Comparison of Nailfold Videocapillaroscopy with Retinal and Choroidal Vascular Parameters in Patients with Central Serous Chorioretinopathy
Abstract
1. Introduction
2. Materials and Methods
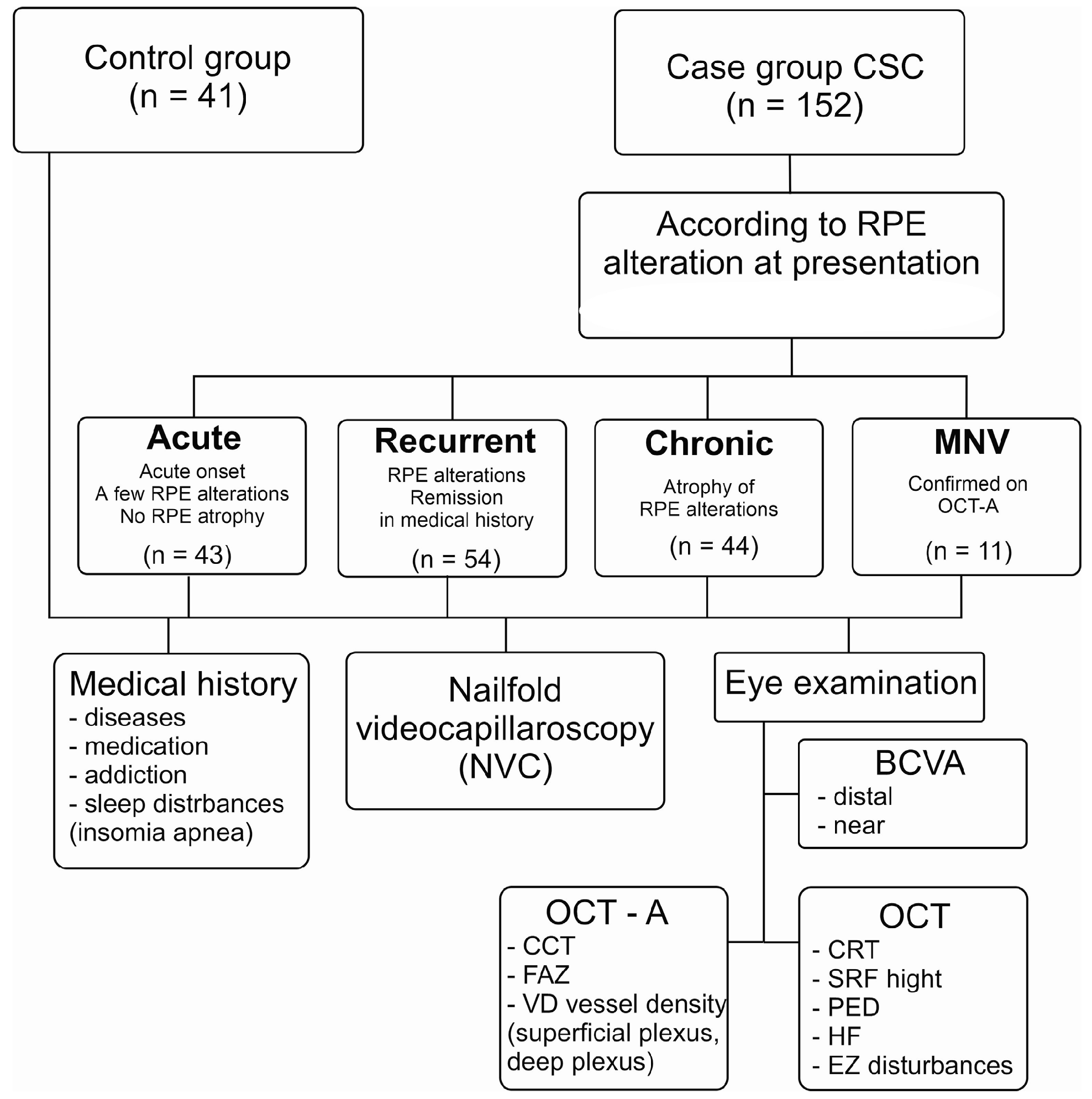
2.1. The Study Groups
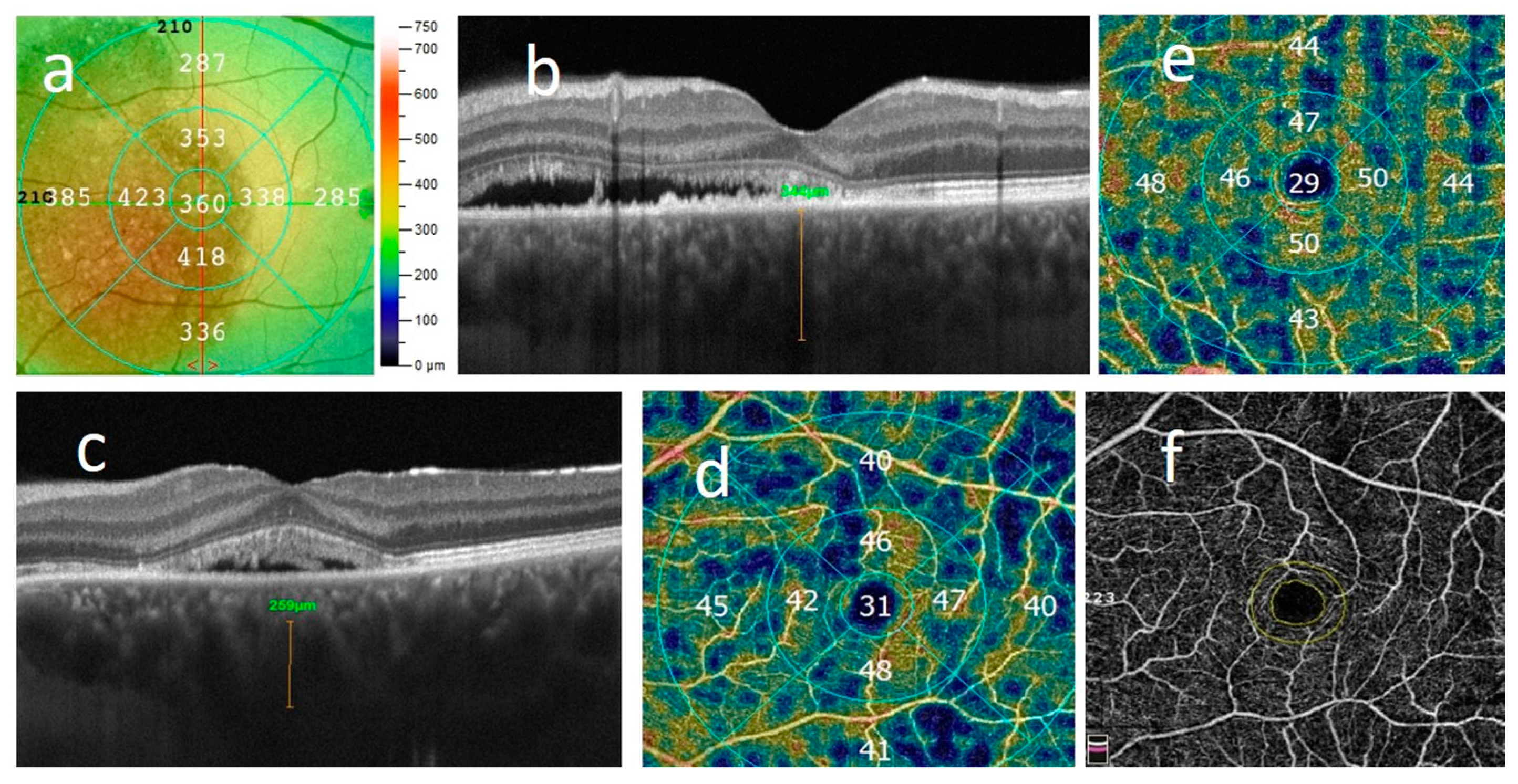
2.2. Ophthalmologic Examination
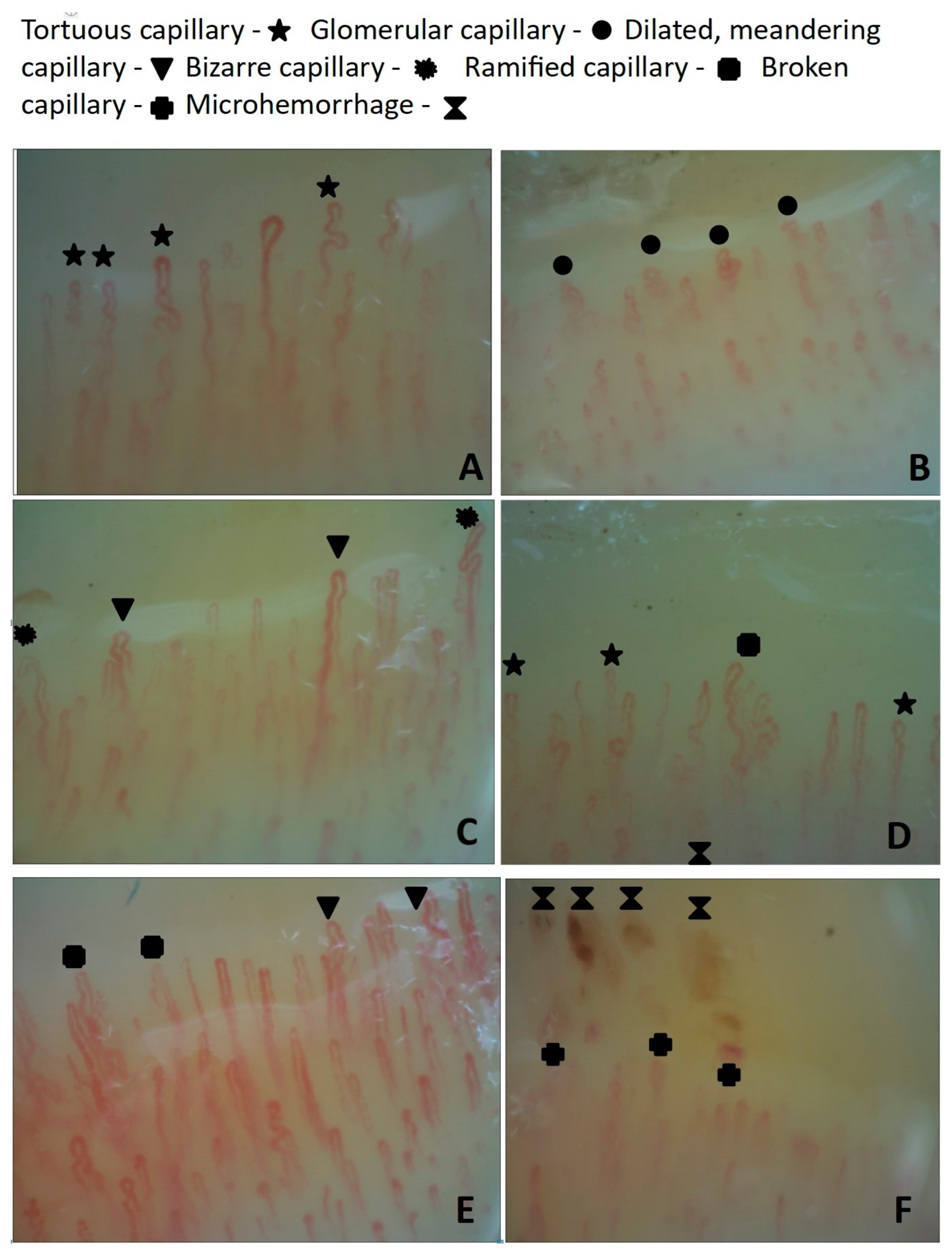
2.3. Nailfold Videocapillaroscopy in the CSC Patients and HCs
2.4. Statistical Analysis
3. Results
3.1. Primary Outcomes
3.1.1. Demographic and Clinical Data
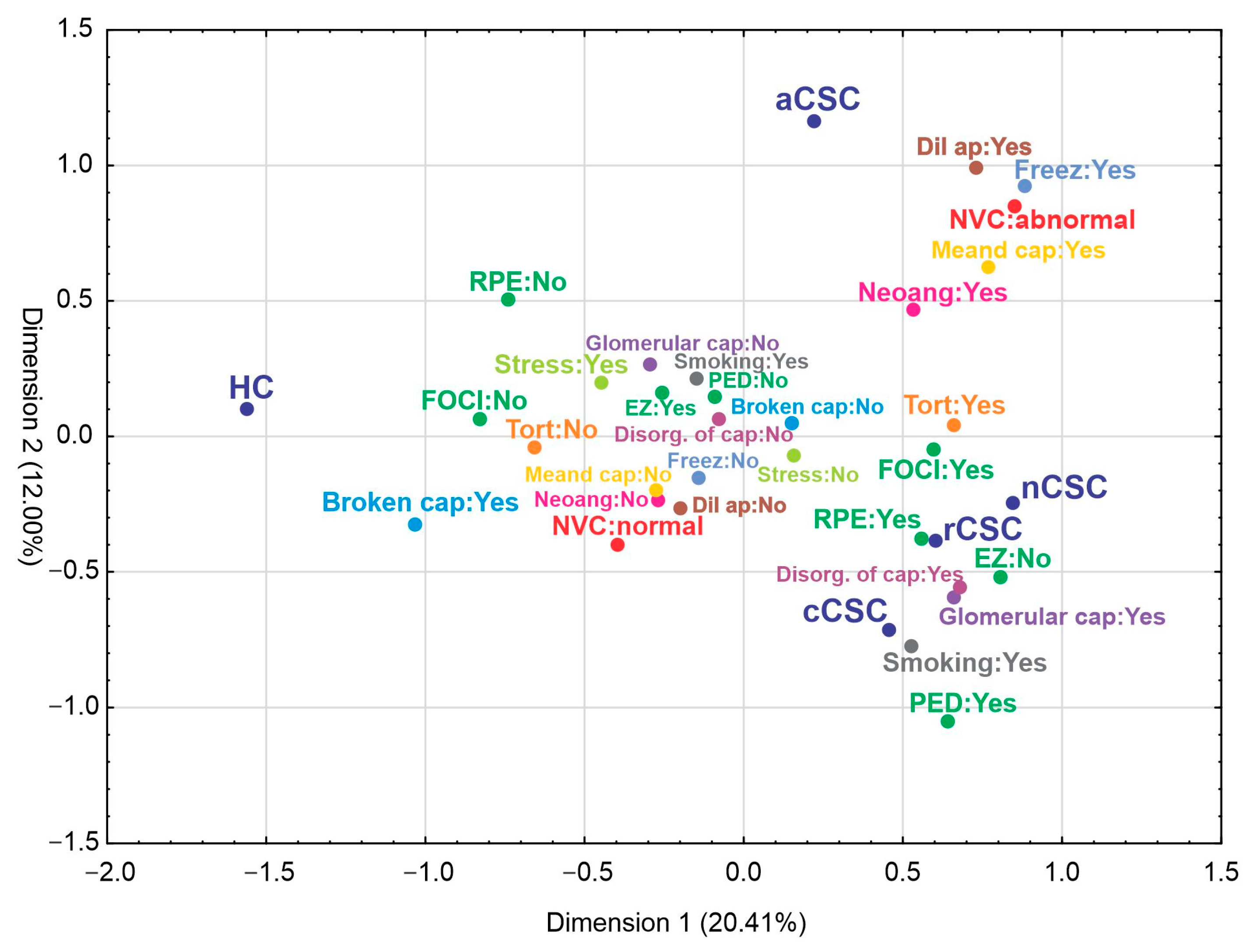
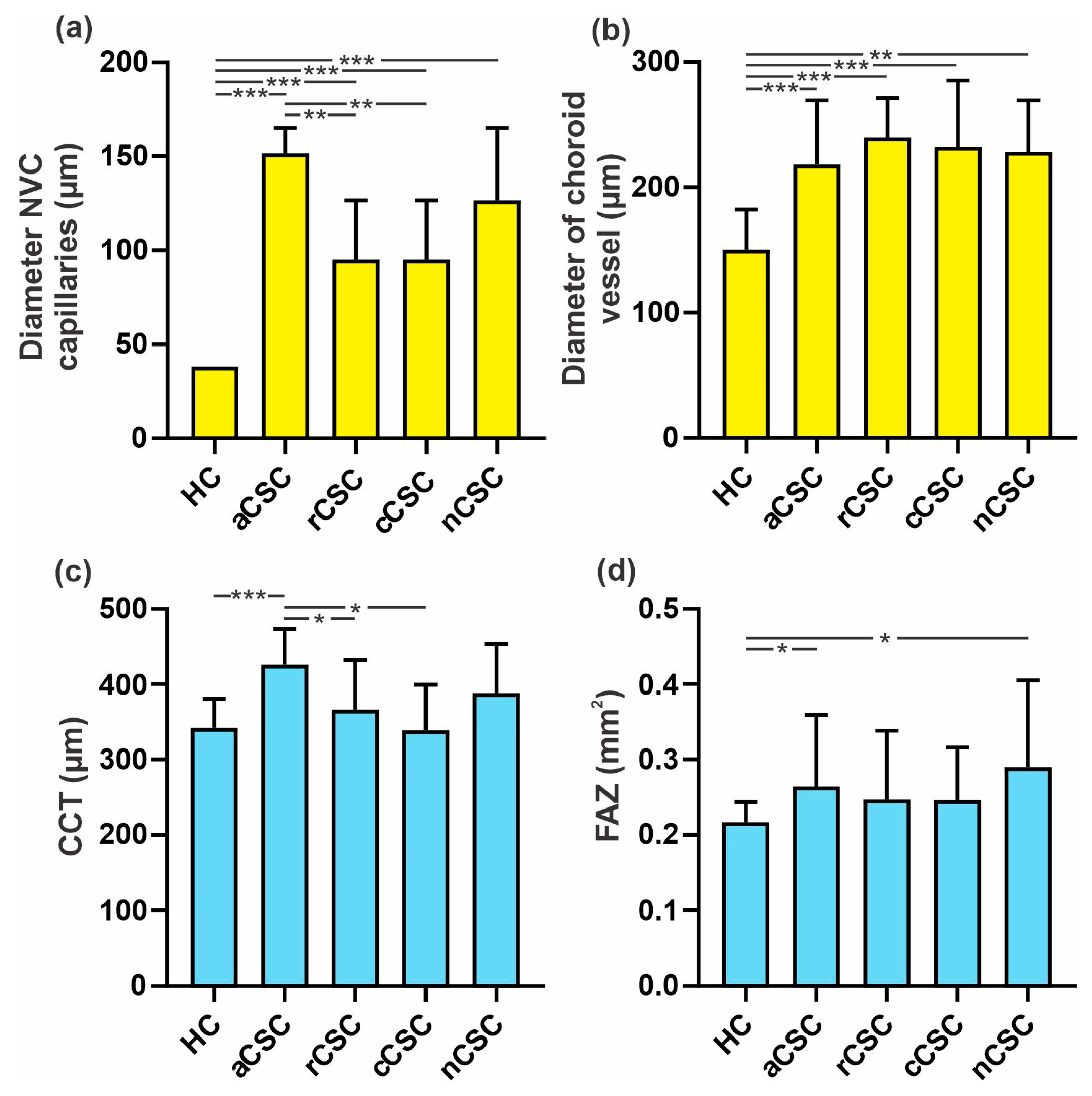
3.1.2. Comparison of NVC Parameters between Acute, Recurrent, Chronic and Neovascular CSC Patients and HCs
3.2. Secondary Outcomes
3.2.1. Correlations between NVC Results and Retinal Vessel Density (VD) of Superficial and Deep Plexus, FAZ, Central Choroid Thickness and Diameter of Choroid Anastomotic Vessel Lumen in CSC Patients
3.2.2. Correlations between NVC and Functional Results, OCT Features and General Risk Factors
3.2.3. Correlation between Retinal Vessel Density (VD) of Superficial and Deep Plexus, FAZ, Central Choroid Thickness and Diameter of Choroid Pachyvessel Lumen in CSC Patients and Functional Results, OCT Features and General Risk Factors
3.2.4. Comparison of the Diameter of Pachyvessel Lumen between Healthy Controls and CSC Groups
3.2.5. Analysis of Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaye, R.; Chandra, S.; Sheth, J.; Boon, C.J.F.; Sivaprasad, S.; Lotery, A. Central Serous Chorioretinopathy: An Update on Risk Factors, Pathophysiology and Imaging Modalities. Prog. Retin. Eye Res. 2020, 79, 100865. [Google Scholar] [CrossRef] [PubMed]
- Von Graefe, A. Ueber Centrale Recidivierende Retinitis. Graefes Arch. Clin. Exp. Ophthalmol. 1866, 12, 211–215. [Google Scholar]
- Spaide, R.F.; Gemmy Cheung, C.M.; Matsumoto, H.; Kishi, S.; Boon, C.J.F.; van Dijk, E.H.C.; Mauget-Faysse, M.; Behar-Cohen, F.; Hartnett, M.E.; Sivaprasad, S.; et al. Venous Overload Choroidopathy: A Hypothetical Framework for Central Serous Chorioretinopathy and Allied Disorders. Prog. Retin. Eye Res. 2022, 86, 100973. [Google Scholar] [CrossRef] [PubMed]
- Brinks, J.; van Dijk, E.H.C.; Meijer, O.C.; Schlingemann, R.O.; Boon, C.J.F. Choroidal Arteriovenous Anastomoses: A Hypothesis for the Pathogenesis of Central Serous Chorioretinopathy and Other Pachychoroid Disease Spectrum Abnormalities. Acta Ophthalmol. 2022, 100, 946–959. [Google Scholar] [CrossRef]
- Spaide, R.F.; Ledesma-Gil, G.; Gemmy Cheung, C.M. Intervortex Venous Anastomosis in Pachychoroid-Related Disorders. Retina 2021, 41, 997–1004. [Google Scholar] [CrossRef]
- Pang, C.E.; Shah, V.P.; Sarraf, D.; Freund, K.B. Ultra-Widefield Imaging with Autofluorescence and Indocyanine Green Angiography in Central Serous Chorioretinopathy. Am. J. Ophthalmol. 2014, 158, 362–371.e2. [Google Scholar] [CrossRef] [PubMed]
- Bacci, T.; Oh, D.J.; Singer, M.; Sadda, S.; Freund, K.B. Ultra-Widefield Indocyanine Green Angiography Reveals Patterns of Choroidal Venous Insufficiency Influencing Pachychoroid Disease. Investig. Ophthalmol. Vis. Sci. 2022, 63, 17. [Google Scholar] [CrossRef] [PubMed]
- Zarnegar, A.; Ong, J.; Matsyaraja, T.; Arora, S.; Chhablani, J. Pathomechanisms in Central Serous Chorioretinopathy: A Recent Update. Int. J. Retina Vitr. 2023, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Nicolò, M.; Rosa, R.; Musetti, D.; Musolino, M.; Saccheggiani, M.; Traverso, C.E. Choroidal Vascular Flow Area in Central Serous Chorioretinopathy Using Swept-Source Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xin, Z.; Yang, J.; Lu, H.; Wang, H.; Zhu, L. Choriocapillaris Ischemia at the Leakage Point of Patients with Acute Central Serous Chorioretinopathy. Front. Med. 2021, 8, 675876. [Google Scholar] [CrossRef]
- Spaide, R.F.; Ledesma-Gil, G. Novel Method for Image Averaging of Optical Coherence Tomography Angiography Images. Retina 2020, 40, 2099–2105. [Google Scholar] [CrossRef] [PubMed]
- Erol, M.K.; Balkarli, A.; Toslak, D.; Dogan, B.; Durmaz, D.; Süren, E.; Altun, S.; Bulut, M.; Cobankara, V. Evaluation of Nailfold Videocapillaroscopy in Central Serous Chorioretinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Latalska, M.; Bartosińska, J.; Kosior-Jarecka, E.; Krasowska, D.; Mackiewicz, J. Nailfold Videocapillaroscopy in Patients with Central Serous Chorioretinopathy and Its Relationship to Morphological and Functional Findings. J. Clin. Med. 2020, 9, 3891. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Pizzorni, C.; Secchi, M.E.; Sulli, A. Capillaroscopy. Best Pract. Res. Clin. Rheumatol. 2008, 22, 1093–1108. [Google Scholar] [CrossRef] [PubMed]
- Ingegnoli, F.; Gualtierotti, R.; Lubatti, C.; Bertolazzi, C.; Gutierrez, M.; Boracchi, P.; Fornili, M.; De Angelis, R. Nailfold Capillary Patterns in Healthy Subjects: A Real Issue in Capillaroscopy. Microvasc. Res. 2013, 90, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Kosior-Jarecka, E.; Bartosińska, J.; Łukasik, U.; Wróbel-Dudzińska, D.; Krasowska, D.; Chodorowska, G.; Żarnowski, T. Results of Nailfold Capillaroscopy in Patients with Normal-Tension Glaucoma. Curr. Eye Res. 2018, 43, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Piccolino, F.C.; Fruttini, D.; Eandi, C.; Nicolò, M.; Mariotti, C.; Tito, S.; Lupidi, M. Vigorous Physical Activity as a Risk Factor for Central Serous Chorioretinopathy. Am. J. Ophthalmol. 2022, 244, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Ngo, W.K.; Barbazetto, I.; Sorenson, J.A. Sausaging and Bulbosities of the Choroidal Veins in Central Serous Chorioretinopathy. Retina 2022, 42, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Vienola, K.V.; Lejoyeux, R.; Gofas-Salas, E.; Snyder, V.C.; Zhang, M.; Dansingani, K.K.; Sahel, J.-A.; Chhablani, J.; Rossi, E.A. Autofluorescent Hyperreflective Foci on Infrared Autofluorescence Adaptive Optics Ophthalmoscopy in Central Serous Chorioretinopathy. Am. J. Ophthalmol. Case Rep. 2022, 28, 101741. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, J.; Chung, H.; Kim, H.C. Baseline Spectral Domain Optical Coherence Tomographic Hyperreflective Foci as a Predictor of Visual Outcome and Recurrence for Central Serous Chorioretinopathy. Retina 2016, 36, 1372–1380. [Google Scholar] [CrossRef] [PubMed]

| Total | Missing | Control (a) | Acute aCSC (b) | Recurrent rCSC (c) | Chronic cCSC (d) | MNV nCSC (e) | p < 0.05 | |
|---|---|---|---|---|---|---|---|---|
| Number of patients (n, %) | 193 (100.00) | 0 | 41 (21.24) | 43 (22.28) | 54 (27.98) | 44 (22.80) | 11 (5.70) | 0.988 |
| Age | 193 (100.00) | 0 | 47.12 ± 0.76 | 46.30 ± 0.90 | 46.61 ± 0.94 | 47.50 ± 1.01 | 47.45 ± 2.08 | 0.901 |
| Sex, male | 147 (76.17) | 0 | 29 (70.73) | 35 (81.4) | 43 (79.63) | 31 (70.45) | 9 (81.82) | 0.988 |
| Residence area, urban | 118 (61.14) | 0 | 27 (65.85) | 20 (46.51) | 31 (57.41) | 31 (70.45) | 9 (81.82) | 0.613 |
| Stress, y | 50 (25.91) | 0 | 18 (43.9) | 8 (18.6) | 15 (27.78) | 6 (13.64) | 3 (27.27) | 0.020 |
| p(a-b) *; p(a-c) ns; p(a-d) **; p(a-e) ns; p(b-c) ns; p(b-d) ns; p(b-e) ns; p(c-d) ns; p(c-e) ns; p(d-e) ns | ||||||||
| Smoking history, y | 40 (20.73) | 1 (0.52) | 0 | 7 (16.28) | 15 (27.78) | 15 (34.09) | 3 (27.27) | 0.018 |
| p(a-b) **; p(a-c) ***; p(a-d) ***; p(a-e) ***; p(b-c) ns; p(b-d) ns; p(b-e) ns; p(c-d) ns; p(c-e) ns; p(d-e) ns | ||||||||
| Optical coherence tomography (OCT) variables (n, %) | ||||||||
| RPE alteration, y | 112 (58.03) | 1 (0.52) | 0 (0.00) | 14 (32.56) | 52 (98.11) | 37 (84.09) | 9 (81.82) | 0.000 |
| p(a-b) ***; p(a-c) ***; p(a-d) ***; p(a-e) ***; p(b-c) ***; p(b-d) ***; p(b-e) **; p(c-d) *; p(c-e) *; p(d-e) ns | ||||||||
| PED, y | 26 (13.47) | 1 (0.52) | 0 | 3 (6.98) | 12 (22.64) | 10 (22.73) | 1 (9.09) | 0.004 |
| p(a-b) ns; p(a-c) **; p(a-d) **; p(a-e) ns; p(b-c) *; p(b-d) *; p(b-e) ns; p(c-d) ns; p(c-e) ns; p(d-e) ns | ||||||||
| HF, y | 108 (55.96) | 10 (5.18) | 0 | 27 (65.85) | 37 (80.43) | 35 (79.55) | 9 (81.82) | 0.000 |
| p(a-b) ***; p(a-c) ***; p(a-d) ***; p(a-e) ***; p(b-c) ns; p(b-d) ns; p(b-e) ns; p(c-d) ns; p(c-e) ns; p(d-e) ns | ||||||||
| EZ disturbances, y | 48 (24.87) | 0 | 1 (2.44) | 6 (13.95) | 18 (33.33) | 17 (38.64) | 6 (54.55) | 0.004 |
| p(a-b) *; p(a-c) **; p(a-d) ***; p(a-e) ***; p(b-c) *; p(b-d) **; p(b-e) **; p(c-d) ns; p(c-e) ns; p(d-e) ns | ||||||||
| Nailfold videocapillaroscopy (NVC) parameters (n, %) | ||||||||
| NVC pattern, abnormal | 68 (35.23) | 0 | 0 | 24 (55.81) | 22 (40.74) | 15 (34.09) | 7 (63.64) | 0.000 |
| p(a-b) ***; p(a-c) ***; p(a-d) ***; p(a-e) ***; p(b-c) ns; p(b-d) *; p(b-e) ns; p(c-d) ns; p(c-e) ns; p(d-e) ns | ||||||||
| Disorganization of the capillary architecture, y | 54 (27.9) | 0 | 5 (12.20) | 19 (44.19) | 18 (33.33) | 8 (18.18) | 4 (36.36) | 0.176 |
| Hands freezing, y | 27 (13.99) | 2 (1.04) | 0 | 15 (34.88) | 8 (14.81) | 0 | 4 (36.36) | 0.000 |
| p(a-b) ***; p(a-c) **; p(a-d) ns; p(a-e) ***; p(b-c) *; p(b-d) ***; p(b-e) ns; p(c-d) **; p(c-e) ns; p(d-e) *** | ||||||||
| Dilated apical part of capillaries, y | 48 (24.87) | 0 | 0 | 18 (41.86) | 17 (31.48) | 12 (27.27) | 1 (9.09) | 0.010 |
| p(a-b) ***; p(a-c) ***; p(a-d) ***; p(a-e) ns; p(b-c) ns; p(b-d) ns; p(b-e) *; p(c-d) ns; p(c-e) ns; p(d-e) ns | ||||||||
| Dilated capillaries (diameter > 14 um), y | 81 (41.7) | 1 (0.52) | 9 (21.95) | 23 (53.49) | 28 (51.85) | 17 (38.64) | 4 (36.36) | 0.143 |
| Ramified capillaries and neoangiogenesis, y | 70 (36.27) | 2 (1.04) | 2 (4.88) | 25 (58.14) | 22 (42.31%) | 16 (36.36) | 5 (45.45) | 0.000 |
| p(a-b) ***; p(a-c) ***; p(a-d) ***; p(a-e) ***; p(b-c) ns; p(b-d) *; p(b-e) ns; p(c-d) ns; p(c-e) ns; p(d-e) ns | ||||||||
| Tortuous capillaries, y | 98 (50.78) | 0 | 3 (7.32) | 25 (58.14) | 37 (68.52) | 25 (56.82) | 8 (72.73) | 0.000 |
| p(a-b) ***; p(a-c) ***; p(a-d) ***; p(a-e) ***; p(b-c) ns; p(b-d) ns; p(b-e) ns; p(c-d) ns; p(c-e) ns; p(d-e) ns | ||||||||
| Glomerular capillaries, y | 59 (30.57) | 0 | 1 (2.44) | 8 (18.60) | 17 (31.48) | 25 (56.82) | 8 (72.73) | 0.000 |
| p(a-b) *; p(a-c) ***; p(a-d) ***; p(a-e) ***; p(b-c) ns; p(b-d) ***; p(b-e) ***; p(c-d) *; p(c-e) *; p(d-e) ns | ||||||||
| Aneurysmal dilatations, y | 18 (9.33) | 0 | 0 | 3 (6.98) | 8 (14.81) | 3 (6.82) | 4 (36.36) | 0.002 |
| p(a-b) ns; p(a-c) *; p(a-d) ns; p(a-e) ***; p(b-c) ns; p(b-d) ns; p(b-e) **; p(c-d) ns; p(c-e) ns; p(d-e) ** | ||||||||
| Broken capillaries, y | 22 (11.40) | 0 | 15 (36.59) | 0 | 3 (6.82) | 2 (3.70) | 3 (27.27) | 0.000 |
| p(a-b) ***; p(a-c) ***; p(a-d) ***; p(a-e) ns; p(b-c) ns; p(b-d) ns; p(b-e) ***; p(c-d) ns; p(c-e) *; p(d-e) ** | ||||||||
| Microhemorrhages, y | 42 (21.76) | 0 | 9 (21.95) | 10 (23.26) | 15 (27.78) | 6 (13.64) | 2 (18.18) | 0.981 |
| Megacapillaries, y | 6 (3.11) | 0 | 0 | 2 (4.65) | 0 | 2 (4.55) | 2 (18.18) | 0.285 |
| Meandering capillaries, y | 55 (28.50) | 0 | 3 (7.32) | 18 (41.86) | 22 (40.74) | 8 (18.18) | 4 (36.36) | 0.035 |
| p(a-b) ***; p(a-c) ***; p(a-d) ns; p(a-e) *; p(b-c) ns; p(b-d) *; p(b-e) ns; p(c-d) *; p(c-e) ns; p(d-e) ns | ||||||||
| Bizarre capillaries, y | 14 (7.25) | 0 | 1 (2.44) | 2 (4.65) | 9 (16.67) | 2 (4.55) | 0 | 0.414 |
| Variables | Sectors of VD | |||||||
|---|---|---|---|---|---|---|---|---|
| CSC Eye | Fellow Eye | |||||||
| Superficial Plexus | ||||||||
| Factor r (p) | Superior | Inferior | Temporal | Nasal | Superior | Inferior | Temporal | Nasal |
| BCDVA | 0.290 * (0.000) | 0.172 * (0.017) | 0.211 * (0.003) | 0.248 * (0.001) | 0.205 * (0.004) | 0.116 (0.111) | 0.164 * (0.024) | 0.188 * (0.010) |
| RPE alteration | −0.243 * (0.010) | −0.183 * (0.011) | −0.217 * (0.002) | −0.227 * (0.001) | −0.177 * (0.015) | −0.103 (0.157) | −0.113 (0.121) | −0.151 * (0.039) |
| HF | −0.133 (0.073) | −0.123 (0.097) | −0.097 (0.191) | −0.135 (0.067) | −0.122 (0.101) | −0.073 (0.328) | −0.073 (0.328) | −0.097 (0.197) |
| EZ disturbances | 0.168 * (0.019) | 0.081 (0.258) | 0.154 * (0.032) | 0.185 * (0.010) | 0.189 * (0.009) | 0.111 (0.128) | 0.090 (0.217) | 0.166 * (0.023) |
| CRT | 0.142 * (0.050) | 0.135 (0.062) | 0.157 * (0.030) | 0.189 * (0.009) | 0.144 * (0.049) | 0.127 (0.083) | 0.080 (0.277) | 0.164 * (0.026) |
| Gender | −0.124 (0.085) | −0.119 (0.097) | −0.170 * (0.018) | −0.008 (0.907) | −0.098 (0.174) | −0.062 (0.394) | −0.167 * (0.022) | −0.144 * (0.048) |
| Smoking | −0.134 (0.064) | −0.118 (0.101) | −0.101 (0.162) | −0.191 * (0.008) | −0.249 * (0.001) | −0.201 * (0.006) | −0.246 * (0.001) | −0.250 * (0.001) |
| Deep Plexus | ||||||||
| BCDVA | 0.161 * (0.027) | 0.295 * (0.000) | 0.305 * (0.000) | 0.263 * (0.000) | 0.019 (0.790) | −0.116 (0.109) | −0.015 (0.829) | −0.042 (0.562) |
| RPE alteration | −0.106 (0.148 | −0.303 * (0.000) | −0.300 * (0.000) | −0.246 * (0.001) | −0.050 (0.485) | 0.085 (0.240) | 0.067 (0.354) | 0.006 (0.932) |
| HF | −0.075 (0.318) | −0.196 * (0.009) | −0.170 * (0.023) | −0.147 * (0.050) | −0.010 (0.891) | 0.113 (0.128) | −0.045 (0.542) | 0.041 (0.574) |
| CRT | 0.973 (0.187) | 0.060 (0.418) | 0.136 (0.064) | 0.163 * (0.026) | 0.066 (0.361) | 0.062 (0.391) | 0.081 (0.265) | 0.064 (0.376) |
| EZ disturbances | −0.030 (0.678) | 0.150 * (0.040) | 0.191 * (0.008) | 0.109 (0.136) | 0.086 (0.235) | −0.038 (0.602) | 0.058 (0.423) | 0.036 (0.619) |
| Gender | −0.102 (0.163) | 0.081 (0.266) | 0.034 (0.640) | −0.088 (0.231) | −0.082 (0.254) | −0.062 (0.393) | −0.135 (0.060) | −0.181 * (0.012) |
| Smoking | −0.044 (0.548) | −0.114 (0.118) | −0.075 (0.304) | −0.139 (0.057) | −0.014 (0.847) | 0.039 (0.589) | −0.039 (0.592) | 0.007 (0.918) |
| Analysis of Risk Factors for aCSC | ||||
| Fit statistics | ||||
| Hosmer–Lemeshow test (p value) | 0.409 | |||
| R2 Cox–Snell | 0.559 | |||
| R2 Nagelkerka | 0.745 | |||
| AIC | 57.009 | |||
| Risk Factor | β | Wlad χ2 | p values | OR |
| Intercept | −9.369 | 1.261 | 0.2614 | |
| VD parafovea deep fellow eye | 0.387 | 8.575 | 0.0034 | 1.472 |
| VD parafovea deep | −0.353 | 7.384 | 0.0065 | 0.702 |
| Diameter pachyvessel | 0.037 | 11.839 | 0.0006 | 1.038 |
| Tortuous capillary; yes (1) | 4.144 | 11.742 | 0.0006 | 63.092 |
| Analysis of Risk Factors for rCSC | ||||
| Fit statistics | ||||
| Hosmer–Lemeshow test (p value) | 0.457 | |||
| R2 Cox–Snell | 0.663 | |||
| R2 Nagelkerka | 0.888 | |||
| AIC | 37.189 | |||
| Risk Factor | β | Wlad χ2 | p values | OR |
| Intercept | 32.822 | 3.640 | 0.0563 | |
| Diameter pachyvessel | 0.045 | 12.906 | 0.0003 | 1.046 |
| VD superior superficial fellow eye | −0.629 | 5.491 | 0.0191 | 0.533 |
| VD parafovea deep | −0.582 | 7.840 | 0.0051 | 0.559 |
| VD parafovea deep fellow eye | 0.455 | 5.003 | 0.0253 | 1.576 |
| Tortuous capillary; yes (1) | 2.135 | 0.681 | 0.0017 | 71.498 |
| Analysis of Risk Factors for cCSC | ||||
| Fit statistics | ||||
| Hosmer–Lemeshow test (p value) | 0.963 | |||
| R2 Cox–Snell | 0.668 | |||
| R2 Nagelkerka | 0.891 | |||
| AIC | 33.222 | |||
| Risk Factor | β | Wlad χ2 | p values | OR |
| Intercept | 27.873 | 5.481 | 0.0192 | |
| Diameter pachyvessel | 0.049 | 6.755 | 0.0093 | 1.051 |
| VD parafovea deep | −0.551 | 6.077 | 0.0137 | 0.576 |
| VD fovea deep fellow eye | −0.142 | 3.402 | 0.0650 | 0.867 |
| Glomerular capillary; yes (1) | 2.064 | 5.747 | 0.0165 | 62.100 |
| Tortuous capillary; yes (1) | 2.179 | 5.372 | 0.0204 | 78.137 |
| Analysis of Risk Factors for nCSC | ||||
| Fit statistics | ||||
| Hosmer–Lemeshow test (p value) | 0.993 | |||
| R2 Cox–Snell | 0.592 | |||
| R2 Nagelkerka | 0.919 | |||
| AIC | 13.056 | |||
| Risk Factor | β | Wlad χ2 | p values | OR |
| Intercept | 47.903 | 4.612 | 0.0317 | |
| VD nasal deep | −1.239 | 6.015 | 0.0142 | 0.2895 |
| CCT | 0.048 | 3.863 | 0.0493 | 1.0497 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latalska, M.; Bartosińska, J.; Dresler, S.; Toro, M.D.; Krasowska, D.; Rejdak, R. Comparison of Nailfold Videocapillaroscopy with Retinal and Choroidal Vascular Parameters in Patients with Central Serous Chorioretinopathy. J. Clin. Med. 2023, 12, 4817. https://doi.org/10.3390/jcm12144817
Latalska M, Bartosińska J, Dresler S, Toro MD, Krasowska D, Rejdak R. Comparison of Nailfold Videocapillaroscopy with Retinal and Choroidal Vascular Parameters in Patients with Central Serous Chorioretinopathy. Journal of Clinical Medicine. 2023; 12(14):4817. https://doi.org/10.3390/jcm12144817
Chicago/Turabian StyleLatalska, Małgorzata, Joanna Bartosińska, Sławomir Dresler, Mario Damiano Toro, Dorota Krasowska, and Robert Rejdak. 2023. "Comparison of Nailfold Videocapillaroscopy with Retinal and Choroidal Vascular Parameters in Patients with Central Serous Chorioretinopathy" Journal of Clinical Medicine 12, no. 14: 4817. https://doi.org/10.3390/jcm12144817
APA StyleLatalska, M., Bartosińska, J., Dresler, S., Toro, M. D., Krasowska, D., & Rejdak, R. (2023). Comparison of Nailfold Videocapillaroscopy with Retinal and Choroidal Vascular Parameters in Patients with Central Serous Chorioretinopathy. Journal of Clinical Medicine, 12(14), 4817. https://doi.org/10.3390/jcm12144817








