fonseca, S.F.; Amorim, M.R.; Teixeira, A.L.; Teixeira, M.M.; Miranda, A.S.; Coimbra, C.C.; et al. Effect of exercise on the plasma BDNF levels in elderly women with knee osteoarthritis. Rheumatol. Int. 2014, 34, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Vasto, S.; Candore, G.; Balistreri, C.R.; Caruso, M.; Colonna-Romano, G.; Grimaldi, M.P.; Listi, F.; Nuzzo, D.; Lio, D.; Caruso, C. Inflammatory networks in ageing, age-related diseases and longevity. Mech. Ageing Dev. 2007, 128, 83–91. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).