COVID-19-Induced Acute Respiratory Distress Syndrome Treated with Hyperbaric Oxygen: Interim Safety Report from a Randomized Clinical Trial (COVID-19-HBO)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Randomization
2.4. Procedures
2.5. Outcomes
- Mean oxygen dose per day including HBO2 and cumulative pulmonary oxygen toxicity expressed as UPTD and CPTD from day 1 to day 30.
- Number of secondary infections, number of events and patients from day 1 to day 30.
- Diagnosed PE needing treatment, number of events and patients from day 1 to day 30.
2.6. Statistical Analysis
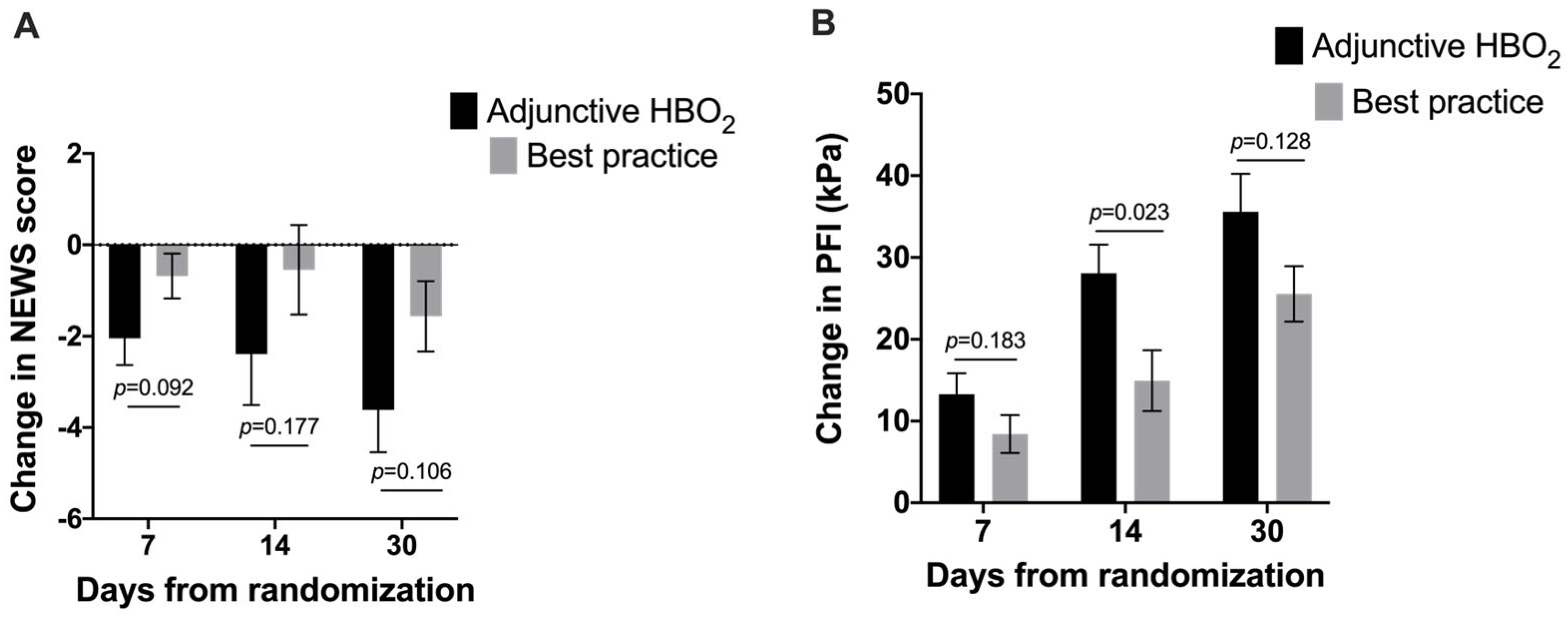
3. Results
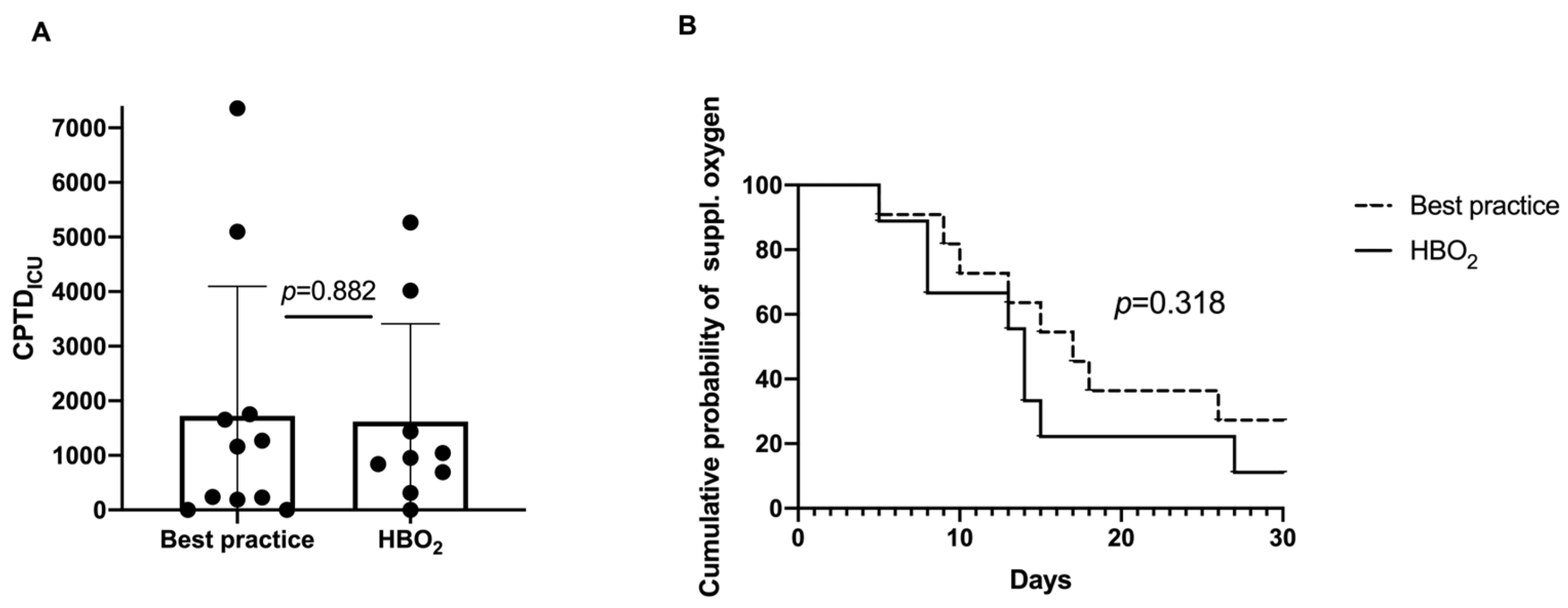
- Oxygen toxicity and total oxygen dose in a subgroup (all patients from the Karolinska University Hospital, n = 20): The cumulative oxygen burden expressed as CPTDICU was not significantly different between the groups with the mean (SD) HBOT 1618 (1791) and best practice 1724 (2374), (p = 0.882) (Figure 3A). There was a trend towards faster recovery in terms of days with supplemental oxygen in the HBOT group (p = 0.318) (Figure 3B).
- Secondary infections: two ventilatory-associated pneumonias (VAPs), two bacteraemia/sepsis and one abscess in m. obturatorius in the control group. One urinary tract infection in the HBOT group.
- Thrombotic events: One patient in the HBOT group had a small pulmonary embolism.
- Barotrauma: One subject in the control group had a pneumothorax. Five subjects had pneumomediastinum, four in the control group and one in the HBOT group.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferguson, N.D.; Fan, E.; Camporota, L.; Antonelli, M.; Anzueto, A.; Beale, R.; Brochard, L.; Brower, R.; Esteban, A.; Gattinoni, L.; et al. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Med. 2012, 38, 1573–1582. [Google Scholar] [CrossRef]
- Novak, S.; Drenjancevic, I.; Vukovic, R.; Kellermayer, Z.; Cosic, A.; Tolusic Levak, M.; Balogh, P.; Culo, F.; Mihalj, M. Anti-Inflammatory Effects of Hyperbaric Oxygenation during DSS-Induced Colitis in BALB/c Mice Include Changes in Gene Expression of HIF-1alpha, Proinflammatory Cytokines, and Antioxidative Enzymes. Mediat. Inflamm. 2016, 2016, 7141430. [Google Scholar] [CrossRef]
- Salhanick, S.D.; Belikoff, B.; Orlow, D.; Holt, D.; Reenstra, W.; Buras, J.A. Hyperbaric oxygen reduces acetaminophen toxicity and increases HIF-1alpha expression. Acad. Emerg. Med. 2006, 13, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, R. Hypoxia and hyperbaric oxygen therapy: A review. Int. J. Gen. Med. 2018, 11, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.H.; Feldmeier, J.; Hampson, N.B.; Smee, R.; Milross, C. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst. Rev. 2016, 4, CD005005. [Google Scholar] [CrossRef]
- Oscarsson, N.; Muller, B.; Rosen, A.; Lodding, P.; Molne, J.; Giglio, D.; Hjelle, K.M.; Vaagbo, G.; Hyldegaard, O.; Vangedal, M.; et al. Radiation-induced cystitis treated with hyperbaric oxygen therapy (RICH-ART): A randomised, controlled, phase 2-3 trial. Lancet Oncol. 2019, 20, 1602–1614. [Google Scholar] [CrossRef]
- Kirby, J.P. Hyperbaric Oxygen Therapy and Radiation-Induced Injuries. Mo. Med. 2019, 116, 198–200. [Google Scholar]
- Dulai, P.S.; Raffals, L.E.; Hudesman, D.; Chiorean, M.; Cross, R.; Ahmed, T.; Winter, M.; Chang, S.; Fudman, D.; Sadler, C.; et al. A phase 2B randomised trial of hyperbaric oxygen therapy for ulcerative colitis patients hospitalised for moderate to severe flares. Aliment. Pharmacol. Ther. 2020, 52, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Kirby, J.P. Hyperbaric Oxygen Indications: Diabetic Foot Ulcers and Intractable Management. Mo. Med. 2019, 116, 188–191. [Google Scholar]
- Devaney, B.; Frawley, G.; Frawley, L.; Pilcher, D.V. Necrotising soft tissue infections: The effect of hyperbaric oxygen on mortality. Anaesth Intensive Care 2015, 43, 685–692. [Google Scholar] [CrossRef]
- Bartek, J., Jr.; Jakola, A.S.; Skyrman, S.; Forander, P.; Alpkvist, P.; Schechtmann, G.; Glimaker, M.; Larsson, A.; Lind, F.; Mathiesen, T. Hyperbaric oxygen therapy in spontaneous brain abscess patients: A population-based comparative cohort study. Acta Neurochir. 2016, 158, 1259–1267. [Google Scholar] [CrossRef]
- Moon, R.D.; Weaver, L.K. Hyperbaric oxygen as a treatment for COVID-19 infection? Undersea Hyperb Med. 2020, 47, 177–179. [Google Scholar] [CrossRef]
- Singer, M.; Young, P.J.; Laffey, J.G.; Asfar, P.; Taccone, F.S.; Skrifvars, M.B.; Meyhoff, C.S.; Radermacher, P. Dangers of hyperoxia. Crit. Care 2021, 25, 440. [Google Scholar] [CrossRef] [PubMed]
- Sadler, C.; Alvarez Villela, M.; Van Hoesen, K.; Grover, I.; Lang, M.; Neuman, T.; Lindholm, P. Diving after SARS-CoV-2 (COVID-19) infection: Fitness to dive assessment and medical guidance. Diving Hyperb. Med. J. South Pac. Underw. Med. Soc. 2020, 50, 278–287. [Google Scholar] [CrossRef]
- Paganini, M.; Bosco, G.; Perozzo, F.A.G.; Kohlscheen, E.; Sonda, R.; Bassetto, F.; Garetto, G.; Camporesi, E.M.; Thom, S.R. The Role of Hyperbaric Oxygen Treatment for COVID-19: A Review. Adv. Exp. Med. Biol. 2021, 1289, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Tao, X.; Tang, Y.; Chen, R. The effect of hyperbaric oxygen therapy on hypoxia in patients with severe new coronavirus pneumonia: The first report. Chin. J. Naut. Med. Hyperb. Med. 2020, 27, 132–135. [Google Scholar] [CrossRef]
- Thibodeaux, K.; Speyrer, M.; Raza, A.; Yaakov, R.; Serena, T.E. Hyperbaric oxygen therapy in preventing mechanical ventilation in COVID-19 patients: A retrospective case series. J. Wound Care 2020, 29, S4–S8. [Google Scholar] [CrossRef]
- Gorenstein, S.A.; Castellano, M.L.; Slone, E.S.; Gillette, B.; Liu, H.; Alsamarraie, C.; Jacobson, A.M.; Wall, S.P.; Adhikari, S.; Swartz, J.L.; et al. Hyperbaric oxygen therapy for COVID-19 patients with respiratory distress: Treated cases versus propensity-matched controls. Undersea Hyperb. Med. 2020, 47, 405–413. [Google Scholar] [CrossRef]
- Cannellotto, M.; Duarte, M.; Keller, G.; Larrea, R.; Cunto, E.; Chediack, V.; Mansur, M.; Brito, D.M.; Garcia, E.; Di Salvo, H.E.; et al. Hyperbaric oxygen as an adjuvant treatment for patients with COVID-19 severe hypoxaemia: A randomised controlled trial. Emerg. Med. J. EMJ 2021, 39, 88–93. [Google Scholar] [CrossRef]
- Oliaei, S.; SeyedAlinaghi, S.; Mehrtak, M.; Karimi, A.; Noori, T.; Mirzapour, P.; Shojaei, A.; MohsseniPour, M.; Mirghaderi, S.P.; Alilou, S.; et al. The effects of hyperbaric oxygen therapy (HBOT) on coronavirus disease-2019 (COVID-19): A systematic review. Eur. J. Med. Res. 2021, 26, 96. [Google Scholar] [CrossRef] [PubMed]
- Siewiera, J.; Brodaczewska, K.; Jermakow, N.; Lubas, A.; Kłos, K.; Majewska, A.; Kot, J. Effectiveness of Hyperbaric Oxygen Therapy in SARS-CoV-2 Pneumonia: The Primary Results of a Randomised Clinical Trial. J. Clin. Med. 2023, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, T.; Shaikh, A.; Kirthika, N. Effective Outcome of HBOT as an Adjuvant Therapy in Patients Diagnosed with COVID-19 in a Tertiary Care Hospital – A Preliminary Study. J. Crit. Care Med. 2022, 8, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Kjellberg, A.; De Maio, A.; Lindholm, P. Can hyperbaric oxygen safely serve as an anti-inflammatory treatment for COVID-19? Med. Hypotheses 2020, 144, 110224. [Google Scholar] [CrossRef]
- De Maio, A.; Hightower, L.E. COVID-19, acute respiratory distress syndrome (ARDS), and hyperbaric oxygen therapy (HBOT): What is the link? Cell Stress Chaperones 2020, 25, 717–720. [Google Scholar] [CrossRef]
- Kjellberg, A.; Douglas, J.; Pawlik, M.T.; Kraus, M.; Oscarsson, N.; Zheng, X.; Bergman, P.; Franberg, O.; Kowalski, J.H.; Nyren, S.P.; et al. Randomised, controlled, open label, multicentre clinical trial to explore safety and efficacy of hyperbaric oxygen for preventing ICU admission, morbidity and mortality in adult patients with COVID-19. BMJ Open 2021, 11, e046738. [Google Scholar] [CrossRef]
- Winter, P.M.; Smith, G. The toxicity of oxygen. Anesthesiology 1972, 37, 210–241. [Google Scholar] [CrossRef]
- Wingelaar, T.T.; Brinkman, P.; de Vries, R.; van Ooij, P.A.M.; Hoencamp, R.; Maitland-van der Zee, A.H.; Hollmann, M.W.; van Hulst, R.A. Detecting Pulmonary Oxygen Toxicity Using eNose Technology and Associations between Electronic Nose and Gas Chromatography-Mass Spectrometry Data. Metabolites 2019, 9, 286. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.M.; Peltan, I.D.; Barkauskas, C.; Rogers, A.J.; Kan, V.; Gelijns, A.; Thompson, B.T. What Does Acute Respiratory Distress Syndrome Mean during the COVID-19 Pandemic? Ann. Am. Thorac Soc. 2021, 18, 1948–1950. [Google Scholar] [CrossRef] [PubMed]
- Bos, L.D.J.; Sjoding, M.; Sinha, P.; Bhavani, S.V.; Lyons, P.G.; Bewley, A.F.; Botta, M.; Tsonas, A.M.; Serpa Neto, A.; Schultz, M.J.; et al. Longitudinal respiratory subphenotypes in patients with COVID-19-related acute respiratory distress syndrome: Results from three observational cohorts. Lancet Respir. Med. 2021, 9, 1377–1386. [Google Scholar] [CrossRef]
- Lascarrou, J.B. COVID-19-related ARDS: One disease, two trajectories, and several unanswered questions. Lancet Respir. Med. 2021, 9, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- Nasa, P.; Azoulay, E.; Khanna, A.K.; Jain, R.; Gupta, S.; Javeri, Y.; Juneja, D.; Rangappa, P.; Sundararajan, K.; Alhazzani, W.; et al. Expert consensus statements for the management of COVID-19-related acute respiratory failure using a Delphi method. Crit. Care 2021, 25, 106. [Google Scholar] [CrossRef]
- Bessereau, J.; Aboab, J.; Hullin, T.; Huon-Bessereau, A.; Bourgeois, J.L.; Brun, P.M.; Chevret, S.; Annane, D. Safety of hyperbaric oxygen therapy in mechanically ventilated patients. Int. Marit. Health 2017, 68, 46–51. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J. Absorption atelectasis: Incidence and clinical implications. AANA J. 2013, 81, 205–208. [Google Scholar] [PubMed]
- Joyce, C.J.; Baker, A.B. What is the role of absorption atelectasis in the genesis of perioperative pulmonary collapse? Anaesth Intensive Care 1995, 23, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L. The pathological effects due to increase of oxygen tension in the air breathed. J. Physiol. 1899, 24, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Wingelaar, T.T.; van Ooij, P.A.M.; van Hulst, R.A. Oxygen Toxicity and Special Operations Forces Diving: Hidden and Dangerous. Front Psychol. 2017, 8, 1263. [Google Scholar] [CrossRef]
- Michalski, J.E.; Kurche, J.S.; Schwartz, D.A. From ARDS to pulmonary fibrosis: The next phase of the COVID-19 pandemic? Transl. Res. 2021, 241, 13–24. [Google Scholar] [CrossRef]
- Kallet, R.H.; Branson, R.D. Should Oxygen Therapy Be Tightly Regulated to Minimize Hyperoxia in Critically Ill Patients? Respir. Care 2016, 61, 801–817. [Google Scholar] [CrossRef]
- Arieli, R.; Yalov, A.; Goldenshluger, A. Modeling pulmonary and CNS O(2) toxicity and estimation of parameters for humans. J. Appl. Physiol. 2002, 92, 248–256. [Google Scholar] [CrossRef]
- Wingelaar, T.T.; Brinkman, P.; Hoencamp, R.; van Ooij, P.A.; Maitland-van der Zee, A.H.; Hollmann, M.W.; van Hulst, R.A. Assessment of pulmonary oxygen toxicity in special operations forces divers under operational circumstances using exhaled breath analysis. Diving Hyperb. Med. J. South Pac. Underw. Med. Soc. 2020, 50, 2–7. [Google Scholar] [CrossRef]
- Chavko, M.; Mahon, R.T.; McCarron, R.M. Mechanisms of protection against pulmonary hyperbaric O(2) toxicity by intermittent air breaks. Eur. J. Appl. Physiol. 2008, 102, 525–532. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Wang, Z.; Chen, Y.; Li, R. Intermittent hyperbaric oxygen exposure mobilizing peroxiredoxin 6 to prevent oxygen toxicity. J. Physiol. Sci. 2019, 69, 779–790. [Google Scholar] [CrossRef]
- Hensley, M.K.; Sjoding, M.W.; Prescott, H.C. COUNTERPOINT: Should Corticosteroids Be Routine Treatment in Early ARDS? No. Chest 2021, 159, 29–33. [Google Scholar] [CrossRef]

| Baseline Variable | HBOT + Best Practice N = 14 | Best Practice N = 15 |
|---|---|---|
| Age | 67.4 (10.8) | 63.3 (8.2) |
| Male sex | 8 (57.1%) | 8 (53.3%) |
| Caucasian ethnicity | 13 (92.8%) | 15 (100%) |
| BMI | 29.4 (4.5) | 29.2 (5.0) |
| Number of risk factors | 2.93 (0.96) | 3.13 (1.06) |
| Smoker (every day) | 1 (7.1%) | 0 (0%) |
| Former smoker | 5 (35.7%) | 5 (33.3%) |
| Never smoked | 8 (57.1%) | 10 (66.7%) |
| Time since initial symptoms (days) | 9.93 (3.58) | 11.67 (3.62) |
| NEWS at randomization | 5.3 (2.0) | 5.4 (1.7) |
| PFI at randomization | 14.0 (3.5) | 17.3 (6.4) |
| Group | HBOT (N = 14) n (%) AE | Best Practice (N = 15) n (%) AE |
|---|---|---|
| Adverse events | 14 (100%) 40 | 13 (87%) 55 |
| Serious adverse events | 6 (43%) 9 | 6 (40%) 14 |
| Severe adverse events | 3 (21%) 4 | 2 (13%) 3 |
| Deaths | 2 (14%) | 1 (7%) |
| Life-threatening | 1 | 2 |
| Persistent or significant disability/incapacity | 2 | 0 |
| Initial or prolonged hospitalization | 5 (36%) 7 | 5 (33%) 9 |
| Congenital anomaly/birth defect | 0 | 0 |
| Relationship to IMP *—possible | 2 | 0 |
| Action taken regarding IMP (discontinued) | 2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kjellberg, A.; Douglas, J.; Hassler, A.; Al-Ezerjawi, S.; Boström, E.; Abdel-Halim, L.; Liwenborg, L.; Hetting, E.; Jonasdottir Njåstad, A.D.; Kowalski, J.; et al. COVID-19-Induced Acute Respiratory Distress Syndrome Treated with Hyperbaric Oxygen: Interim Safety Report from a Randomized Clinical Trial (COVID-19-HBO). J. Clin. Med. 2023, 12, 4850. https://doi.org/10.3390/jcm12144850
Kjellberg A, Douglas J, Hassler A, Al-Ezerjawi S, Boström E, Abdel-Halim L, Liwenborg L, Hetting E, Jonasdottir Njåstad AD, Kowalski J, et al. COVID-19-Induced Acute Respiratory Distress Syndrome Treated with Hyperbaric Oxygen: Interim Safety Report from a Randomized Clinical Trial (COVID-19-HBO). Journal of Clinical Medicine. 2023; 12(14):4850. https://doi.org/10.3390/jcm12144850
Chicago/Turabian StyleKjellberg, Anders, Johan Douglas, Adrian Hassler, Sarah Al-Ezerjawi, Emil Boström, Lina Abdel-Halim, Lovisa Liwenborg, Eric Hetting, Anna Dora Jonasdottir Njåstad, Jan Kowalski, and et al. 2023. "COVID-19-Induced Acute Respiratory Distress Syndrome Treated with Hyperbaric Oxygen: Interim Safety Report from a Randomized Clinical Trial (COVID-19-HBO)" Journal of Clinical Medicine 12, no. 14: 4850. https://doi.org/10.3390/jcm12144850
APA StyleKjellberg, A., Douglas, J., Hassler, A., Al-Ezerjawi, S., Boström, E., Abdel-Halim, L., Liwenborg, L., Hetting, E., Jonasdottir Njåstad, A. D., Kowalski, J., Catrina, S.-B., Rodriguez-Wallberg, K. A., & Lindholm, P. (2023). COVID-19-Induced Acute Respiratory Distress Syndrome Treated with Hyperbaric Oxygen: Interim Safety Report from a Randomized Clinical Trial (COVID-19-HBO). Journal of Clinical Medicine, 12(14), 4850. https://doi.org/10.3390/jcm12144850








