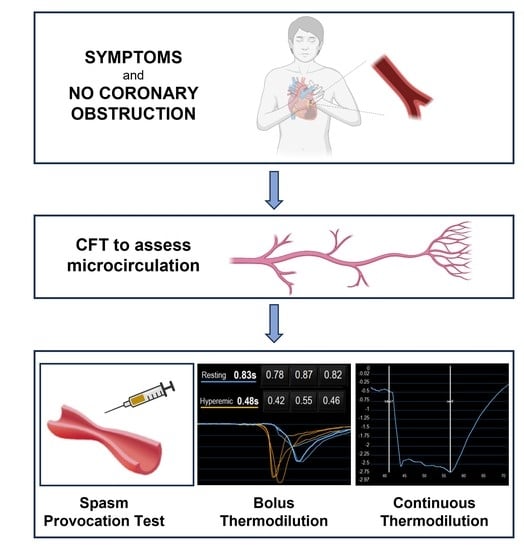
Coronary Vascular (DYS) Function and Invasive Physiology Assessment: Insights into Bolus and Continuous Thermodilution Methods
Abstract
1. Introduction
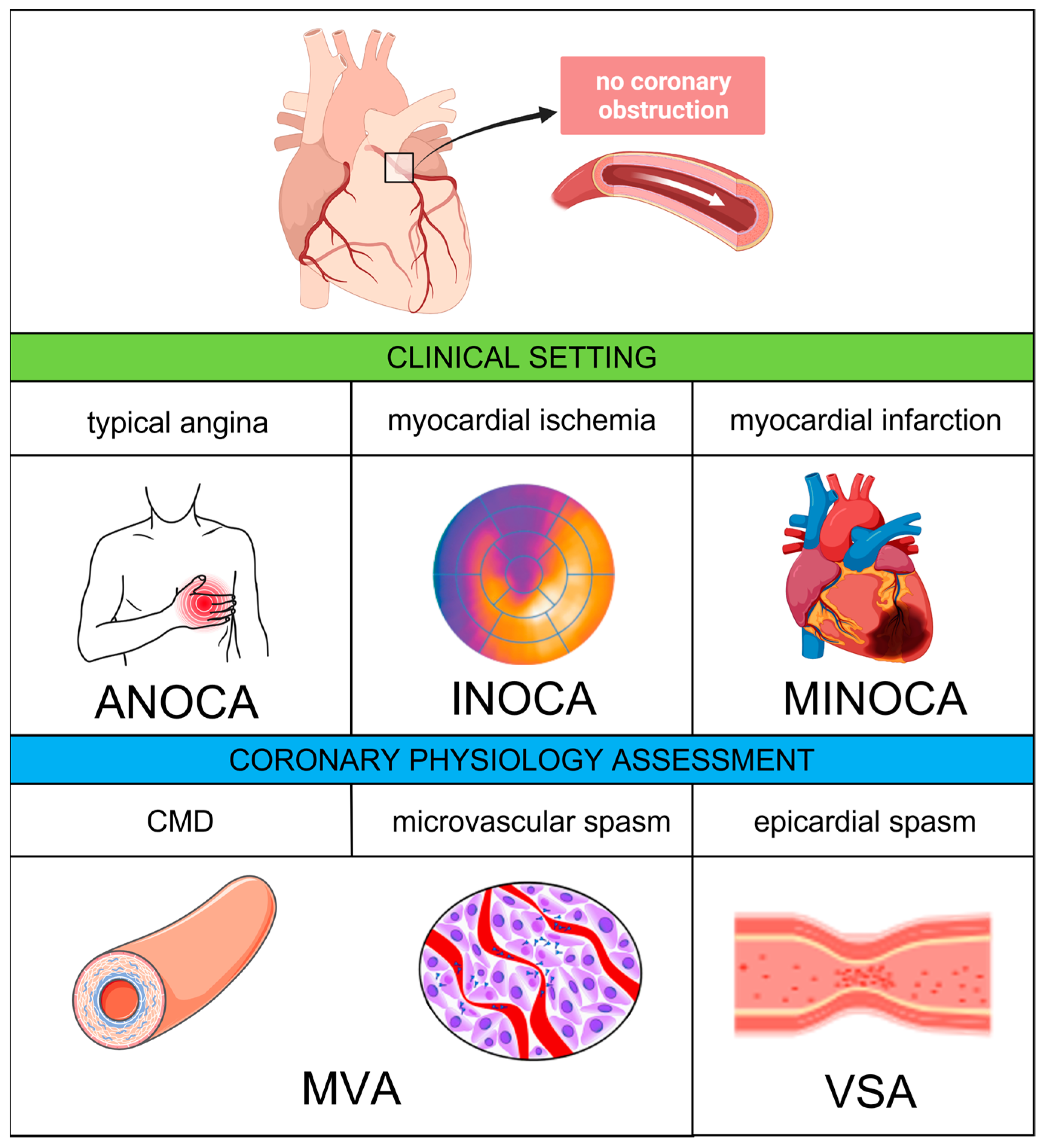
2. Definitions
3. Epidemiology and Prognostic Implications
4. Pathophysiology
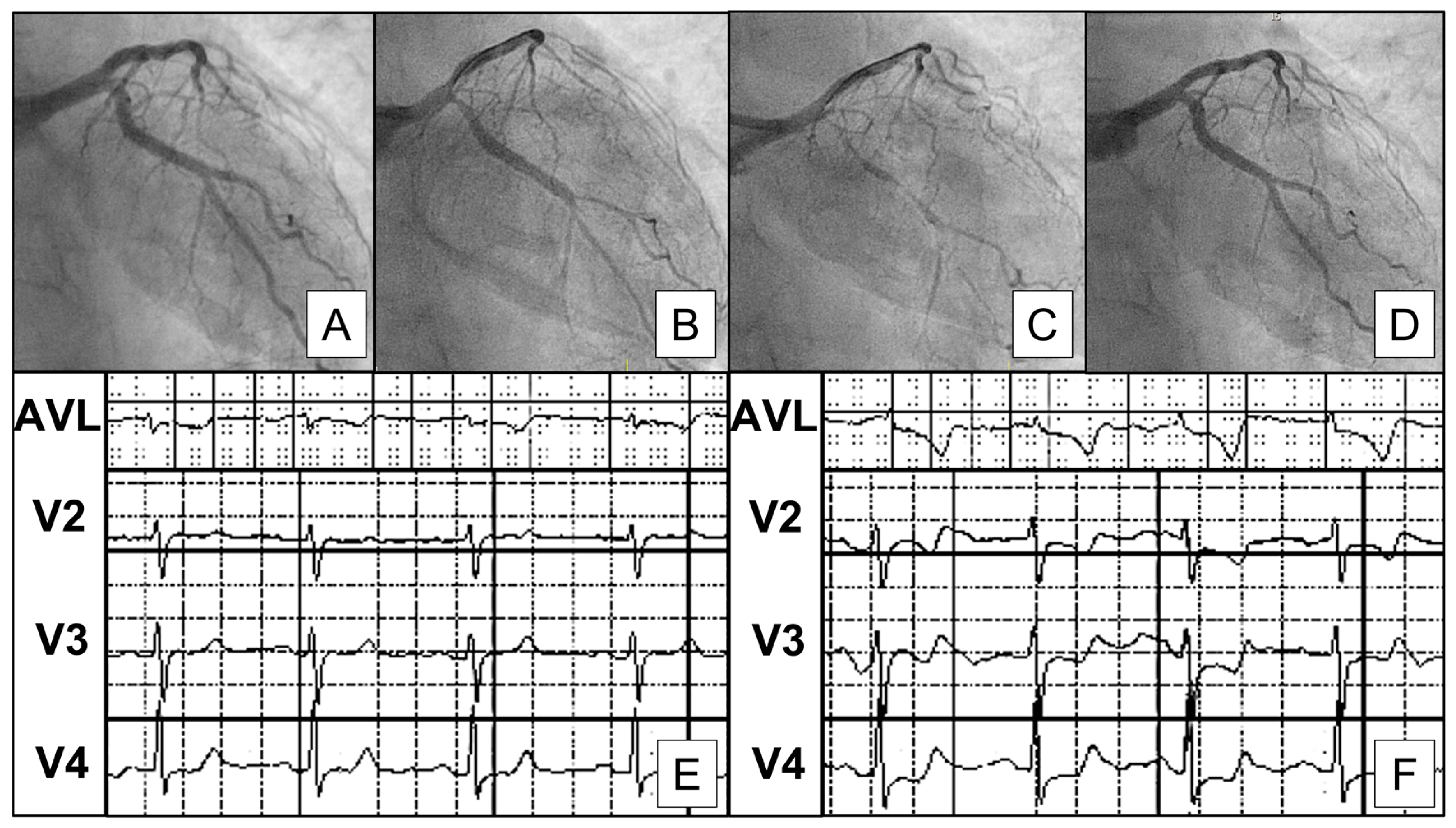
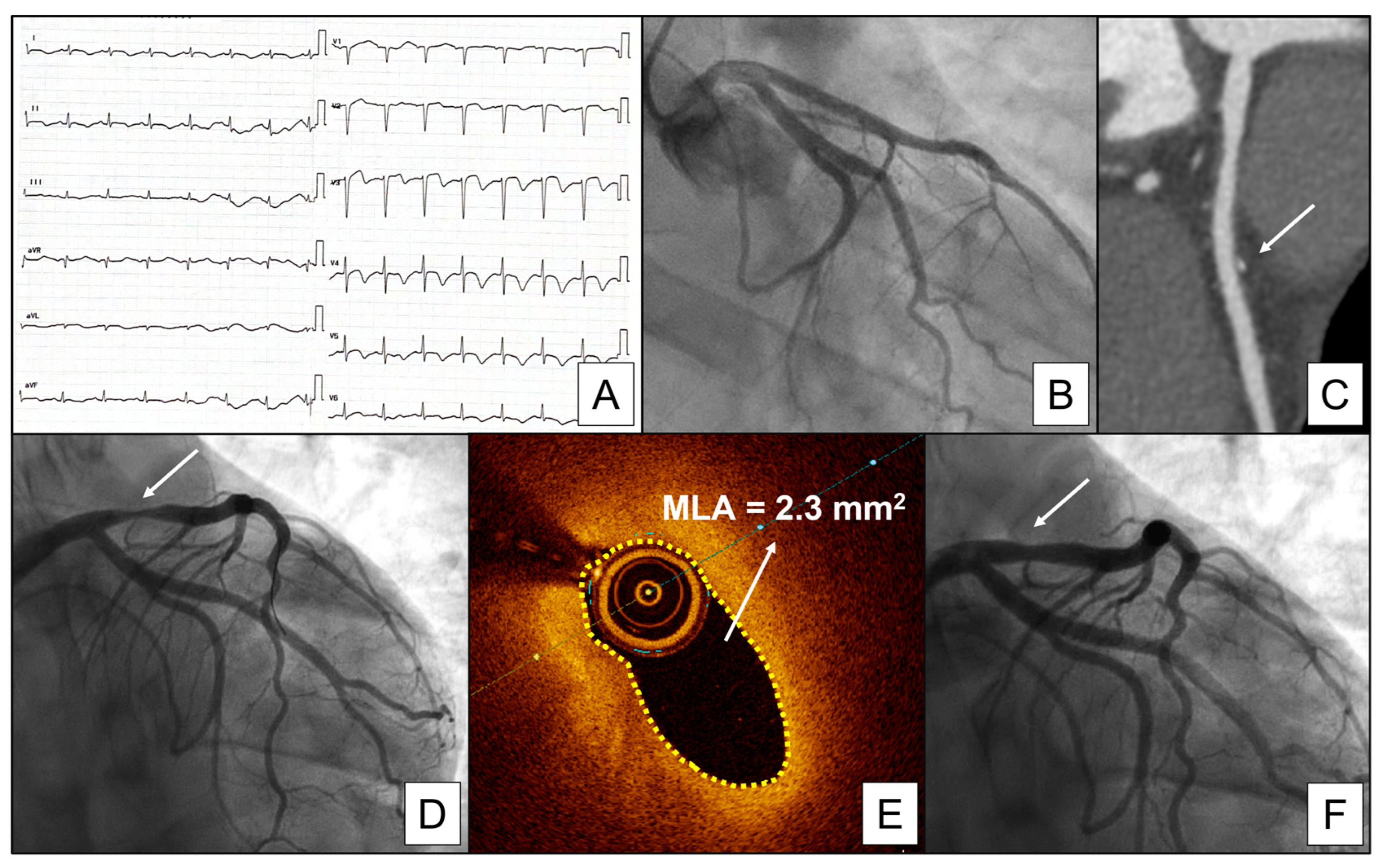
4.1. Epicardial and Microvascular Vasospasm
4.2. Coronary Microvascular Dysfunction
5. Diagnosis
5.1. Spasm Provocation Test
5.2. Assessing CMD—General Concepts
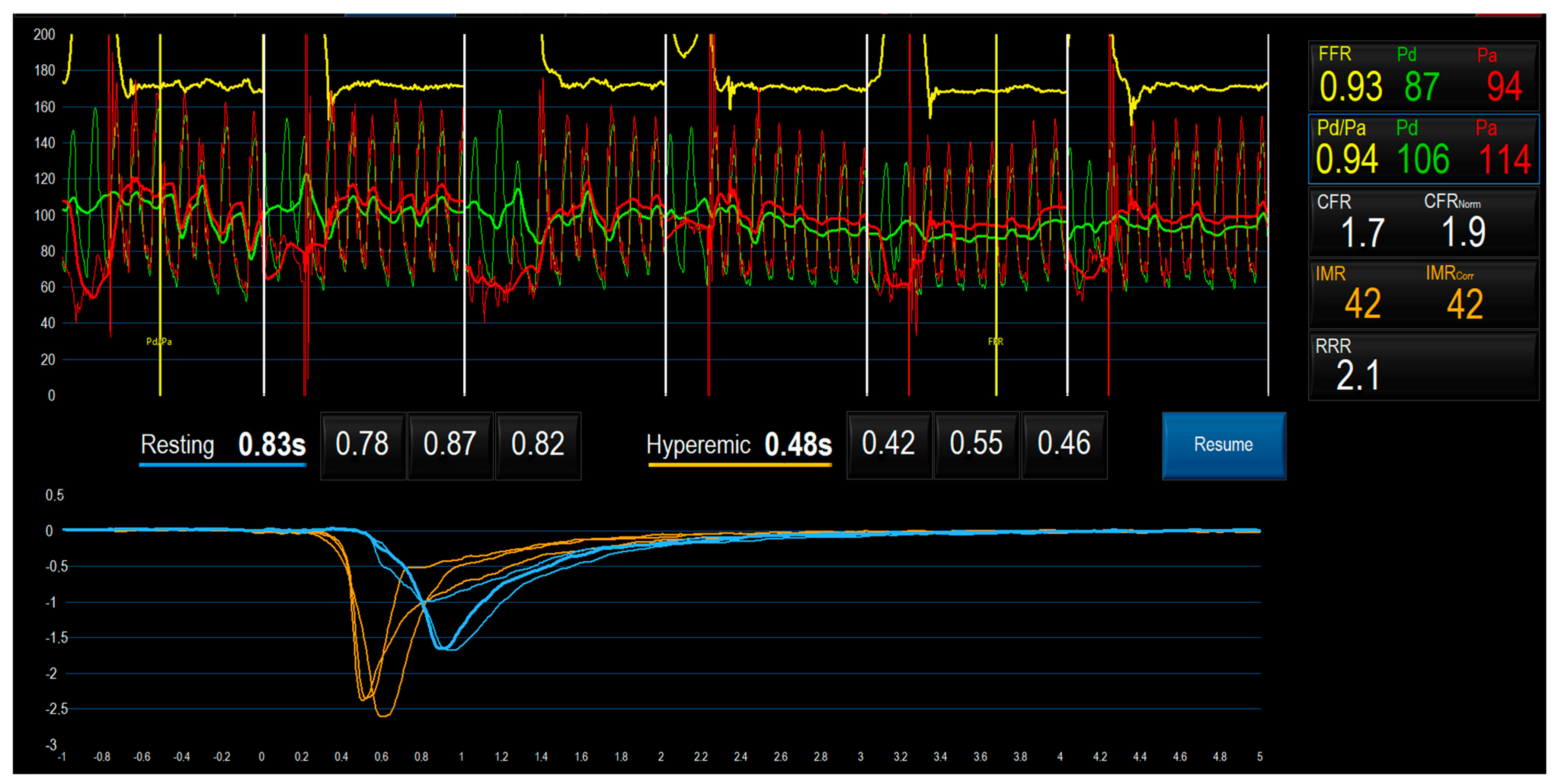
5.3. Bolus Thermodilution Method
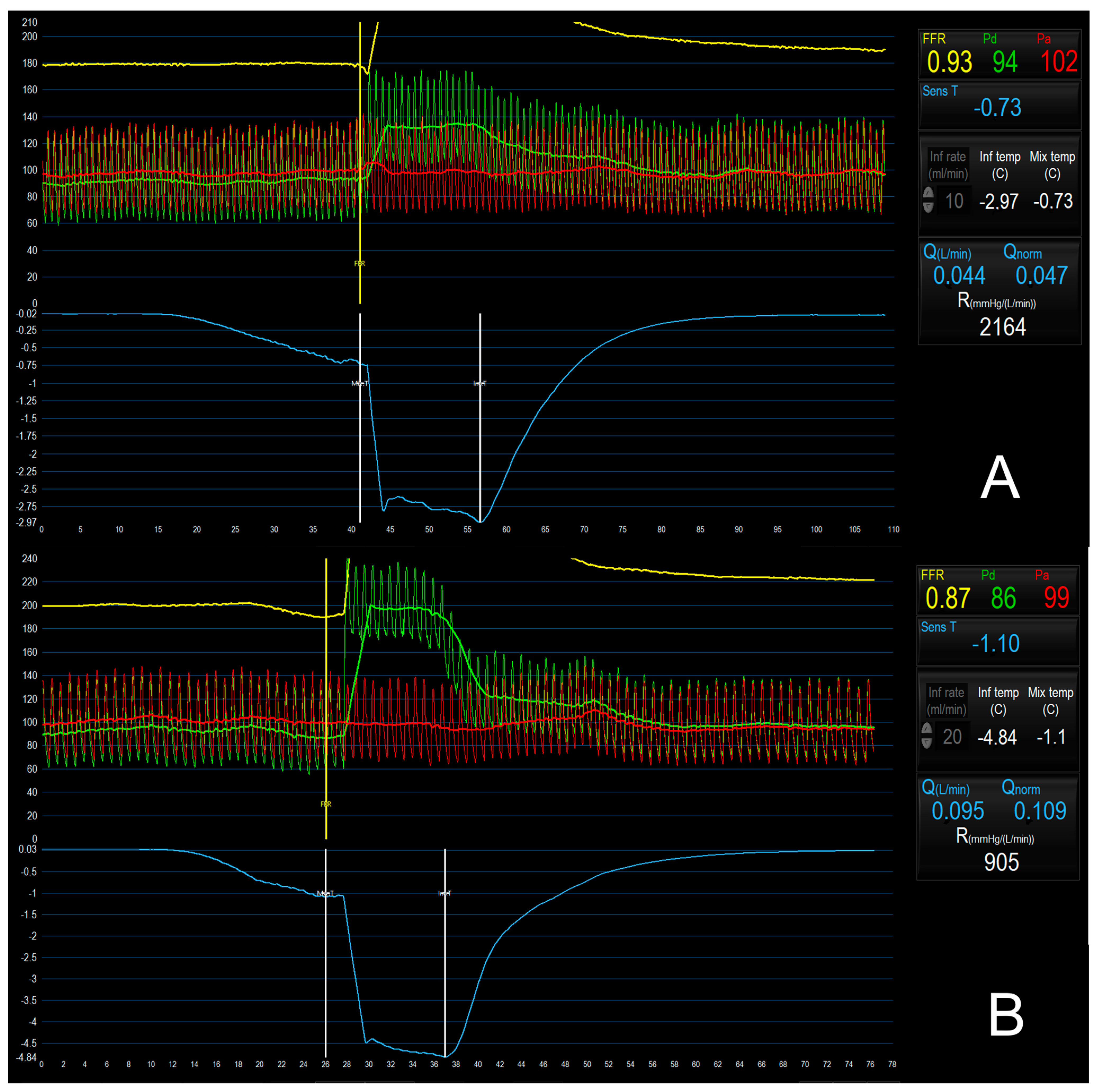
5.4. Continuous Thermodilution Method
5.5. Bolus vs. Continuous Thermodilution Method to Assess CMD
6. Treatment
Therapy of ANOCA
7. Gaps in Knowledge
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, M.R.; Peterson, E.D.; Dai, D.; Brennan, J.M.; Redberg, R.F.; Anderson, H.V.; Brindis, R.G.; Douglas, P.S. Low Diagnostic Yield of Elective Coronary Angiography. N. Engl. J. Med. 2010, 362, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.; Shufelt, C.; Merz, C.N.B. Persistent Chest Pain and No Obstructive Coronary Artery Disease. JAMA 2009, 301, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Mehta, P.K.; Bairey Merz, C.N. Cardiac Syndrome X: Update 2014. Cardiol. Clin. 2014, 32, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Villano, A.; Lanza, G.A.; Crea, F. Microvascular angina: Prevalence, pathophysiology and therapy. J. Cardiovasc. Med. 2018, 19 (Suppl. 1), e36–e39. [Google Scholar] [CrossRef]
- Jansen, T.P.; Konst, R.E.; Elias-Smale, S.E.; Oord, S.C.v.D.; Ong, P.; de Vos, A.M.; van de Hoef, T.P.; Paradies, V.; Smits, P.C.; van Royen, N.; et al. Assessing Microvascular Dysfunction in Angina With Unobstructed Coronary Arteries. J. Am. Coll. Cardiol. 2021, 78, 1471–1479. [Google Scholar] [CrossRef]
- Crooijmans, C.; Jansen, T.; Konst, R.; Woudstra, J.; Appelman, Y.; Ruijter, H.D.; Onland-Moret, N.; Meeder, J.; de Vos, A.; Paradies, V.; et al. Design and rationale of the NetherLands registry of invasive Coronary vasomotor Function Testing (NL-CFT). Int. J. Cardiol. 2023, 379, 1–8. [Google Scholar] [CrossRef]
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Merz, C.N.B.; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int. J. Cardiol. 2018, 250, 16–20. [Google Scholar] [CrossRef]
- Picard, F.; Sayah, N.; Spagnoli, V.; Adjedj, J.; Varenne, O. Vasospastic angina: A literature review of current evidence. Arch. Cardiovasc. Dis. 2019, 112, 44–55. [Google Scholar] [CrossRef]
- Vancheri, F.; Longo, G.; Vancheri, S.; Henein, M. Coronary Microvascular Dysfunction. J. Clin. Med. 2020, 9, 2880. [Google Scholar] [CrossRef]
- Prinzmetal, M.; Kennamer, R.; Merliss, R.; Wada, T.; Bor, N. Angina pectoris I. A variant form of angina pectoris: Preliminary report. Am. J. Med. 1959, 27, 375–388. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Fung, M.; Liang, Z.; Butalia, S.; Anderson, T.J. Temporal Trends of the Prevalence of Angina With No Obstructive Coronary Artery Disease (ANOCA). Can. J. Cardiol. 2023, 39, 63–70. [Google Scholar] [CrossRef]
- Patel, M.R.; Dai, D.; Hernandez, A.F.; Douglas, P.S.; Messenger, J.; Garratt, K.N.; Maddox, T.M.; Peterson, E.D.; Roe, M.T. Prevalence and predictors of nonobstructive coronary artery disease identified with coronary angiography in contemporary clinical practice. Am. Heart J. 2014, 167, 846–852.e2. [Google Scholar] [CrossRef]
- Jespersen, L.; Hvelplund, A.; Abildstrøm, S.Z.; Pedersen, F.; Galatius, S.; Madsen, J.K.; Jørgensen, E.; Kelbaek, H.; Prescott, E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur. Heart J. 2012, 33, 734–744. [Google Scholar] [CrossRef]
- Sharaf, B.; Wood, T.; Shaw, L.; Johnson, B.D.; Kelsey, S.; Anderson, R.D.; Pepine, C.J.; Merz, C.N.B. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: Findings from the National Heart, Lung, and Blood Institute–sponsored Women’s Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am. Heart J. 2013, 166, 134–141. [Google Scholar] [CrossRef]
- Mileva, N.; Nagumo, S.; Mizukami, T.; Sonck, J.; Berry, C.; Gallinoro, E.; Monizzi, G.; Candreva, A.; Munhoz, D.; Vassilev, D.; et al. Prevalence of Coronary Microvascular Disease and Coronary Vasospasm in Patients With Nonobstructive Coronary Artery Disease: Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2022, 11, e023207. [Google Scholar] [CrossRef]
- Buchthal, S.D.; Hollander, J.A.D.; Merz, C.N.B.; Rogers, W.J.; Pepine, C.J.; Reichek, N.; Sharaf, B.L.; Reis, S.; Kelsey, S.F.; Pohost, G.M. Abnormal Myocardial Phosphorus-31 Nuclear Magnetic Resonance Spectroscopy in Women with Chest Pain but Normal Coronary Angiograms. N. Engl. J. Med. 2000, 342, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Gehrie, E.R.; Reynolds, H.R.; Chen, A.Y.; Neelon, B.H.; Roe, M.T.; Gibler, W.B.; Ohman, E.M.; Newby, L.K.; Peterson, E.D.; Hochman, J.S. Characterization and outcomes of women and men with non–ST-segment elevation myocardial infarction and nonobstructive coronary artery disease: Results from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) Quality Improvement Initiative. Am. Heart J. 2009, 158, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.Y.; Jeong, M.H.; Ahn, Y.K.; Kim, J.H.; Chae, S.C.; Kim, Y.J.; Hur, S.H.; Seong, I.W.; Hong, T.J.; Choi, D.H.; et al. Are patients with angiographically near-normal coronary arteries who present as acute myocardial infarction actually safe? Int. J. Cardiol. 2011, 146, 207–212. [Google Scholar] [CrossRef]
- Larsen, A.I.; Galbraith, P.D.; Ghali, W.A.; Norris, C.M.; Graham, M.M.; Knudtson, M.L. Characteristics and outcomes of patients with acute myocardial infarction and angiographically normal coronary arteries. Am. J. Cardiol. 2005, 95, 261–263. [Google Scholar] [CrossRef]
- Bugiardini, R.; Manfrini, O.; De Ferrari, G.M. Unanswered Questions for Management of Acute Coronary Syndrome. Arch. Intern. Med. 2006, 166, 1391–1395. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, S.; Air, T.; Dreyer, R.P.; Tavella, R.; Beltrame, J.F. Systematic Review of Patients Presenting With Suspected Myocardial Infarction and Nonobstructive Coronary Arteries. Circulation 2015, 131, 861–870. [Google Scholar] [CrossRef] [PubMed]
- DeWood, M.A.; Spores, J.; Notske, R.; Mouser, L.T.; Burroughs, R.; Golden, M.S.; Lang, H.T. Prevalence of Total Coronary Occlusion during the Early Hours of Transmural Myocardial Infarction. N. Engl. J. Med. 1980, 303, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Cooper-DeHoff, R.M.; McClure, C.; Johnson, B.D.; Shaw, L.J.; Handberg, E.M.; Zineh, I.; Kelsey, S.F.; Arnsdorf, M.F.; Black, H.R.; et al. Adverse Cardiovascular Outcomes in Women With Nonobstructive Coronary Artery Disease. Arch. Intern. Med. 2009, 169, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, L.; Abildstrom, S.Z.; Hvelplund, A.; Madsen, J.K.; Galatius, S.; Pedersen, F.; Hojberg, S.; Prescott, E. Burden of Hospital Admission and Repeat Angiography in Angina Pectoris Patients with and without Coronary Artery Disease: A Registry-Based Cohort Study. PLoS ONE 2014, 9, e93170. [Google Scholar] [CrossRef] [PubMed]
- Brainin, P.; Frestad, D.; Prescott, E. The prognostic value of coronary endothelial and microvascular dysfunction in subjects with normal or non-obstructive coronary artery disease: A systematic review and meta-analysis. Int. J. Cardiol. 2018, 254, 1–9. [Google Scholar] [CrossRef]
- Kenkre, T.S.; Malhotra, P.; Johnson, B.D.; Handberg, E.M.; Thompson, D.V.; Marroquin, O.C.; Rogers, W.J.; Pepine, C.J.; Merz, C.N.B.; Kelsey, S.F.; et al. Ten-Year Mortality in the WISE Study (Women’s Ischemia Syndrome Evaluation). Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003863. [Google Scholar] [CrossRef]
- Patel, M.R.; Chen, A.Y.; Peterson, E.D.; Newby, L.K.; Pollack, C.V.; Brindis, R.G.; Gibson, C.M.; Kleiman, N.S.; Saucedo, J.F.; Bhatt, D.L.; et al. Prevalence, predictors, and outcomes of patients with non–ST-segment elevation myocardial infarction and insignificant coronary artery disease: Results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines (CRUSADE) initiative. Am. Heart J. 2006, 152, 641–647. [Google Scholar] [CrossRef]
- Larsen, A.I.; Nilsen, D.W.; Yu, J.; Mehran, R.; Nikolsky, E.; Lansky, A.J.; Caixeta, A.; Parise, H.; Fahy, M.; Cristea, E.; et al. Long-Term Prognosis of Patients Presenting With ST-Segment Elevation Myocardial Infarction With No Significant Coronary Artery Disease (from The HORIZONS-AMI Trial). Am. J. Cardiol. 2013, 111, 643–648. [Google Scholar] [CrossRef]
- Beltrame, J.F.; Crea, F.; Kaski, J.C.; Ogawa, H.; Ong, P.; Sechtem, U.; Shimokawa, H.; Merz, C.N.B. International standardization of diagnostic criteria for vasospastic angina. Eur. Heart J. 2015, 38, 2565–2568. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H.; Seto, M.; Katsumata, N.; Amano, M.; Kozai, T.; Yamawaki, T.; Kuwata, K.; Kandabashi, T.; Egashira, K.; Ikegaki, I.; et al. Rho-kinase-mediated pathway induces enhanced myosin light chain phosphorylations in a swine model of coronary artery spasm. Cardiovasc. Res. 1999, 43, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Kandabashi, T.; Shimokawa, H.; Miyata, K.; Kunihiro, I.; Kawano, Y.; Fukata, Y.; Higo, T.; Egashira, K.; Takahashi, S.; Kaibuchi, K.; et al. Inhibition of myosin phosphatase by upregulated rho-kinase plays a key role for coronary artery spasm in a porcine model with interleukin-1beta. Circulation 2000, 101, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, A.; Mohri, M.; Shimokawa, H.; Urakami, L.; Usui, M.; Takeshita, A. Suppression of Coronary Artery Spasm by the Rho-Kinase Inhibitor Fasudil in Patients With Vasospastic Angina. Circulation 2002, 105, 1545–1547. [Google Scholar] [CrossRef] [PubMed]
- Miwa, K.; Igawa, A.; Miyagi, Y.; Nakagawa, K.; Inoue, H. Alterations of autonomic nervous activity preceding nocturnal variant angina: Sympathetic augmentation with parasympathetic impairment. Am. Heart J. 1998, 135, 762–771. [Google Scholar] [CrossRef]
- Laughlin, M.H.; Davis, M.J.; Secher, N.H.; Lieshout, J.J.; Arce-Esquivel, A.A.; Simmons, G.H.; Bender, S.B.; Padilla, J.; Bache, R.J.; Merkus, D.; et al. Peripheral Circulation. Compr. Physiol. 2012, 2, 321–447. [Google Scholar] [CrossRef]
- Slavich, M.; Patel, R.S. Coronary artery spasm: Current knowledge and residual uncertainties. IJC Heart Vasc. 2016, 10, 47–53. [Google Scholar] [CrossRef]
- Mohri, M.; Koyanagi, M.; Egashira, K.; Tagawa, H.; Ichiki, T.; Shimokawa, H.; Takeshita, A. Angina pectoris caused by coronary microvascular spasm. Lancet 1998, 351, 1165–1169. [Google Scholar] [CrossRef]
- Sun, H.; Mohri, M.; Shimokawa, H.; Usui, M.; Urakami, L.; Takeshita, A. Coronary microvascular spasm causes myocardial ischemia in patients with vasospastic angina. J. Am. Coll. Cardiol. 2002, 39, 847–851. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef]
- Pries, A.R.; Badimon, L.; Bugiardini, R.; Camici, P.G.; Dorobantu, M.; Duncker, D.J.; Escaned, J.; Koller, A.; Piek, J.J.; de Wit, C. Coronary vascular regulation, remodelling, and collateralization: Mechanisms and clinical implications on behalf of the working group on coronary pathophysiology and microcirculation. Eur. Heart J. 2015, 36, 3134–3146. [Google Scholar] [CrossRef]
- Crea, F.; Montone, R.A.; Rinaldi, R. Pathophysiology of Coronary Microvascular Dysfunction. Circ. J. 2022, 86, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Demir, O.M.; Khan, F.; Ryan, M.; Ellis, H.; Mills, M.T.; Chiribiri, A.; Webb, A.; Perera, D. Physiological Stratification of Patients With Angina Due to Coronary Microvascular Dysfunction. J. Am. Coll. Cardiol. 2020, 75, 2538–2549. [Google Scholar] [CrossRef] [PubMed]
- Camici, P.G.; Crea, F. Coronary Microvascular Dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Camici, P.G.; Merz, C.N.B. Coronary microvascular dysfunction: An update. Eur. Heart J. 2014, 35, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Kovarnik, T.; Hitoshi, M.; Kral, A.; Jerabek, S.; Zemanek, D.; Kawase, Y.; Omori, H.; Tanigaki, T.; Pudil, J.; Vodzinska, A.; et al. Fractional Flow Reserve Versus Instantaneous Wave-Free Ratio in Assessment of Lesion Hemodynamic Significance and Explanation of their Discrepancies. International, Multicenter and Prospective Trial: The FiGARO Study. J. Am. Heart Assoc. 2022, 11, e021490. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.M.; Jeremias, A.; Petraco, R.; Sen, S.; Nijjer, S.; Shun-Shin, M.J.; Ahmad, Y.; de Waard, G.; van de Hoef, T.; Echavarria-Pinto, M.; et al. Fractional Flow Reserve/Instantaneous Wave-Free Ratio Discordance in Angiographically Intermediate Coronary Stenoses. JACC Cardiovasc. Interv. 2017, 10, 2514–2524. [Google Scholar] [CrossRef] [PubMed]
- Nardone, M.; McCarthy, M.; Ardern, C.I.; Nield, L.E.; Toleva, O.; Cantor, W.J.; Miner, S.E. Concurrently Low Coronary Flow Reserve and Low Index of Microvascular Resistance Are Associated With Elevated Resting Coronary Flow in Patients With Chest Pain and Nonobstructive Coronary Arteries. Circ. Cardiovasc. Interv. 2022, 15, e011323. [Google Scholar] [CrossRef]
- Taqueti, V.R.; Di Carli, M.F. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options. J. Am. Coll. Cardiol. 2018, 72, 2625–2641. [Google Scholar] [CrossRef]
- Sueda, S.; Kohno, H.; Ochi, T.; Uraoka, T.; Tsunemitsu, K. Overview of the pharmacological spasm provocation test: Comparisons between acetylcholine and ergonovine. J. Cardiol. 2017, 69, 57–65. [Google Scholar] [CrossRef]
- Suzuki, S.; Kaikita, K.; Yamamoto, E.; Jinnouchi, H.; Tsujita, K. Role of acetylcholine spasm provocation test as a pathophysiological assessment in nonobstructive coronary artery disease. Cardiovasc. Interv. Ther. 2021, 36, 39–51. [Google Scholar] [CrossRef]
- Ong, P.; Athanasiadis, A.; Sechtem, U. Intracoronary Acetylcholine Provocation Testing for Assessment of Coronary Vasomotor Disorders. J. Vis. Exp. 2016, 114, e54295. [Google Scholar] [CrossRef]
- Takahashi, T.; Samuels, B.A.; Li, W.; Parikh, M.A.; Wei, J.; Moses, J.W.; Fearon, W.F.; Henry, T.D.; Tremmel, J.A.; Kobayashi, Y. Safety of Provocative Testing with Intracoronary Acetylcholine and Implications for Standard Protocols. J. Am. Coll. Cardiol. 2022, 79, 2367–2378. [Google Scholar] [CrossRef] [PubMed]
- Fearon, W.F.; Balsam, L.B.; Farouque, H.M.O.; Robbins, R.C.; Fitzgerald, P.J.; Yock, P.G.; Yeung, A.C. Novel Index for Invasively Assessing the Coronary Microcirculation. Circulation 2003, 107, 3129–3132. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.K.; Yeung, A.C.; Fearon, W.F. Invasive Assessment of the Coronary Microcirculation. Circulation 2006, 113, 2054–2061. [Google Scholar] [CrossRef]
- Doucette, J.W.; Corl, P.D.; Payne, H.M.; Flynn, A.E.; Goto, M.; Nassi, M.; Segal, J. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation 1992, 85, 1899–1911. [Google Scholar] [CrossRef]
- Tsiachris, D.; Tsioufis, C.; Dimitriadis, K.; Syrseloudis, D.; Rousos, D.; Kasiakogias, A.; Papademetriou, V.; Tousoulis, D.; Stefanadis, C. Relation of Impaired Coronary Microcirculation to Increased Urine Albumin Excretion in Patients with Systemic Hypertension and No Epicardial Coronary Arterial Narrowing. Am. J. Cardiol. 2012, 109, 1026–1030. [Google Scholar] [CrossRef]
- Tsiachris, D.; Tsioufis, C.; Syrseloudis, D.; Roussos, D.; Tatsis, I.; Dimitriadis, K.; Toutouzas, K.; Tsiamis, E.; Stefanadis, C. Subendocardial viability ratio as an index of impaired coronary flow reserve in hypertensives without significant coronary artery stenoses. J. Hum. Hypertens. 2012, 26, 64–70. [Google Scholar] [CrossRef]
- Fearon, W.F.; Farouque, H.O.; Balsam, L.B.; Cooke, D.T.; Robbins, R.C.; Fitzgerald, P.J.; Yeung, A.C.; Yock, P.G. Comparison of Coronary Thermodilution and Doppler Velocity for Assessing Coronary Flow Reserve. Circulation 2003, 108, 2198–2200. [Google Scholar] [CrossRef]
- Everaars, H.; de Waard, G.A.; Driessen, R.S.; Danad, I.; van de Ven, P.M.; Raijmakers, P.G.; Lammertsma, A.A.; van Rossum, A.C.; Knaapen, P.; van Royen, N. Doppler Flow Velocity and Thermodilution to Assess Coronary Flow Reserve. JACC Cardiovasc. Interv. 2018, 11, 2044–2054. [Google Scholar] [CrossRef]
- Kern, M.J.; Seto, A.H. The Challenges of Measuring Coronary Flow Reserve: Comparisons of Doppler and Thermodilution to [15O]H2O PET Perfusion. JACC Cardiovasc. Interv. 2018, 11, 2055–2057. [Google Scholar] [CrossRef]
- Layland, J.; Nerlekar, N.; Palmer, S.; Berry, C.; Oldroyd, K. Invasive assessment of the coronary microcirculation in the catheter laboratory. Int. J. Cardiol. 2015, 199, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Konst, R.E.; Elias-Smale, S.E.; Pellegrini, D.; Hartzema-Meijer, M.; van Uden, B.J.; Jansen, T.P.; Vart, P.; Gehlmann, H.; Maas, A.H.; van Royen, N.; et al. Absolute Coronary Blood Flow Measured by Continuous Thermodilution in Patients with Ischemia and Nonobstructive Disease. J. Am. Coll. Cardiol. 2021, 77, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Demir, O.M.; Boerhout, C.K.; de Waard, G.A.; van de Hoef, T.P.; Patel, N.; Beijk, M.A.; Williams, R.; Rahman, H.; Everaars, H.; Kharbanda, R.K.; et al. Comparison of Doppler Flow Velocity and Thermodilution Derived Indexes of Coronary Physiology. JACC Cardiovasc. Interv. 2022, 15, 1060–1070. [Google Scholar] [CrossRef]
- Gallinoro, E.; Candreva, A.; Colaiori, I.; Kodeboina, M.; Fournier, S.; Nelis, O.; Di Gioia, G.; Sonck, J.; Van’t Veer, M.; Pijls, N.H.; et al. Thermodilution-derived volumetric resting coronary blood flow measurement in humans. Eurointervention 2021, 17, e672–e679. [Google Scholar] [CrossRef] [PubMed]
- Barbato, E.; Aarnoudse, W.; Aengevaeren, W.R.; Werner, G.; Klauss, V.; Bojara, W.; Herzfeld, I.; Oldroyd, K.G.; Pijls, N.H.; De Bruyne, B. Validation of coronary flow reserve measurements by thermodilution in clinical practice. Eur. Heart J. 2004, 25, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, A.; Castaldi, G.; Vermeersch, P.; Wilgenhof, A.; Convens, C.; Scott, B.; Verheye, S.; Agostoni, P.; Zivelonghi, C. Clinical implications of coronary microvascular dysfunction in patients with non-obstructive coronary artery disease and role of the thermodilution method. Minerva Cardioangiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Pijls, N.H.; De Bruyne, B.; Smith, L.; Aarnoudse, W.; Barbato, E.; Bartunek, J.; Bech, G.J.W.; Van De Vosse, F. Coronary Thermodilution to Assess Flow Reserve. Circulation 2002, 105, 2482–2486. [Google Scholar] [CrossRef]
- Aarnoudse, W.; van den Berg, P.; van de Vosse, F.; Geven, M.; Rutten, M.; van Turnhout, M.; Fearon, W.; De Bruyne, B.; Pijls, N. Myocardial resistance assessed by guidewire-based pressure-temperature measurement: In vitro validation. Catheter. Cardiovasc. Interv. 2004, 62, 56–63. [Google Scholar] [CrossRef]
- Layland, J.; Carrick, D.; McEntegart, M.; Ahmed, N.; Payne, A.; McClure, J.; Sood, A.; McGeoch, R.; MacIsaac, A.; Whitbourn, R.; et al. Vasodilatory capacity of the coronary microcirculation is preserved in selected patients with non-ST-segment-elevation myocardial infarction. Circ. Cardiovasc. Interv. 2013, 6, 231–236. [Google Scholar] [CrossRef]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eurointervention 2021, 16, 1049–1069. [Google Scholar] [CrossRef]
- Maznyczka, A.M.; Oldroyd, K.G.; Greenwood, J.P.; McCartney, P.J.; Cotton, J.; Lindsay, M.; McEntegart, M.; Rocchiccioli, J.P.; Good, R.; Robertson, K.; et al. Comparative Significance of Invasive Measures of Microvascular Injury in Acute Myocardial Infarction. Circ. Cardiovasc. Interv. 2020, 13, e008505. [Google Scholar] [CrossRef]
- van’t Veer, M.; Adjedj, J.; Wijnbergen, I.; Tóth, G.G.; Rutten, M.C.; Barbato, E.; van Nunen, L.X.; Pijls, N.H.; De Bruyne, B. Novel monorail infusion catheter for volumetric coronary blood flow measurement in humans: In vitro validation. EuroIntervention 2016, 12, 701–707. [Google Scholar] [CrossRef]
- De Bruyne, B.; Adjedj, J.; Xaplanteris, P.; Ferrara, A.; Mo, Y.; Penicka, M.; Floré, V.; Pellicano, M.; Toth, G.; Barbato, E.; et al. Saline-Induced Coronary Hyperemia. Circ. Cardiovasc. Interv. 2017, 10, e004719. [Google Scholar] [CrossRef]
- Xaplanteris, P.; Fournier, S.; Keulards, D.; Adjedj, J.; Ciccarelli, G.; Milkas, A.; Pellicano, M.; Veer, M.V.; Barbato, E.; Pijls, N.H.; et al. Catheter-Based Measurements of Absolute Coronary Blood Flow and Microvascular Resistance. Circ. Cardiovasc. Interv. 2018, 11, e006194. [Google Scholar] [CrossRef]
- de Vos, A.; Jansen, T.P.; van’t Veer, M.; Dimitriu-Leen, A.; Konst, R.E.; Elias-Smale, S.; Paradies, V.; Rodwell, L.; van den Oord, S.; Smits, P.; et al. Microvascular Resistance Reserve to Assess Microvascular Dysfunction in ANOCA Patients. JACC Cardiovasc. Interv. 2023, 16, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Everaars, H.; De Waard, G.A.; Schumacher, S.P.; Zimmermann, F.M.; Bom, M.J.; Van De Ven, P.M.; Raijmakers, P.G.; Lammertsma, A.A.; Götte, M.J.; Van Rossum, A.C.; et al. Continuous thermodilution to assess absolute flow and microvascular resistance: Validation in humans using [15O]H2O positron emission tomography. Eur. Heart J. 2019, 40, 2350–2359. [Google Scholar] [CrossRef] [PubMed]
- Gallinoro, E.; Bertolone, D.T.; Fernandez-Peregrina, E.; Paolisso, P.; Bermpeis, K.; Esposito, G.; Gomez-Lopez, A.; Candreva, A.; Mileva, N.; Belmonte, M.; et al. Reproducibility of bolus versus continuous thermodilution for assessment of coronary microvascular function in patients with ANOCA. Eurointervention 2023, 19, e155–e166. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.P.J.; De Vos, A.; Damman, P.; Paradies, V.; Konst, R.E.; Teerenstra, S.; Oord, S.C.H.V.D.; Dimitriu-Leen, A.; Maas, A.H.E.M.; Smits, P.C.; et al. Absolute flow and resistance have a lower variability in repeated testing as compared to CFR and IMR: An EDIT-CMD substudy. Eur. Heart J. 2022, 43, ehac544-1209. [Google Scholar] [CrossRef]
- de Vos, A.; Paradies, V.; Dimitriu-Leen, A.; Rodwell, L.; Smits, P.; Pijls, N.; van Royen, N.; Damman, P.; Jansen, T. TCT-303 Continuous Versus Bolus Thermodilution Derived CFR and MRR and Their Association With Symptoms of CMD: A Head-to-Head Comparison. J. Am. Coll. Cardiol. 2022, 80, B121. [Google Scholar] [CrossRef]
- Hambrecht, R.; Fiehn, E.; Weigl, C.; Gielen, S.; Hamann, C.; Kaiser, R.; Yu, J.; Adams, V.; Niebauer, J.; Schuler, G. Regular Physical Exercise Corrects Endothelial Dysfunction and Improves Exercise Capacity in Patients with Chronic Heart Failure. Circulation 1998, 98, 2709–2715. [Google Scholar] [CrossRef]
- Maron, D.J.; Fazio, S.; Linton, M.F. Current Perspectives on Statins. Circulation 2000, 101, 207–213. [Google Scholar] [CrossRef]
- Caliskan, M.; Erdogan, D.; Gullu, H.; Topcu, S.; Ciftci, O.; Yildirir, A.; Muderrisoglu, H. Effects of Atorvastatin on Coronary Flow Reserve in Patients with Slow Coronary Flow. Clin. Cardiol. 2007, 30, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.J.; Hwang, E.; Jang, J.Y.; Kim, D.-H.; Song, J.-M.; Kang, D.-H. Effect of Rosuvastatin on Coronary Flow Reserve in Patients with Systemic Hypertension. Am. J. Cardiol. 2014, 114, 1234–1237. [Google Scholar] [CrossRef]
- Yang, Y.; Hwang, E.; Lee, S.-A.; Lee, S.; Kim, D.-H.; Song, J.-M.; Kang, D.-H. Effect of Rosuvastatin on Coronary Flow Reserve in Hypertensive Patients at Cardiovascular Risk. J. Cardiovasc. Imaging 2021, 29, 255–262. [Google Scholar] [CrossRef]
- Pizzi, C.; Manfrini, O.; Fontana, F.; Bugiardini, R. Angiotensin-Converting Enzyme Inhibitors and 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase in Cardiac Syndrome X. Circulation 2004, 109, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-W.; Hsu, N.-W.; Wu, T.-C.; Lin, S.-J.; Chang, M.-S. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. Am. J. Cardiol. 2002, 90, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D.F.; Johnson, B.D.; Anderson, R.D.; Handberg, E.M.; Smith, K.M.; Cooper-DeHoff, R.M.; Sopko, G.; Sharaf, B.M.; Kelsey, S.F.; Merz, C.N.B.; et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: A double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am. Heart J. 2011, 162, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.J.; Stanley, B.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; Robertson, K.; et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina. J. Am. Coll. Cardiol. 2018, 72, 2841–2855. [Google Scholar] [CrossRef]
- Ford, T.J.; Stanley, B.; Sidik, N.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; et al. 1-Year Outcomes of Angina Management Guided by Invasive Coronary Function Testing (CorMicA). JACC Cardiovasc. Interv. 2019, 13, 33–45. [Google Scholar] [CrossRef]
- Ford, T.; Berry, C. How to Diagnose and Manage Angina Without Obstructive Coronary Artery Disease: Lessons from the British Heart Foundation CorMicA Trial. Interv. Cardiol. Rev. Res. Resour. 2019, 14, 76–82. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Lanza, G.A.; Colonna, G.; Pasceri, V.; Maseri, A. Atenolol versus amlodipine versus isosorbide-5-mononitrate on anginal symptoms in syndrome X. Am. J. Cardiol. 1999, 84, 854–856. [Google Scholar] [CrossRef]
- Bugiardini, R.; Borghi, A.; Biagetti, L.; Puddu, P. Comparison of verapamil versus propranolol therapy in syndrome X. Am. J. Cardiol. 1989, 63, 286–290. [Google Scholar] [CrossRef]
- Togni, M.; Vigorito, F.; Windecker, S.; Abrecht, L.; Wenaweser, P.; Cook, S.; Billinger, M.; Meier, B.; Hess, O.M. Does the β-Blocker Nebivolol Increase Coronary Flow Reserve? Cardiovasc. Drugs Ther. 2007, 21, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Sütsch, G.; Oechslin, E.; Mayer, I.; Hess, O.M. Effect of diltiazem on coronary flow reserve in patients with microvascular angina. Int. J. Cardiol. 1995, 52, 135–143. [Google Scholar] [CrossRef]
- Merz, C.N.B.; Pepine, C.J.; Walsh, M.N.; Fleg, J.L.; Camici, P.G.; Chilian, W.M.; Clayton, J.A.; Cooper, L.S.; Crea, F.; Di Carli, M.; et al. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation 2017, 135, 1075–1092. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Di Franco, A.; Lamendola, P.; Tarzia, P.; Nerla, R.; Stazi, A.; Villano, A.; Sestito, A.; Lanza, G.A.; Crea, F. Lack of Effect of Nitrates on Exercise Stress Test Results in Patients with Microvascular Angina. Cardiovasc. Drugs Ther. 2013, 27, 229–234. [Google Scholar] [CrossRef]
- Merz, C.N.B.; Handberg, E.M.; Shufelt, C.L.; Mehta, P.K.; Minissian, M.B.; Wei, J.; Thomson, L.E.; Berman, D.S.; Shaw, L.J.; Petersen, J.W.; et al. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): Impact on angina and myocardial perfusion reserve. Eur. Heart J. 2016, 37, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Skalidis, E.I.; Hamilos, M.I.; Chlouverakis, G.; Zacharis, E.A.; Vardas, P.E. Ivabradine improves coronary flow reserve in patients with stable coronary artery disease. Atherosclerosis 2011, 215, 160–165. [Google Scholar] [CrossRef]
- Villano, A.; Di Franco, A.; Nerla, R.; Sestito, A.; Tarzia, P.; Lamendola, P.; Di Monaco, A.; Sarullo, F.M.; Lanza, G.A.; Crea, F. Effects of Ivabradine and Ranolazine in Patients With Microvascular Angina Pectoris. Am. J. Cardiol. 2013, 112, 8–13. [Google Scholar] [CrossRef]
- Nishigaki, K.; Inoue, Y.; Yamanouchi, Y.; Fukumoto, Y.; Yasuda, S.; Sueda, S.; Urata, H.; Shimokawa, H.; Minatoguchi, S. Prognostic Effects of Calcium Channel Blockers in Patients With Vasospastic Angina—A Meta-Analysis. Circ. J. 2010, 74, 1943–1950. [Google Scholar] [CrossRef]
- Jansen, T.P.; Konst, R.E.; de Vos, A.; Paradies, V.; Teerenstra, S.; Oord, S.C.V.D.; Dimitriu-Leen, A.; Maas, A.H.; Smits, P.C.; Damman, P.; et al. Efficacy of Diltiazem to Improve Coronary Vasomotor Dysfunction in ANOCA. JACC Cardiovasc. Imaging 2022, 15, 1473–1484. [Google Scholar] [CrossRef]
- Lombardi, M.; Morales, M.A.; Michelassi, C.; Moscarelli, E.; Distante, A.; L’Abbate, A. Efficacy of isosorbide-5-mononitrate versus nifedipine in preventing spontaneous and ergonovine-induced myocardial ischaemia. A double-blind, placebo-controlled study. Eur. Heart J. 1993, 14, 845–851. [Google Scholar] [CrossRef]
- Task Force Members; Montalescot, G.; Sechtem, U.; Achenbach, S.; Andreotti, F.; Arden, C.; Budaj, A.; Bugiardini, R.; Crea, F.; Cuisset, T.; et al. 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 2013, 34, 2949–3003. [Google Scholar]
- Lee, J.M.; Jung, J.-H.; Hwang, D.; Park, J.; Fan, Y.; Na, S.-H.; Doh, J.-H.; Nam, C.-W.; Shin, E.-S.; Koo, B.-K. Coronary Flow Reserve and Microcirculatory Resistance in Patients With Intermediate Coronary Stenosis. J. Am. Coll. Cardiol. 2016, 67, 1158–1169. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, K.H.; Hwang, D.; Park, J.; Jung, J.-H.; Kim, H.Y.; Jung, H.W.; Cho, Y.-K.; Yoon, H.-J.; Bin Song, Y.; et al. Prognostic Implication of Thermodilution Coronary Flow Reserve in Patients Undergoing Fractional Flow Reserve Measurement. JACC Cardiovasc. Interv. 2018, 11, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Kelshiker, M.A.; Seligman, H.; Howard, J.P.; Rahman, H.; Foley, M.; Nowbar, A.N.; Rajkumar, C.A.; Shun-Shin, M.J.; Ahmad, Y.; Sen, S.; et al. Coronary flow reserve and cardiovascular outcomes: A systematic review and meta-analysis. Eur. Heart J. 2022, 43, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Boerhout, C.B.; Waard, G.d.W.d.; Lee, J.M.; Mejia-Renteria, H.; Lee, S.H.; Jung, J.-H.; Hoshino, M.; Echavarria-Pinto, M.; Meuwissen, M.; Matsuo, H.; et al. Prognostic value of structural and functional coronary microvascular dysfunction in patients with non-obstructive coronary artery disease; from the multicentre international ILIAS registry. Eurointervention 2022, 18, 719–728. [Google Scholar] [CrossRef] [PubMed]
| Invasive Method | Parameter | Formula | Cutoff for CMD | Meaning |
|---|---|---|---|---|
| Bolus thermodilution | Coronary flow reserve (CFRbolus) | CFRbolus | <2.0 (validated) | The capacity to increase CBF from resting to stress conditions (derived from transit time). |
| Index of microvascular resistance (IMR) | IMR = | ≥25 (validated) | Dimensionless index which represents MVR during stress conditions. | |
| Resistive reserve ratio (RRR) | RRR = | Unknown | The ability of microcirculation to reduce MVR during stress conditions (derived from transit time). | |
| Continuous thermodilution | Absolute coronary flow (Q) | Q = | <200 mL/min (under investigation) | Direct measurement of CBF. |
| Absolute microvascular resistance (R) | R = | >500 UW (under investigation) | True MVR derived from CBF. | |
| Coronary flow reserve (CFRcontinuous) | CFRcontinuous = | <2.0–2.5 (under investigation) | The capacity to increase CBF from resting to stress conditions (derived from CBF). | |
| Microvascular resistance reserve (MRR) | <2.1 (under investigation) | The ability of microcirculation to reduce MVR during stress conditions (derived from CBF). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurina, M.; Benedetti, A.; Stefanini, G.; Condorelli, G.; Collet, C.; Zivelonghi, C.; Smits, P.C.; Paradies, V. Coronary Vascular (DYS) Function and Invasive Physiology Assessment: Insights into Bolus and Continuous Thermodilution Methods. J. Clin. Med. 2023, 12, 4864. https://doi.org/10.3390/jcm12144864
Maurina M, Benedetti A, Stefanini G, Condorelli G, Collet C, Zivelonghi C, Smits PC, Paradies V. Coronary Vascular (DYS) Function and Invasive Physiology Assessment: Insights into Bolus and Continuous Thermodilution Methods. Journal of Clinical Medicine. 2023; 12(14):4864. https://doi.org/10.3390/jcm12144864
Chicago/Turabian StyleMaurina, Matteo, Alice Benedetti, Giulio Stefanini, Gianluigi Condorelli, Carlos Collet, Carlo Zivelonghi, Pieter C. Smits, and Valeria Paradies. 2023. "Coronary Vascular (DYS) Function and Invasive Physiology Assessment: Insights into Bolus and Continuous Thermodilution Methods" Journal of Clinical Medicine 12, no. 14: 4864. https://doi.org/10.3390/jcm12144864
APA StyleMaurina, M., Benedetti, A., Stefanini, G., Condorelli, G., Collet, C., Zivelonghi, C., Smits, P. C., & Paradies, V. (2023). Coronary Vascular (DYS) Function and Invasive Physiology Assessment: Insights into Bolus and Continuous Thermodilution Methods. Journal of Clinical Medicine, 12(14), 4864. https://doi.org/10.3390/jcm12144864