Optical Performance of a Segmented Extended-Depth-of-Focus Intraocular Lens under the Influence of Different Values of Spherical Aberration Generated by Refractive Surgery
Abstract
1. Introduction
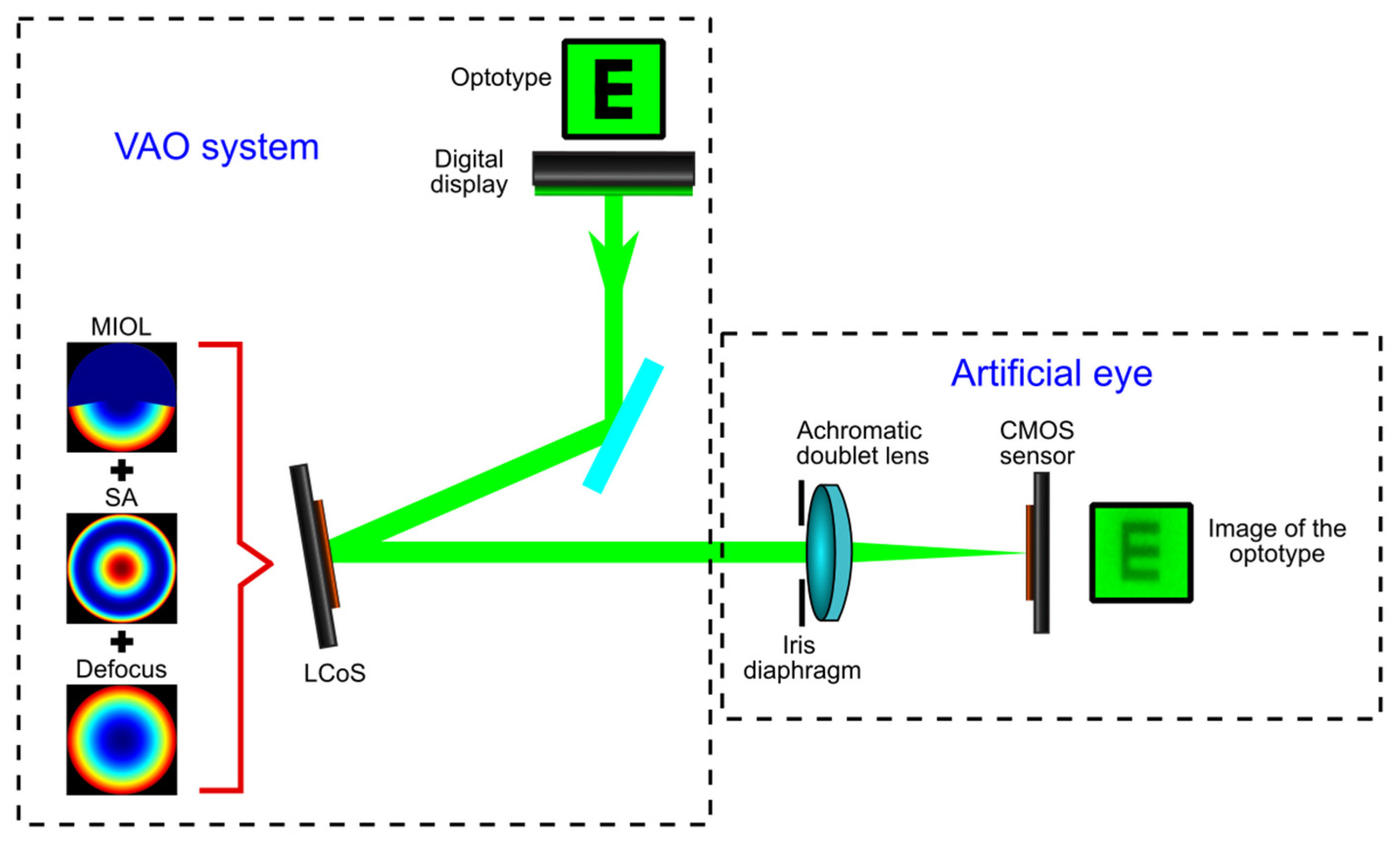
2. Materials and Methods
2.1. Multifocal Intraocular Lens Description
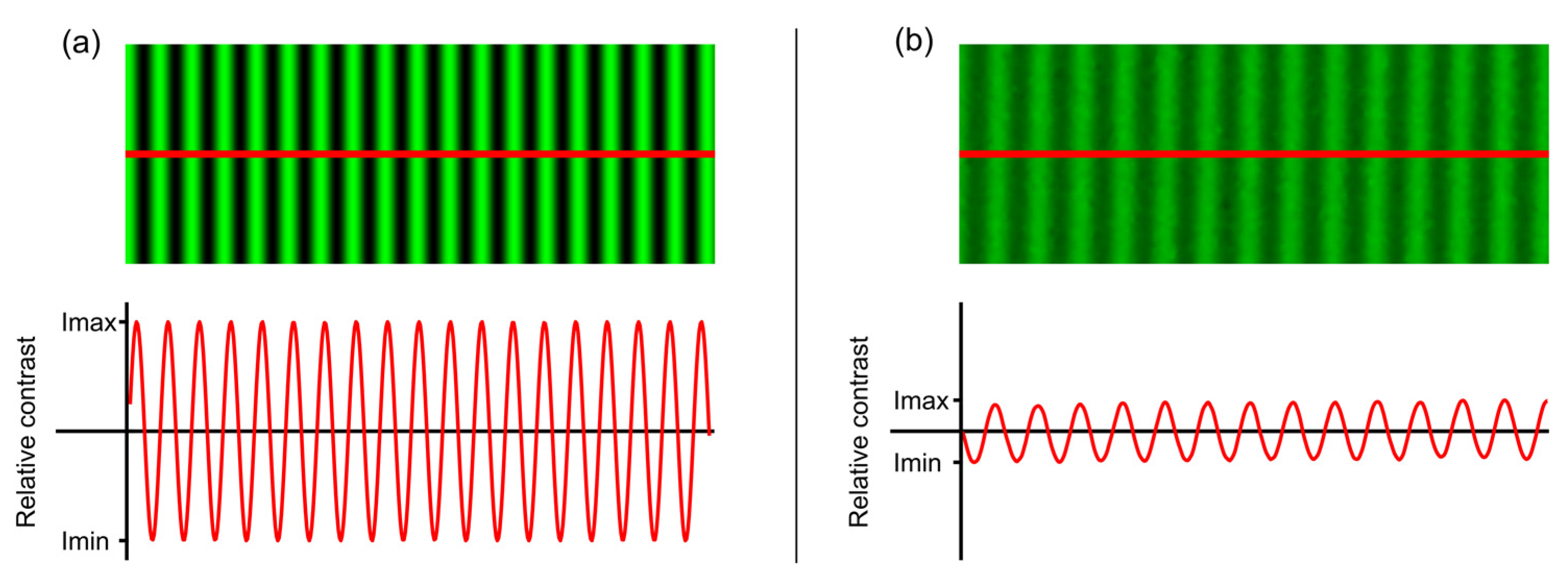
2.2. Experimental Procedure
3. Results
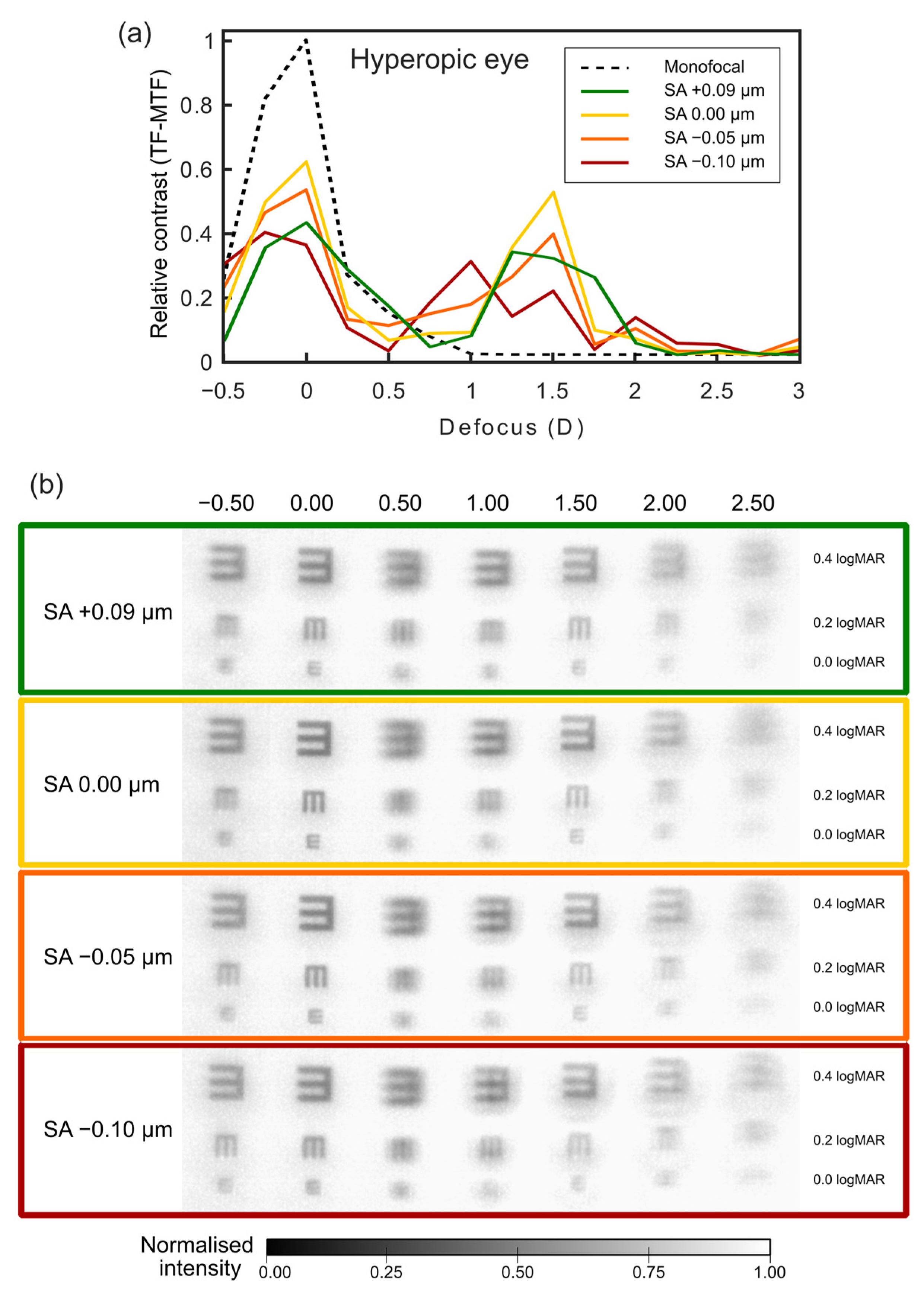
3.1. Prediction of the SA Effect on Hyperopic Eyes after Surgery
3.2. Prediction of the SA Effect on Myopic Eyes after Surgery
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moshirfar, M.; Tukan, A.N.; Bundogji, N.; Liu, H.Y.; McCabe, S.E.; Ronquillo, Y.C.; Hoopes, P.C. Ectasia after corneal refractive surgery: A systematic review. Ophthalmol. Ther. 2021, 10, 753–776. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Dou, R. Aberration Compensation between Anterior and Posterior Corneal Surfaces after Small Incision Lenticule Extraction and Femtosecond Laser-assisted Laser In-situ Keratomileusis. Ophthalmic Physiol. Opt. 2015, 35, 540–551. [Google Scholar] [CrossRef]
- Moreno-Barriuso, E.; Lloves, J.M.; Marcos, S.; Navarro, R.; Llorente, L.; Barbero, S. Ocular Aberrations before and after Myopic Corneal Refractive Surgery: LASIK-Induced Changes Measured with Laser Ray Tracing. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1396–1403. [Google Scholar]
- Kohnen, T.; Bühren, J. Corneal First-Surface Aberration Analysis of the Biomechanical Effects of Astigmatic Keratotomy and a Microkeratome Cut after Penetrating Keratoplasty. J. Cataract Refract. Surg. 2005, 31, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Bottos, K.M.; Leite, M.T.; Aventura-Isidro, M.; Bernabe-Ko, J.; Wongpitoonpiya, N.; Ong-Camara, N.H.; Purcell, T.L.; Schanzlin, D.J. Corneal Asphericity and Spherical Aberration after Refractive Surgery. J. Cataract Refract. Surg. 2011, 37, 1109–1115. [Google Scholar] [CrossRef]
- Courtin, R.; Saad, A.; Grise-Dulac, A.; Guilbert, E.; Gatinel, D. Changes to Corneal Aberrations and Vision after Monovision in Patients with Hyperopia after Using a Customized Aspheric Ablation Profile to Increase Corneal Asphericity (Q-Factor). J. Refract. Surg. 2016, 32, 734–741. [Google Scholar] [CrossRef]
- Gatinel, D.; Adam, P.-A.; Chaabouni, S.; Munck, J.; Thevenot, M.; Hoang-Xuan, T.; Pieger, S.; Fujieda, M.; Bains, H.S. Comparison of Corneal and Total Ocular Aberrations before and after Myopic LASIK. J. Refract. Surg. 2010, 26, 333–340. [Google Scholar] [CrossRef]
- Ivarsen, A.; Hjortdal, J. Seven-Year Changes in Corneal Power and Aberrations after PRK or LASIK. Investig. Opthalmol. Vis. Sci. 2012, 53, 6011. [Google Scholar] [CrossRef]
- Liu, T.; Chen, T.; Dan, T.; Shi, R.; Linghu, S.; Li, H. Four-Year Follow-up of Corneal Aberrations and Visual Functionsof Myopic Patients after Laser in Situ Keratomileusis. Pak. J. Med. Sci. 2015, 31, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wei, H.; Jhanji, V.; Sun, L.; Li, J.; Jiang, J.; Zhuang, S.; Zhang, M. Comparison of Corneal Aberrations and Refractive Outcomes after Small-Incision Lenticule Extraction and Femtosecond-Assisted Laser-Assisted In Situ Keratomileusis. Int. Ophthalmol. 2021, 41, 2521–2531. [Google Scholar] [CrossRef]
- Piao, J.; Whang, W.; Joo, C. Comparison of Visual Outcomes after Femtosecond Laser-Assisted LASIK versus Flap-off Epipolis LASIK for Myopia. BMC Ophthalmol. 2020, 20, 310. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, S.; Wang, G.; Zhao, S.; Wei, R.; Huang, Y. Corneal Asphericity and Higher-Order Aberrations after FS-LASIK and Trans-PRK for Myopia. J. Ophthalmol. 2021, 2021, 3765046. [Google Scholar] [CrossRef] [PubMed]
- Al-Zeraid, F.M.; Osuagwu, U.L. Induced higher-order aberrations after laser in situ keratomileusis (LASIK) performed with wavefront-guided IntraLase femtosecond laser in moderate to high astigmatism. BMC Ophthalmol. 2016, 16, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Camps, V.J.; Miret, J.J.; García, C.; Tolosa, A.; Piñero, D.P. Simulation of the Effect of Different Presbyopia-Correcting Intraocular Lenses with Eyes with Previous Laser Refractive Surgery. J. Refract. Surg. 2018, 34, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Charman, W.N. Developments in the correction of presbyopia II: Surgical approaches. Ophthalmic Physiol. Opt. 2014, 34, 397–426. [Google Scholar] [CrossRef] [PubMed]
- Gatinel, D.; Houbrechts, Y. Comparison of Bifocal and Trifocal Diffractive and Refractive Intraocular Lenses Using an Optical Bench. J. Cataract Refract. Surg. 2013, 39, 1093–1099. [Google Scholar] [CrossRef]
- Rampat, R.; Gatinel, D. Multifocal and Extended Depth-of-Focus Intraocular Lenses in 2020. Ophthalmology 2021, 128, e164–e185. [Google Scholar] [CrossRef]
- MacRae, S.; Holladay, J.T.; Glasser, A.; Calogero, D.; Hilmantel, G.; Masket, S.; Stark, W.; Tarver, M.E.; Nguyen, T.; Eydelman, M. Special Report: American Academy of Ophthalmology Task Force Consensus Statement for Extended Depth of Focus Intraocular Lenses. Ophthalmology 2017, 124, 139–141. [Google Scholar] [CrossRef]
- Wang, L.; Koch, D.D. Intraocular Lens Power Calculations in Eyes with Previous Corneal Refractive Surgery. Ophthalmology 2021, 128, e121–e131. [Google Scholar] [CrossRef]
- Darian-Smith, E.; Versace, P. Visual Performance and Positional Stability of a Capsulorhexis-Fixated Extended Depth-of-Focus Intraocular Lens. J. Cataract Refract. Surg. 2020, 46, 179–187. [Google Scholar] [CrossRef]
- Liu, X.; Xie, L.; Huang, Y. Effects of Decentration and Tilt at Different Orientations on the Optical Performance of a Rotationally Asymmetric Multifocal Intraocular Lens. J. Cataract Refract. Surg. 2019, 45, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Wang, Y.-C.; Zhao, T.-Y.; Wang, Z.-Z.; Wang, W. Tilt and Decentration with Various Intraocular Lenses: A Narrative Review. World J. Clin. Cases 2022, 10, 3639–3646. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, J.W.; Vrijman, V.; El-Saady, R.; van der Meulen, I.J.; Mourits, M.P.; Lapid-Gortzak, R. Autorefraction versus Subjective Refraction in a Radially Asymmetric Multifocal Intraocular Lens. Acta Ophthalmol. 2014, 92, 764–768. [Google Scholar] [CrossRef]
- Martínez-Espert, A.; Montagud-Martínez, D.; Ferrando, V.; Furlan, W.D.; Monsoriu, J.A. Assessment of a New Trifocal Diffractive Corneal Inlay for Presbyopia Correction Using an Adaptive Optics Visual Simulator. Photonics 2022, 9, 135. [Google Scholar] [CrossRef]
- García, S.; Salvá, L.; García-Delpech, S.; Martínez-Espert, A.; Ferrando, V.; Montagud-Martínez, D. Polychromatic Assessment of a Refractive Segmented EDOF Intraocular Lens. J. Clin. Med. 2022, 11, 1480. [Google Scholar] [CrossRef]
- Shajari, M.; Sonntag, R.; Niermann, T.; Holland, D.; Kohnen, T.; Priglinger, S.; Mayer, W.J. Determining and Comparing the Effective Lens Position and Refractive Outcome of a Novel Rhexis-Fixated Lens to Established Lens Designs. Am. J. Ophthalmol. 2020, 213, 62–68. [Google Scholar] [CrossRef] [PubMed]
- VAO. Visual Adaptive Optics Simulator. Available online: https://voptica.com/vao/ (accessed on 12 June 2023).
- Furlan, W.D.; Montagud-Martínez, D.; Ferrando, V.; García-Delpech, S.; Monsoriu, J.A. A new trifocal corneal inlay for presbyopia. Sci. Rep. 2021, 11, 6620. [Google Scholar] [CrossRef]
- Furlan, W.D.; Martínez-Espert, A.; Montagud-Martínez, D.; Ferrando, V.; García-Delpech, S.; Monsoriu, J.A. Optical performance of a new design of a trifocal intraocular lens based on the Devil’s diffractive lens. Biomed. Opt. Express 2023, 14, 2365–2374. [Google Scholar] [CrossRef]
- Piers, P.A.; Fernandez, E.J.; Manzanera, S.; Norrby, S.; Artal, P. Adaptive optics simulation of intraocular lenses with modified spherical aberration. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4601–4610. [Google Scholar] [CrossRef]
- Fernández, E.J.; Manzanera, S.; Piers, P.; Artal, P. Adaptive optics visual simulator. J. Refract. Surg. 2002, 18, S634–S638. [Google Scholar] [CrossRef]
- Tabernero, J.; Otero, C.; Pardhan, S.A. Comparison between refraction from an adaptive optics visual simulator and clinical refractions. Transl. Vis. Sci. Technol. 2020, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.M.; Fernández, E.J.; Manzanera, S.; Artal, P. Adaptive optics with a programmable phase modulator: Applications in the human eye. Opt. Express 2004, 12, 4059–4071. [Google Scholar] [CrossRef] [PubMed]
- Manzanera, S.; Prieto, P.M.; Ayala, D.B.; Lindacher, J.M.; Artal, P. Liquid crystal adaptive optics visual simulator: Application to testing and design of ophthalmic optical elements. Opt. Express 2007, 15, 16177–16188. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dai, E.; Koch, D.D.; Nathoo, A. Optical Aberrations of the Human Anterior Cornea. J. Cataract Refract. Surg. 2003, 29, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Schwiegerling, J. Scaling Zernike Expansion Coefficients to Different Pupil Sizes. J. Opt. Soc. Am. A 2002, 19, 1937. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, A.; Remón, L.; Martos, J.; Furlan, W.D.; Monsoriu, J.A. Imaging Quality of Multifocal Intraocular Lenses: Automated Assessment Setup. Ophthalmic Physiol. Opt. 2013, 33, 420–426. [Google Scholar] [CrossRef]
- Rodríguez-Vallejo, M.; Burguera, N.; Rocha-de-Lossada, C.; Aramberri, J.; Fernández, J. Refraction and Defocus Curves in Eyes with Monofocal and Multifocal Intraocular Lenses. J. Optom. 2023, 16, 236–243. [Google Scholar] [CrossRef]
- Hong, X.; Choi, M. Influence of Ocular Longitudinal Chromatic Aberration on the Selection of Aspheric Intraocular Lenses. Opt. Express 2010, 18, 26175. [Google Scholar] [CrossRef]
- Montagud-Martínez, D.; Ferrando, V.; Martínez-Espert, A.; Garcia-Delpech, S.; Monsoriu, J.A.; Furlan, W.D. In vitro chromatic performance of three presbyopia-correcting intraocular lenses with different optical designs. J. Clin. Med. 2022, 11, 1212. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Dorronsoro, C.; Marcos, S. Differences in visual quality with orientation of a rotationally asymmetric bifocal intraocular lens design. J. Cataract Refract. Surg. 2016, 42, 1276–1287. [Google Scholar] [CrossRef]
- Marcos, S.; Rosales, P.; Llorente, L.; Barbero, S.; Jiménez-Alfaro, I. Balance of corneal horizontal coma by internal optics in eyes with intraocular artificial lenses: Evidence of a passive mechanism. Vis. Res. 2008, 48, 70–77. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvá, L.; García, S.; García-Delpech, S.; Martínez-Espert, A.; Ferrando, V. Optical Performance of a Segmented Extended-Depth-of-Focus Intraocular Lens under the Influence of Different Values of Spherical Aberration Generated by Refractive Surgery. J. Clin. Med. 2023, 12, 4758. https://doi.org/10.3390/jcm12144758
Salvá L, García S, García-Delpech S, Martínez-Espert A, Ferrando V. Optical Performance of a Segmented Extended-Depth-of-Focus Intraocular Lens under the Influence of Different Values of Spherical Aberration Generated by Refractive Surgery. Journal of Clinical Medicine. 2023; 12(14):4758. https://doi.org/10.3390/jcm12144758
Chicago/Turabian StyleSalvá, Luís, Scott García, Salvador García-Delpech, Anabel Martínez-Espert, and Vicente Ferrando. 2023. "Optical Performance of a Segmented Extended-Depth-of-Focus Intraocular Lens under the Influence of Different Values of Spherical Aberration Generated by Refractive Surgery" Journal of Clinical Medicine 12, no. 14: 4758. https://doi.org/10.3390/jcm12144758
APA StyleSalvá, L., García, S., García-Delpech, S., Martínez-Espert, A., & Ferrando, V. (2023). Optical Performance of a Segmented Extended-Depth-of-Focus Intraocular Lens under the Influence of Different Values of Spherical Aberration Generated by Refractive Surgery. Journal of Clinical Medicine, 12(14), 4758. https://doi.org/10.3390/jcm12144758





