Vascular Schizophrenia-like Psychosis in Older Adults
Abstract
1. Introduction
- -
- to analyze the prevalence and severity of vascular risk factors in a population of older patients referred to our clinic due to the onset of VLOSLP;
- -
- to create a specific phenotype based on pathophysiological insight rather than age of onset.
2. Materials and Methods
2.1. Study Sample
2.2. Study Design
2.3. Cognitive, Neuropsychiatric, and Functional Assessment
2.4. Genotyping
2.5. Value Quantification of Biochemical Concentrations
2.6. Vascular Risk Factor Assessment
2.7. Statistical Analyses
3. Results
3.1. Demographic, Cognitive, Functional, Neuropsychiatric, and Clinical Characteristics
3.2. Vascular Risk Frequency
3.3. Current Use of Antipsychotic and Antidepressant Drugs
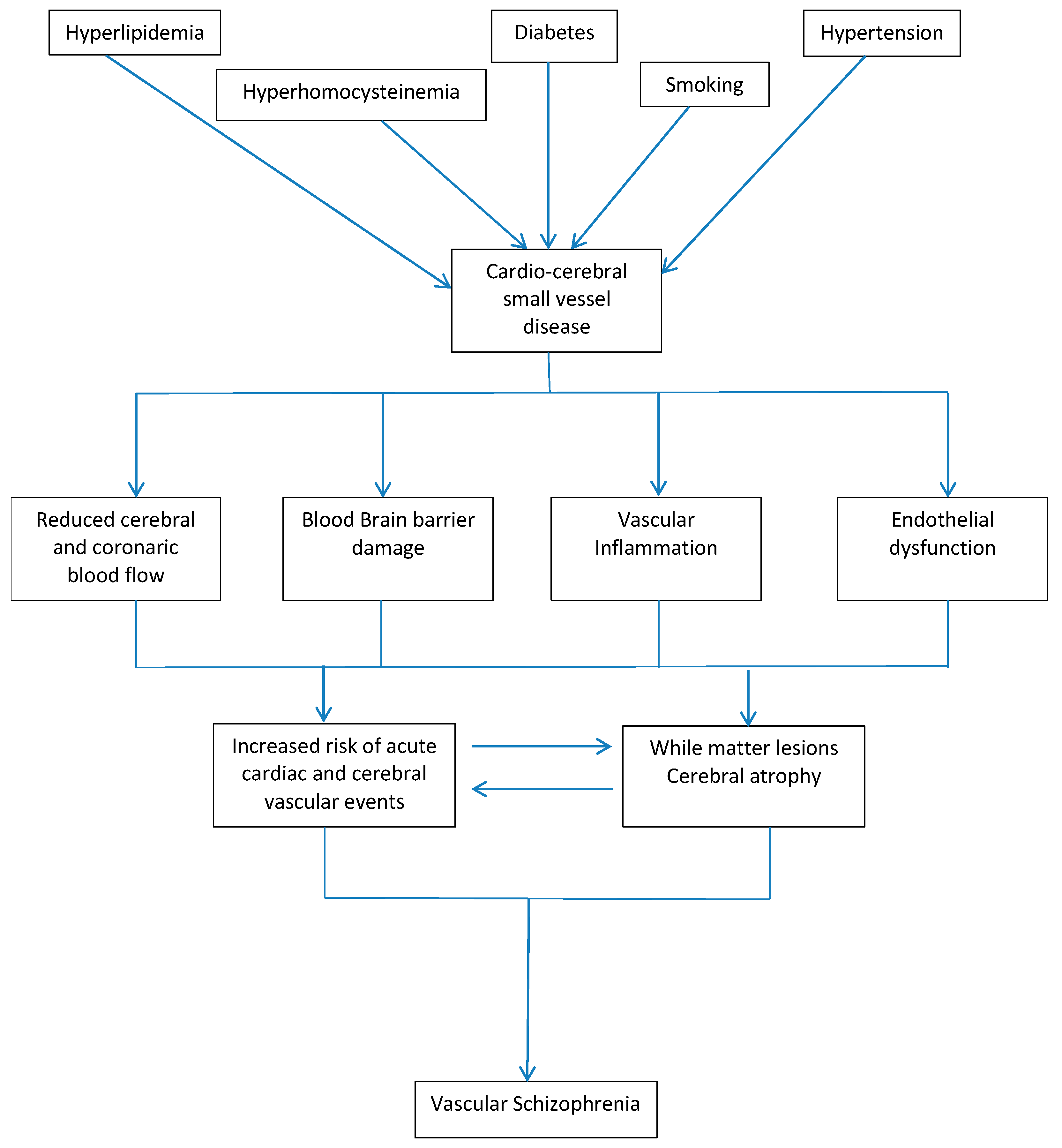
4. Discussion
4.1. Homocisteine and ApoE Gene
4.2. Endothelial Dysfunction
4.3. Neurovascular Unit Dysfunction
4.4. Vascular Schizophrenia-like Psychosis as Specific Phenotype
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Insel, T.R. Rethinking schizophrenia. Nature 2010, 468, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Rossler, W.; Salize, H.J.; van Os, J.; Riecher-Rossler, A. Size of burden of schizophrenia and psychotic disorders. Eur. Neuropsychopharmacol. 2005, 15, 399–409. [Google Scholar] [CrossRef]
- Jordan, G.; MacDonald, K.; Pope, M.A.; Schorr, E.; Malla, A.K.; Iyer, S.N. Positive changes experienced after a first episode of psychosis: A systematic review. Psychiatr. Serv. 2018, 69, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Maglione, J.E.; Thomas, S.E.; Jeste, D.V. Late-onset schizophrenia: Do recent studies support categorizing LOS as a subtype of schizophrenia? Curr. Opin. Psychiatry 2014, 27, 173–178. [Google Scholar] [CrossRef]
- Jeste, D.V.; Blazer, D.G.; First, M. Aging-related diagnostic variations: Need for diagnostic criteria appropriate for elderly psychiatric patients. Biol. Psychiatry 2005, 58, 265–271. [Google Scholar] [CrossRef]
- Mühlbauer, V.; Möhler, R.; Dichter, M.N.; Zuidema, S.U.; Köpke, S.; Luijendijk, H.J. Antipsychotics for agitation and psychosis in people with Alzheimer’s disease and vascular dementia. Cochrane Database Syst. Rev. 2021, 12, CD013304. [Google Scholar] [CrossRef]
- Kessler, R.C.; Amminger, G.P.; Aguilar-Gaxiola, S.; Alonso, J.; Lee, S.; Ustün, T.B. Age of onset of mental disorders: A review of recent literature. Curr. Opin. Psychiatry 2007, 20, 359–364. [Google Scholar] [CrossRef]
- Blöchl, M.; Schaare, H.L.; Kunzmann, U.; Nestler, S. The Age-Dependent Association between Vascular Risk Factors and Depressed Mood. J. Gerontol. B Psychol. Sci. Soc. Sci. 2022, 77, 284–294. [Google Scholar] [CrossRef]
- Mast, B.T.; Neufeld, S.; MacNeill, S.E.; Lichtenberg, P.A. Longitudinal support for the relationship between vascular risk factors and late-life depressive symptoms. Am. J. Geriatr. Psychiatry 2004, 12, 93–101. [Google Scholar] [CrossRef]
- Howard, R.; Cox, T.; Almeida, O.; Mullen, R.; Graves, P.; Reveley, A.; Levy, R. White matter signal hyperintensities in the brains of patients with late paraphrenia and the normal, community-living elderly. Biol. Psychiatry 1995, 38, 86–91. [Google Scholar] [CrossRef]
- Kim, J.T.; Park, M.S.; Yoon, G.J.; Jung, H.J.; Choi, K.H.; Nam, T.S.; Lee, S.H.; Choi, S.M.; Kim, B.C.; Kim, M.K.; et al. White matter hyperintensity as a factor associated with delayed mood disorders in patients with acute ischemic stroke. Eur. Neurol. 2011, 66, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, A.; Sundström, A.; Lindgren, L.; Stomby, A.; Aarsland, D.; Westman, E.; Winblad, B.; Olsson, T.; Nyberg, L. Longitudinal relationships among depressive symptoms, cortisol, and brain atrophy in the neocortex and the hippocampus. Acta Psychiatr. Scand. 2018, 137, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Utumi, Y.; Iseki, E.; Arai, H. Three patients with mood disorders showing catatonia and frontotemporal lobes atrophy. Psychogeriatrics 2013, 13, 254–259. [Google Scholar] [CrossRef]
- Breitner, J.C.; Husain, M.M.; Figiel, G.S.; Krishnan, K.R.; Boyko, O.B. Cerebral white matter disease in late-onset paranoid psychosis. Biol. Psychiatry 1990, 28, 266–274. [Google Scholar] [CrossRef]
- Jeste, D.V.; McAdams, L.A.; Palmer, B.W.; Braff, D.; Jernigan, T.L.; Paulsen, J.S.; Stout, J.C.; Symonds, L.L.; Bailey, A.; Heaton, R.K. Relationship of neuropsychological and MRI measures to age of onset of schizophrenia. Acta Psychiatr. Scand. 1998, 98, 156–164. [Google Scholar] [CrossRef]
- Swayze, V.W., 2nd; Andreasen, N.C.; Alliger, R.J.; Yuh, W.T.; Ehrhardt, J.C. Subcortical and temporal structures in affective disorder and schizophrenia: A magnetic resonance imaging study. Biol. Psychiatry 1992, 31, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, L.R.; Hegtmann, C.L.; Straszek, S.P.V.; Høyer, C.; Polcwiartek, C.; Petersen, L.J.; Dalgaard, M.K.; Jensen, S.E.; Nielsen, R.E. Peripheral artery disease in patients with schizophrenia as compared to controls. BMC Cardiovasc. Disord. 2023, 23, 126. [Google Scholar] [CrossRef]
- Wilkowska, A.; Kujawska-Danecka, H.; Hajduk, A. Risk and prophylaxis of venous thromboembolism in hospitalized psychiatric patients. A review. Psychiatr. Pol. 2018, 52, 421–435. [Google Scholar] [CrossRef]
- van der Heijden, F.M.; Zeebregts, C.J.; Reijnen, M.M. Does extracranial arterial pathology play a role in late-onset psychiatric disorders? Cogn. Behav. Neurol. 2010, 23, 147–151. [Google Scholar] [CrossRef]
- Osby, U.; Westman, J.; Hallgren, J.; Gissler, M. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987–2010. Eur. J. Public Health 2016, 26, 867–871. [Google Scholar] [CrossRef]
- Fan, Z.; Wu, Y.; Shen, J.; Ji, T.; Zhan, R. Schizophrenia and the risk of cardiovascular diseases: A meta-analysis of thirteen cohort studies. J. Psychiatr. Res. 2013, 47, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Moises, H.W.; Wollschläger, D.; Binder, H. Functional genomics indicate that schizophrenia may be an adult vascular-ischemic disorder. Transl. Psychiatry 2015, 5, e616. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Fleming, I.; Busse, R. Endothelial aging. Cardiovasc. Res. 2005, 66, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and vascular endothelial function in humans. Clin. Sci. 2011, 120, 357–375. [Google Scholar] [CrossRef]
- Müller, N. Inflammation in Schizophrenia: Pathogenetic Aspects and Therapeutic Considerations. Schizophr. Bull. 2018, 44, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Korann, V.; Suhas, S.; Appaji, A.; Nagendra, B.; Padmanabha, A.; Jacob, A.; Devi, P.; Bharath, R.D.; Kumar, V.; Varambally, S.; et al. Association between retinal vascular measures and brain white matter lesions in schizophrenia. Asian J. Psychiatr. 2022, 70, 103042. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Dmitrieva, E.M.; Parshukova, D.A.; Kazantseva, D.V.; Vasilieva, A.R.; Smirnova, L.P. Oxidative Stress-Related Mechanisms in Schizophrenia Pathogenesis and New Treatment Perspectives. Oxid. Med. Cell Longev. 2021, 2021, 8881770. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Dev, S.I.; Chen, G.; Liou, S.C.; Martin, A.S.; Irwin, M.R.; Carroll, J.E.; Tu, X.; Jeste, D.V.; Eyler, L.T. Abnormal levels of vascular endothelial biomarkers in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2018, 268, 849–860. [Google Scholar] [CrossRef]
- Howard, R.; Rabins, P.V.; Seeman, M.V.; Jeste, D.V. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: An international consensus. The International Late-Onset Schizophrenia Group. Am. J. Psychiatry 2000, 157, 172–178. [Google Scholar] [CrossRef]
- Sachdev, P.S.; Mohan, A.; Taylor, L.; Jeste, D.V. DSM-5 and Mental Disorders in Older Individuals: An Overview. Harv. Rev. Psychiatry 2015, 23, 320–328. [Google Scholar] [CrossRef]
- Chen, L.; Selvendra, A.; Stewart, A.; Castle, D. Risk factors in early and late onset schizophrenia. Compr. Psychiatry 2018, 80, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.J.; Graham, C.; Sham, P.; Dennehey, J.; Castle, D.J.; Levy, R.; Murray, R. A controlled family study of late-onset non-affective psychosis (late paraphrenia). Br. J. Psychiatry 1997, 170, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Meesters, P.D.; de Haan, L.; Comijs, H.C.; Stek, M.L.; Smeets-Janssen, M.M.; Weeda, M.R.; Eikelenboom, P.; Smit, J.H.; Beekman, A.T. Schizophrenia spectrum disorders in later life: Prevalence and distribution of age at onset and sex in a dutch catchment area. Am. J. Geriatr. Psychiatry 2012, 20, 18–28. [Google Scholar] [CrossRef]
- Hanssen, M.; van der Werf, M.; Verkaaik, M.; Arts, B.; Myin-Germeys, I.; van Os, J.; Verhey, F.; Köhler, S.; Genetic Risk and Outcome in Psychosis Study Group. Comparative study of clinical and neuropsychological characteristics between early-, late and very-late-onset schizophrenia-spectrum disorders. Am. J. Geriatr. Psychiatry 2015, 23, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Reeves, S.; Stewart, R.; Howard, R. Service contact and psychopathology in very-late-onset schizophrenia-like psychosis: The effects of gender and ethnicity. Int. J. Geriatr. Psychiatry 2002, 17, 473–479. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; (DSM-5); American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Folstein, M.; Folstein, S.; McHugh, P. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Cummings, J.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.; Gornbein, J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994, 44, 2308–2314. [Google Scholar] [CrossRef]
- Katz, S.; Downs, T.; Cash, H.; Grotz, R. Progress in the development of an index of ADL. Gerontologist 1970, 10, 20–30. [Google Scholar] [CrossRef]
- Lawton, M.; Brody, E. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Gabb, G.M.; Mangoni, A.A.; Arnolda, L. Guideline for the diagnosis and management of hypertension in adults—2016. Med. J. Aust. 2017, 206, 141. [Google Scholar] [CrossRef]
- Jellinger, P.S.; Handelsman, Y.; Rosenblit, P.D.; Bloomgarden, Z.T.; Fonseca, V.A.; Garber, A.J.; Grunberger, G.; Guerin, C.K.; Bell, D.S.H.; Mechanick, J.I.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines For Management of Dyslipidemia And Prevention of Cardiovascular Disease. Endocr. Pr. 2017, 23, 1–87. [Google Scholar] [CrossRef]
- Garber, A.J.; Abrahamson, M.J.; Barzilay, J.I.; Blonde, L.; Bloomgarden, Z.T.; Bush, M.A.; Dagogo-Jack, S.; DeFronzo, R.A.; Einhorn, D.; Fonseca, V.A.; et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2017 Executive Summary. Endocr. Pr. 2017, 23, 207–238. [Google Scholar] [CrossRef]
- Talonen, S.; Väänänen, J.; Kaltiala-Heino, R. Gender differences in first onset Schizophrenia spectrum psychoses. Nord. J. Psychiatry 2017, 71, 131–138. [Google Scholar] [CrossRef]
- Seeman, M.V. Sex differences in schizophrenia relevant to clinical care. Expert. Rev. Neurother. 2021, 21, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Hung, C.F.; Lin, P.Y.; Lee, Y.; Wu, C.C.; Hsu, S.T.; Chen, C.C.; Chong, M.Y.; Lin, C.H.; Wang, L.J. Gender differences in susceptibility to schizophrenia: Potential implication of neurosteroids. Psychoneuroendocrinology 2017, 84, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Wolters, F.J.; de Bruijn, R.F.; Hofman, A.; Koudstaal, P.J.; Ikram, M.A.; Heart Brain Connection Collaborative Research Group. Cerebral Vasoreactivity, Apolipoprotein E, and the Risk of Dementia: A Population-Based Study. Arter. Thromb. Vasc. Biol. 2016, 36, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Refsum, H.; Bottiglieri, T.; Fenech, M.; Hooshmand, B.; McCaddon, A.; Miller, J.W.; Rosenberg, I.H.; Obeid, R. Homocysteine and Dementia: An International Consensus Statement. J. Alzheimers Dis. 2018, 62, 561–570. [Google Scholar] [CrossRef]
- McCulloch, Y.; Clare, L.; Howard, R.; Peters, E. Psychological processes underlying delusional thinking in late-onset psychosis: A preliminary investigation. Int. J. Geriatr. Psychiatry 2006, 21, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Giblin, S.; Clare, L.; Livingston, G.; Howard, R. Psychosocial correlates of late-onset psychosis: Life experiences, cognitive schemas, and attitudes to ageing. Int. J. Geriatr. Psychiatry 2004, 19, 611–623. [Google Scholar] [CrossRef]
- Jones, D.K.; Catani, M.; Pierpaoli, C.; Reeves, S.J.; Shergill, S.S.; O’Sullivan, M.; Maguire, P.; Horsfield, M.A.; Simmons, A.; Williams, S.C.; et al. A diffusion tensor magnetic resonance imaging study of frontal cortex connections in very-late-onset schizophrenia-like psychosis. Am. J. Geriatr. Psychiatry 2005, 13, 1092–1099. [Google Scholar] [CrossRef]
- Barak, Y.; Aizenberg, D.; Mirecki, I.; Mazeh, D.; Achiron, A. Very late-onset schizophrenia-like psychosis: Clinical and imaging characteristics in comparison with elderly patients with schizophrenia. J. Nerv. Ment. Dis. 2002, 190, 733–736. [Google Scholar] [CrossRef]
- Van Assche, L.; Van Aubel, E.; Van de Ven, L.; Bouckaert, F.; Luyten, P.; Vandenbulcke, M. The Neuropsychological Profile and Phenomenology of Late Onset Psychosis: A Cross-sectional Study on the Differential Diagnosis of Very-Late-Onset Schizophrenia-Like Psychosis, Dementia with Lewy Bodies and Alzheimer’s Type Dementia with Psychosis. Arch. Clin. Neuropsychol. 2019, 34, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.; Blackwood, N.; Corcoran, R.; Rowse, G.; Kinderman, P.; Bentall, R.; Howard, R. Misunderstanding the intentions of others: An exploratory study of the cognitive etiology of persecutory delusions in very late-onset schizophrenia-like psychosis. Am. J. Geriatr. Psychiatry 2006, 14, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.S.; Kotsopoulos, E.J.; Yamin, S. Phenotypic cognitive impairment in late-onset delusional disorder. Int. Psychogeriatr. 2014, 26, 965–975. [Google Scholar] [CrossRef]
- Brodaty, H.; Sachdev, P.; Koschera, A.; Monk, D.; Cullen, B. Long-term outcome of late-onset schizophrenia: 5-year follow-up study. Br. J. Psychiatry 2003, 183, 213–219. [Google Scholar] [CrossRef]
- Bravo Baptista, S.; Faustino, M.; Brizida, L.; Loureiro, J.; Augusto, J.; Abecasis, J.; Monteiro, C.; Leal, P.; Nédio, M.; Farto, E.; et al. Early peripheral endothelial dysfunction predicts myocardial infarct extension and microvascular obstruction in patients with ST-elevation myocardial infarction. Rev. Port. Cardiol. 2017, 36, 731–742. [Google Scholar] [CrossRef]
- Israel, A.K.; Seeck, A.; Boettger, M.K.; Rachow, T.; Berger, S.; Voss, A.; Bär, K.J. Peripheral endothelial dysfunction in patients suffering from acute schizophrenia: A potential marker for cardiovascular morbidity? Schizophr. Res. 2011, 128, 44–50. [Google Scholar] [CrossRef]
- Protopopova, D.; Masopust, J.; Malý, R.; Valis, M.; Dostalova, G.; Ranna, K.; Bažant, J. Peripheral endothelial dysfunction as a marker of cardiovascular risk in physically healthy patients with schizophrenia and related psychoses: A matched case control study. Neuro Endocrinol. Lett. 2014, 35, 503–509. [Google Scholar]
- Radu, G.; Luca, C.; Petrescu, L.; Bordejevic, D.A.; Tomescu, M.C.; Andor, M.; Cîtu, I.; Mavrea, A.; Buda, V.; Tomescu, C.; et al. The Predictive Value of Endothelial Inflammatory Markers in the Onset of Schizophrenia. Neuropsychiatr. Dis. Treat. 2020, 16, 545–555. [Google Scholar] [CrossRef]
- Lyness, J.M.; King, D.A.; Conwell, Y.; Cox, C.; Caine, E.D. Cerebrovascular risk factors and 1-year depression outcome in older primary care patients. Am. J. Psychiatry 2000, 157, 1499–1501. [Google Scholar] [CrossRef] [PubMed]
- Massardo, T.; Quintana, J.C.; Jaimovich, R.; Sáez, C.G.; Risco, L.; Liberman, C.; Araya, A.V.; Galleguillos, T.; Castro-Mora, G.; Pereira, J. Regional Brain Perfusion Is Associated with Endothelial Dysfunction Markers in Major Depressive Disorder. Neuropsychobiology 2021, 80, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Wake, R.; Miyaoka, T.; Araki, T.; Kawakami, K.; Furuya, M.; Limoa, E.; Hashioka, S.; Horiguchi, J. Regional cerebral blood flow in late-onset schizophrenia: A SPECT study using 99mTc-ECD. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 3–12. [Google Scholar] [CrossRef]
- Najjar, S.; Pahlajani, S.; De Sanctis, V.; Stern, J.N.H.; Najjar, A.; Chong, D. Neurovascular Unit Dysfunction and Blood-Brain Barrier Hyperpermeability Contribute to Schizophrenia Neurobiology: A Theoretical Integration of Clinical and Experimental Evidence. Front. Psychiatry 2017, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Friedman, A.; Kaufer, D. Blood-brain barrier breakdown and blood-brain communication in neurological and psychiatric diseases. Cardiovasc. Psychiatry Neurol. 2011, 2011, 431470. [Google Scholar] [CrossRef]
- Uranova, N.A.; Zimina, I.S.; Vikhreva, O.V.; Krukov, N.O.; Rachmanova, V.I.; Orlovskaya, D.D. Ultrastructural damage of capillaries in the neocortex in schizophrenia. World J. Biol. Psychiatry 2010, 11, 567–578. [Google Scholar] [CrossRef]
- Pun, P.B.; Lu, J.; Moochhala, S. Involvement of ROS in BBB dysfunction. Free Radic. Res. 2009, 43, 348–364. [Google Scholar] [CrossRef]
- Yarlagadda, A.; Alfson, E.; Clayton, A.H. The blood brain barrier and the role of cytokines in neuropsychiatry. Psychiatry 2009, 6, 18–22. [Google Scholar]
- Harris, L.W.; Wayland, M.; Lan, M.; Ryan, M.; Giger, T.; Lockstone, H.; Wuethrich, I.; Mimmack, M.; Wang, L.; Kotter, M.; et al. The cerebral microvasculature in schizophrenia: A laser capture microdissection study. PLoS ONE 2008, 3, e3964. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, G.M.; Dantzer, R. Is there a role for immune-to-brain communication in schizophrenia? Psychopharmacology 2016, 233, 1559–1573. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Song, Z.; Yu, S.; Piazza, A.; Nanda, A.; Penninger, J.M.; Granger, D.N.; Li, G. Phosphatidylinositol-3-kinase gamma plays a central role in blood-brain barrier dysfunction in acute experimental stroke. Stroke 2011, 42, 2033–2044. [Google Scholar] [CrossRef] [PubMed]
- Henninger, D.D.; Panés, J.; Eppihimer, M.; Russell, J.; Gerritsen, M.; Anderson, D.C.; Granger, D.N. Cytokine-induced VCAM-1 and ICAM-1 expression in different organs of the mouse. J. Immunol. 1997, 158, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Lencz, T.; Guha, S.; Liu, C.; Rosenfeld, J.; Mukherjee, S.; DeRosse, P.; John, M.; Cheng, L.; Zhang, C.; Badner, J.A.; et al. Genome-wide association study implicates NDST3 in schizophrenia and bipolar disorder. Nat. Commun. 2013, 4, 2739. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, G.S.; Meyers, B.S.; Young, R.C.; Campbell, S.; Silbersweig, D.; Charlson, M. ’Vascular depression’ hypothesis. Arch. Gen. Psychiatry 1997, 54, 915–922. [Google Scholar] [CrossRef]
- Wijeratne, C.; Malhi, G.S. Vascular mania: An old concept in danger of sclerosing? A clinical overview. Acta Psychiatr. Scand. Suppl. 2007, 434, 35–40. [Google Scholar] [CrossRef]
- Steffens, D.C.; Krishnan, K.R. Structural neuroimaging and mood disorders: Recent findings, implications for classification, and future directions. Biol. Psychiatry 1998, 43, 705–712. [Google Scholar] [CrossRef]
- Fischer, C.E.; Namasivayam, A.; Crawford-Holland, L.; Hakobyan, N.; Schweizer, T.A.; Munoz, D.G.; Pollock, B.G. Psychotic Disorders in the Elderly: Diagnosis, Epidemiology, and Treatment. Psychiatr. Clin. North Am. 2022, 45, 691–705. [Google Scholar] [CrossRef] [PubMed]
| ALL N = 103 | Male N = 39 | Female N = 64 | p-Value | |
|---|---|---|---|---|
| Age (years) | 0.639 | |||
| Mean ± SD | 80.32 ± 7.65 | 80.78 ± 8.08 | 80.04 ± 7.42 | |
| Range | 65.00–95.36 | 65.00–95.36 | 65.00–93.45 | |
| MMSE | 0.727 | |||
| Mean ± SD | 20.55 ± 5.98 | 20.81 ± 7.18 | 20.39 ± 5.17 | |
| Range | 0–30.00 | 0–30.00 | 0–30.00 | |
| ADL | 0.348 | |||
| Mean ± SD | 4.57 ± 1.58 | 4.38 ± 1.56 | 4.69 ± 1.59 | |
| Range | 1.00–6.00 | 2.00–6.00 | 1.00–6.00 | |
| IADL | 0.415 | |||
| Mean ± SD | 3.38 ± 2.92 | 3.08 ± 2.75 | 3.56 ± 3.02 | |
| Range | 0–8.00 | 0–8.00 | 0–8.00 | |
| NPI—Total score | 0.770 | |||
| Mean ± SD | 40.73 ± 22.39 | 39.90 ± 19.64 | 41.23 ± 24.04 | |
| Range | 4.00–100.00 | 6.00–84.00 | 4.00–100.00 | |
| NPI—Distress | ||||
| Mean ± SD | 15.78 ± 7.33 | 14.82 ± 7.18 | 16.36 ± 7.41 | 0.304 |
| Range | 0–38.00 | 0–32.00 | 4.00–38.00 | |
| NPI—Delusion | ||||
| Mean ± SD | 5.11 ± 4.74 | 5.00 ± 4.58 | 5.17 ± 4.86 | 0.859 |
| Range | 0–12.00 | 0–12.00 | 0–12.00 | |
| NPI—Hallucination | ||||
| Mean ± SD | 4.73 ± 4.85 | 3.56 ± 4.48 | 5.44 ± 4.97 | 0.057 |
| Range | 0–15.00 | 0–15.00 | 0–15.00 | |
| NPI—Agitation/Aggresion | ||||
| Mean ± SD | 5.57 ± 4.27 | 6.44 ± 4.43 | 5.05 ± 4.11 | 0.109 |
| Range | 0–16.00 | 0–16.00 | 0–12.00 | |
| NPI—Depression | ||||
| Mean ± SD | 5.40 ± 4.38 | 5.13 ± 4.67 | 5.56 ± 4.22 | 0.628 |
| Range | 0–16.00 | 0–16.00 | 0–12.00 | |
| NPI—Anxiety | ||||
| Mean ± SD | 3.93 ± 4.62 | 3.44 ± 4.76 | 4.23 ± 4.54 | 0.397 |
| Range | 0–16.00 | 0–16.00 | 0–12.00 | |
| NPI—Euphoria | ||||
| Mean ± SD | 0.88 ± 2.87 | 0.62 ± 2.38 | 1.05 ± 3.14 | 0.462 |
| Range | 0–12.00 | 0–12.00 | 0–12.00 | |
| NPI—Apathy | ||||
| Mean ± SD | 4.89 ± 4.75 | 3.67 ± 4.62 | 5.64 ± 4.71 | 0.040 |
| Range | 0–12.00 | 0–12.00 | 0–12.00 | |
| NPI—Disinhibition | ||||
| Mean ± SD | 0.79 ± 2.30 | 0.82 ± 2.32 | 0.77 ± 2.31 | 0.907 |
| Range | 0–9.00 | 0–9.00 | 0–9.00 | |
| NPI—Irritability | ||||
| Mean ± SD | 5.04 ± 4.62 | 5.62 ± 5.08 | 4.69 ± 4.31 | 0.325 |
| Range | 0–16.00 | 0–16.00 | 0–12.00 | |
| NPI—Abnormal activity | ||||
| Mean ± SD | 1.01 ± 2.70 | 0.87 ± 2.32 | 1.09 ± 2.93 | 0.688 |
| Range | 0–12.00 | 0–9.00 | 0–12.00 | |
| NPI—Sleep disturbance | ||||
| Mean ± SD | 4.97 ± 4.24 | 4.28 ± 3.95 | 5.39 ± 4.38 | 0.189 |
| Range | 0–12.00 | 0–9.00 | 0–12.00 | |
| NPI—Eating disorders | ||||
| Mean ± SD | 1.86 ± 3.62 | 1.03 ± 2.66 | 2.38 ± 4.03 | 0.066 |
| Range | 0–12.00 | 0–12.00 | 0–12.00 | |
| Psychosis | ||||
| Affective—n (%) | 76(73.8) | 26(34.2) | 50(65.8) | 0.200 |
| No-affective—n (%) | 27(26.2) | 13(48.1) | 14(51.9) |
| ALL N = 103 | Male N = 39 | Female N = 64 | p-Value | |
|---|---|---|---|---|
| Laboratory measurements | ||||
| ApoE | ||||
| ε2/ε3–N (%) | 6 (5.8) | 3 (7.7) | 3 (4.7) | |
| ε3/ε3–N (%) | 61 (59.2) | 24 (61.5) | 37 (57.8) | 0.862 |
| ε3/ε4–N (%) | 27 (26.2) | 9 (23.1) | 18 (28.1) | |
| ε4/ε4–N (%) | 9 (8.7) | 3 (7.7) | 6 (9.4) | |
| Hb, g/dL | 0.019 | |||
| Mean ± SD | 13.40 ± 1.52 | 13.85 ± 1.62 | 13.13 ± 1.39 | |
| Range | 10.00–18.00 | 10.00–18.00 | 10.00–17.00 | |
| MCV, fL | 0.195 | |||
| Mean ± SD | 90.84 ± 6.55 | 91.91 ± 6.58 | 90.18 ± 6.50 | |
| Range | 59.00–100.00 | 65.00–100.00 | 59.00–100.00 | |
| Platelets, mil/mcl | 0.004 | |||
| Mean ± SD | 220.94 ± 79.94 | 192.15 ± 61.82 | 238.48 ± 84.94 | |
| Range | 21.00–595.00 | 21.00–367.00 | 119.00–595.00 | |
| TPT, g/dL | 0.768 | |||
| Mean ± SD | 6.99 ± 0.71 | 6.96 ± 0.63 | 7.01 ± 0.75 | |
| Range | 5.00–9.00 | 5.00–8.00 | 5.00–9.00 | |
| Creatinine, mg/dL | 0.406 | |||
| Mean ± SD | 1.01 ± 0.36 | 1.04 ± 0.33 | 0.98 ± 0.38 | |
| Range | 0–3.00 | 1.00–2.00 | 0–3.00 | |
| Azotemia, mg/dL | ||||
| Mean ± SD | 45.95 ± 16.81 | 45.64 ± 17.49 | 46.14 ± 16.53 | 0.884 |
| Range | 21.00–106.00 | 21.00–105.00 | 22.00–106.00 | |
| Glycemia, mg/dL | ||||
| Mean ± SD | 96.90 ± 24.56 | 95.15 ± 21.40 | 97.98 ± 26.44 | 0.574 |
| Range | 68.00–187.00 | 68.00–164.00 | 71.00–187.00 | |
| TC, mg/dL | ||||
| Mean ± SD | 183.46 ± 45.21 | 175.33 ± 46.09 | 188.41 ± 44.30 | 0.156 |
| Range | 97.00–301.00 | 106.00–301.00 | 97.00–298.00 | |
| TG, mg/dL | ||||
| Mean ± SD | 114.83 ± 85.66 | 130.79 ± 128.31 | 105.09 ± 41.20 | 0.140 |
| Range | 39.00–835.00 | 42.00–835.00 | 39.00–311.00 | |
| UA, mg/dL | ||||
| Mean ± SD | 4.90 ± 1.55 | 5.53 ± 1.52 | 4.53 ± 1.46 | 0.001 |
| Range | 2.00–10.00 | 2.00–10.00 | 2.00–9.00 | |
| Na, mmol/L | ||||
| Mean ± SD | 140.57 ± 3.60 | 139.95 ± 3.03 | 140.95 ± 3.88 | 0.171 |
| Range | 120.00–147.00 | 133.00–146.00 | 120.00–147.00 | |
| K, mmol/L | ||||
| Mean ± SD | 4.28 ± 0.63 | 4.25 ± 0.40 | 4.29 ± 0.74 | 0.780 |
| Range | 3.00–8.00 | 4.00–5.00 | 3.00–8.00 | |
| Cl, mmol/L | ||||
| Mean ± SD | 104.89 ± 3.67 | 104.82 ± 2.94 | 104.94 ± 4.08 | 0.876 |
| Range | 87.00–116.00 | 87.00–116.00 | 87.00–116.00 | |
| Ca, mg/dL | ||||
| Mean ± SD | 9.04 ± 0.59 | 8.82 ± 0.48 | 9.17 ± 0.61 | 0.003 |
| Range | 8.00–11.00 | 8.00–10.00 | 8.00–11.00 | |
| Folate, ng/mL | ||||
| Mean ± SD | 7.62 ± 6.56 | 7.20 ± 7.26 | 7.93 ± 6.06 | 0.629 |
| Range | 2.00–41.00 | 2.00–41.00 | 2.00–41.00 | |
| Vit-B12, pg/mL | ||||
| Mean ± SD | 371.04 ± 286.85 | 323.91 ± 205.32 | 405.60 ± 333.33 | 0.216 |
| Range | 60.00–2000.00 | 60.00–806.00 | 98.00–2000.00 | |
| Homocysteine, μmol/L | ||||
| Mean ± SD | 12.58 ± 5.34 | 13.97 ± 6.83 | 11.66 ± 3.90 | 0.100 |
| Range | 4.00–28.00 | 4.00–28.00 | 5.00–20.00 | |
| Vascular risks | ||||
| Hypertension | ||||
| Yes—N (%) | 57 (55.3) | 20 (51.3) | 37 (57.8) | 0.518 |
| No—N (%) | 46 (44.7) | 19 (48.7) | 27 (42.2) | |
| Hyperlipidemia in treatment | ||||
| Yes—N (%) | 22 (21.4) | 7 (17.9) | 15 (23.4) | 0.510 |
| No—N (%) | 81 (78.6) | 32 (82.1) | 49 (76.6) | |
| Diabetes | ||||
| Yes—N (%) | 20 (19.4) | 6 (15.4) | 14 (21.9) | 0.419 |
| No—N (%) | 83 (80.6) | 33 (84.6) | 50 (78.1) | |
| AF | ||||
| Yes—N (%) | 11 (10.7) | 5 (12.8) | 6 (9.4) | 0.583 |
| No—N (%) | 92 (89.3) | 34 (87.2) | 58 (90.6) | |
| HF | ||||
| Yes—N (%) | 8 (7.8) | 4 (10.3) | 4 (6.2) | 0.461 |
| No—N (%) | 95 (92.2) | 35 (89.7) | 60 (93.8) | |
| Stroke | ||||
| Yes—N (%) | 15 (14.6) | 6 (15.4) | 9 (14.1) | 0.854 |
| No—N (%) | 88 (85.4) | 33 (84.6) | 55 (85.9) | |
| MI | ||||
| Yes—N (%) | 13 (12.6) | 5 (12.8) | 8 (12.5) | 0.962 |
| No—N (%) | 90 (87.4) | 34 (87.2) | 56 (87.5) | |
| Tobacco use | ||||
| Yes—N (%) | 5 (4.9) | 4 (10.3) | 1 (1.6) | 0.046 |
| No—N (%) | 98 (95.1) | 35 (89.7) | 63 (98.4) | |
| Alcohol consumption | ||||
| Yes—N (%) | 3 (2.9) | 3 (7.7) | 0 | 0.024 |
| No—N (%) | 100 (97.1) | 36 (92.3) | 64 (100.0) |
| <80 Years | ≥80 Years | p-Value | |
|---|---|---|---|
| Males | |||
| Hypertension | |||
| Yes—N (%) | 8 (44.4) | 12 (57.1) | 0.429 |
| No—N (%) | 10 (55.6) | 9 (42.9) | |
| Hyperlipidemia in treatment | |||
| Yes—N (%) | 4 (22.2) | 3 (14.3) | 0.520 |
| No—N (%) | 14 (77.8) | 18 (85.7) | |
| Diabetes | |||
| Yes—N (%) | 4 (22.2) | 2 (9.5) | 0.273 |
| No—N (%) | 14 (77.8) | 19 (90.5) | |
| AF | |||
| Yes—N (%) | 1 (5.6) | 4 (19.0) | 0.209 |
| No—N (%) | 17 (94.4) | 17 (81.0) | |
| HF | |||
| Yes—N (%) | 2 (11.1) | 2 (9.5) | 0.871 |
| No—N (%) | 16 (88.9) | 19 (90.5) | |
| Stroke | |||
| Yes—N (%) | 1 (5.6) | 5 (23.8) | 0.115 |
| No—N (%) | 17 (94.4) | 16 (76.2) | |
| MI | |||
| Yes—N (%) | 0 | 5 (23.8) | 0.027 |
| No—N (%) | 18 (100.0) | 16 (76.2) | |
| Tobacco use | |||
| Yes—N (%) | 3 (16.7) | 1 (4.8) | 0.222 |
| No—N (%) | 15 (83.3) | 20 (95.2) | |
| Alcohol consumption | |||
| Yes—N (%) | 1 (5.6) | 2 (9.5) | 0.643 |
| No—N (%) | 17 (94.4) | 19 (90.5) | |
| Females | |||
| Hypertension | |||
| Yes—N (%) | 16 (53.3) | 21 (61.8) | 0.496 |
| No—N (%) | 14 (46.7) | 13 (38.2) | |
| Dyslipidemia | |||
| Yes—N (%) | 9 (30.0) | 6 (17.6) | 0.244 |
| No—N (%) | 21 (70.0) | 28 (82.4) | |
| Diabetes | |||
| Yes—N (%) | 9 (30.0) | 5 (14.7) | 0.140 |
| No—N (%) | 21 (70.0) | 29 (85.3) | |
| AF | |||
| Yes—N (%) | 1 (3.3) | 5 (14.7) | 0.119 |
| No—N (%) | 29 (96.7) | 29 (85.3) | |
| HF | |||
| Yes—N (%) | 0 | 4 (11.8) | 0.052 |
| No—N (%) | 30 (100.0) | 30 (88.2) | |
| Stroke | |||
| Yes—N (%) | 5 (16.7) | 4 (11.8) | 0.573 |
| No—N (%) | 25 (83.3) | 30 (88.2) | |
| MI | |||
| Yes—N (%) | 2 (6.7) | 6 (17.6) | 0.185 |
| No—N (%) | 28 (93.3) | 28 (82.4) | |
| Tobacco use | |||
| Yes—N (%) | 1 (3.3) | 0 | 0.283 |
| No—N (%) | 29 (96.7) | 34 (100.0) | |
| Alcohol consumption | |||
| Yes—N (%) | 0 | 0 | 1.000 |
| No—N (%) | 30 (100.0) | 34 (100.0) |
| Medications | Affective Psychosis | No-Affective Psychosis | p-Value |
|---|---|---|---|
| Untreated—N (%) | 1 (1.3) | 0 | 0.549 |
| Treated—N (%) | 75 (98.7) | 27 (100.0) | |
| SSRI—N (%) | 21 (27.6) | 5 (18.5) | 0.349 |
| AA—N (%) | 41 (53.9) | 17 (63.0) | 0.417 |
| TA—N (%) | 9 (11.8) | 8 (29.6) | 0.032 |
| MS—N (%) | 14 (18.4) | 1 (3.7) | 0.063 |
| Tz—N (%) | 11 (14.5) | 2 (7.4) | 0.342 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauriola, M.; D’Onofrio, G.; Ciccone, F.; Torre, A.l.; Angelillis, V.; Germano, C.; Cascavilla, L.; Greco, A. Vascular Schizophrenia-like Psychosis in Older Adults. J. Clin. Med. 2023, 12, 4831. https://doi.org/10.3390/jcm12144831
Lauriola M, D’Onofrio G, Ciccone F, Torre Al, Angelillis V, Germano C, Cascavilla L, Greco A. Vascular Schizophrenia-like Psychosis in Older Adults. Journal of Clinical Medicine. 2023; 12(14):4831. https://doi.org/10.3390/jcm12144831
Chicago/Turabian StyleLauriola, Michele, Grazia D’Onofrio, Filomena Ciccone, Annamaria la Torre, Valentina Angelillis, Carmela Germano, Leandro Cascavilla, and Antonio Greco. 2023. "Vascular Schizophrenia-like Psychosis in Older Adults" Journal of Clinical Medicine 12, no. 14: 4831. https://doi.org/10.3390/jcm12144831
APA StyleLauriola, M., D’Onofrio, G., Ciccone, F., Torre, A. l., Angelillis, V., Germano, C., Cascavilla, L., & Greco, A. (2023). Vascular Schizophrenia-like Psychosis in Older Adults. Journal of Clinical Medicine, 12(14), 4831. https://doi.org/10.3390/jcm12144831






