Effects of Proton Pump Inhibitors on Patient Survival in Patients Undergoing Maintenance Hemodialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Population
2.2. Study’s Variables
2.3. Statistical Analyses
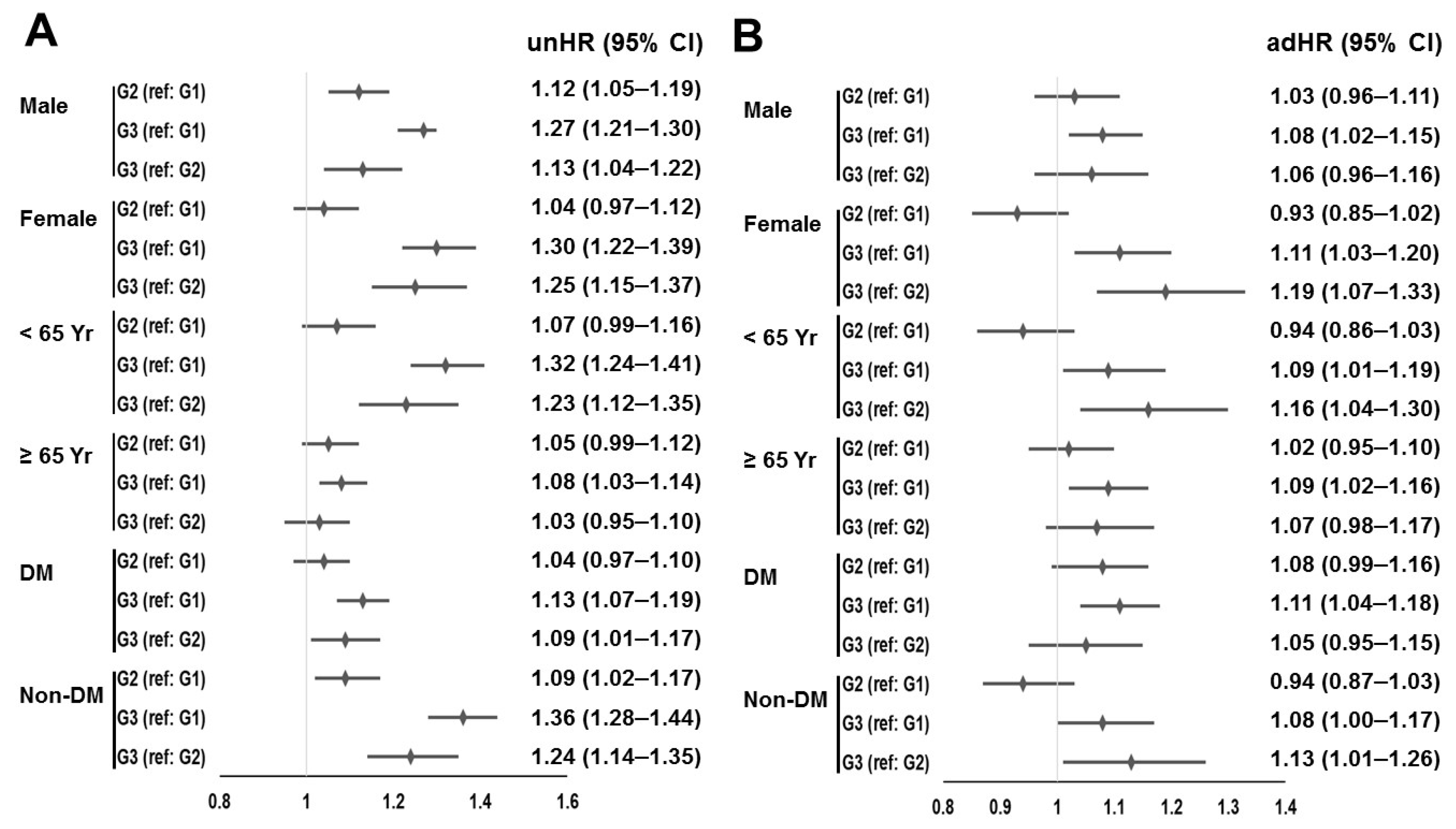
3. Results
3.1. Participants
3.2. Survival
3.3. Analyses That Used Propensity Score Weights
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- US Renal Data System, USRDS 2020 Annual Data Report: Atlas of Chronic Kidney Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. 2020. Available online: https://adr.usrds.org/2020 (accessed on 1 May 2023).
- ESRD Registry Committee: Korean Society of Nephrology. Current Renal Replacement Therapy in Korea. 2022. Available online: https://ksn.or.kr/bbs/index.php?code=report (accessed on 1 May 2023).
- Levey, A.S.; Eknoyan, G. Cardiovascular disease in chronic renal disease. Nephrol. Dial. Transpl. 1994, 14, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Battistella, M.; Jandoc, R.; Ng, J.Y.; McArthur, E.; Garg, A.X. A province-wide, cross-sectional study of demographics and medication use of patients in hemodialysis units across Ontario. Can. J. Kidney Health Dis. 2018, 5, 2054358118760832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, D.; Zhang, R.; Yi, J.; Dong, J.; Sha, L. Relationship between Proton Pump Inhibitors and Adverse Effects in Hemodialysis Patients: A Systematic Review and Meta-Analysis. Kidney Blood Press. Res. 2022, 47, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Kosedo, I.; Tokushige, A.; Takumi, T.; Yoshikawa, A.; Teraguchi, K.; Takenouchi, K.; Shiraishi, K.; Ikeda, D.; Imamura, M.; Sonoda, T.; et al. Use of proton pump inhibitors is associated with an increase in adverse cardiovascular events in patients with hemodialysis: Insight from the kids registry. Eur. J. Intern. Med. 2020, 72, 79–87. [Google Scholar] [CrossRef]
- Vangala, C.; Niu, J.; Lenihan, C.R.; Mitch, W.E.; Navaneethan, S.D.; Winkelmayer, W.C. Proton pump inhibitors, histamine-2 receptor antagonists, and hip fracture risk among patients on hemodialysis. Clin. J. Am. Soc. Nephrol. 2018, 13, 1534–1541. [Google Scholar] [CrossRef]
- Savarino, V.; Dulbecco, P.; De Bortoli, N.; Ottonello, A.; Savarino, E. The appropriate use of proton pump inhibitors (PPIs): Need for a reappraisal. Eur. J. Intern. Med. 2017, 37, 19–24. [Google Scholar] [CrossRef]
- Makunts, T.; Alpatty, S.; Lee, K.C.; Atayee, R.S.; Abagyan, R. Proton-pump inhibitor use is associated with a broad spectrum of neurological adverse events including impaired hearing, vision, and memory. Sci. Rep. 2019, 9, 17280. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Milic, S.; Stimac, D.; Zaputovic, L.; Lukenda Zanko, V.; Gulin, T.; Jakopcic, I.; Klaric, D.; Gulin, M.; Orlic, L. Is there a relationship between hypomagnesemia and proton-pump inhibitors in patients on chronic hemodialysis? Eur. J. Intern. Med. 2016, 30, 99–103. [Google Scholar] [CrossRef]
- Alhosaini, M.; Walter, J.S.; Singh, S.; Dieter, R.S.; Hsieh, A.; Leehey, D.J. Hypomagnesemia in hemodialysis patients: Role of proton pump inhibitors. Am. J. Nephrol. 2014, 39, 204–209. [Google Scholar] [CrossRef]
- Misra, P.S.; Alam, A.; Lipman, M.L.; Nessim, S.J. The relationship between proton pump inhibitor use and serum magnesium concentration among hemodialysis patients: A cross-sectional study. BMC Nephrol. 2015, 16, 136. [Google Scholar] [CrossRef]
- Ago, R.; Shindo, T.; Banshodani, M.; Shintaku, S.; Moriishi, M.; Masaki, T.; Kawanishi, H. Hypomagnesemia as a predictor of mortality in hemodialysis patients and the role of proton pump inhibitors: A cross-sectional, 1-year, retrospective cohort study. Hemodial. Int. 2016, 20, 580–588. [Google Scholar] [CrossRef]
- De Francisco, A.L.M.; Varas, J.; Ramos, R.; Merello, J.I.; Canaud, B.; Stuard, S.; Pascual, J.; Aljama, P.; Optimizing Results in Dialysis (ORD) Group. Optimizing Results in Dialysis (ORD) group. Proton Pump Inhibitor Usage and the Risk of Mortality in Hemodialysis Patients. Kidney Int. Rep. 2017, 3, 374–384. [Google Scholar] [CrossRef]
- Kim, H.W.; Jhee, J.H.; Joo, Y.S.; Yang, K.H.; Jung, J.J.; Shin, J.H.; Han, S.H.; Yoo, T.H.; Kang, S.W.; Park, J.T. Clinical significance of hemodialysis quality of care indicators in very elderly patients with end stage kidney disease. J. Nephrol. 2022, 35, 2351–2361. [Google Scholar] [CrossRef]
- Health Insurance Review & Assessment Service. 6th Hemodialysis Quality Assessment Program. Available online: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020002000100&brdScnBltNo=4&brdBltNo=6619#none (accessed on 1 May 2023).
- Daugirdas, J.T. Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J. Am. Soc. Nephrol. 1993, 4, 1205–1213. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Abraham, N.S.; Hlatky, M.A.; Antman, E.M.; Bhatt, D.L.; Bjorkman, D.J.; Clark, C.B.; Furberg, C.D.; Johnson, D.A.; Kahi, C.J.; Laine, L.; et al. ACCF/ACG/AHA 2010 Expert Consensus Document on the concomitant use of proton pump inhibitors and thienopyridines: A focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation 2010, 122, 2619–2633. [Google Scholar]
- Desbuissons, G.; Mercadal, L. Use of proton pump inhibitors in dialysis patients: A double-edged sword? J. Nephrol. 2021, 34, 661–672. [Google Scholar] [CrossRef]
- Scarpignato, C.; Gatta, L.; Zullo, A.; Blandizzi, C.; SIF-AIGO-FIMMG Group; Italian Society of Pharmacology; the Italian Association of Hospital Gastroenterologists; the Italian Federation of General Practitioners. Effective and safe proton pump inhibitor therapy in acid-related diseases—A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016, 14, 179. [Google Scholar] [CrossRef]
- Nitta, K.; Goto, G.; Masakane, I.; Taniguchi, M.; Hasegawa, T.; Nakai, S.; Wada, A.; Hamano, T.; Hoshino, J.; Joki, N.; et al. Annual dialysis data report for 2018, JSDT Renal Data Registry: Survey method, facility data, incidence, prevalence, and mortality. Ren. Replace. Ther. 2020, 60, 41. [Google Scholar] [CrossRef]
- Drüeke, T.B.; Parfrey, P.S. Summary of the KDIGO guideline on anemia and comment: Reading between the guidelines. Kidney Int. 2012, 82, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Targownik, L.E.; Fisher, D.A.; Saini, S.D. AGA Clinical Practice Update on De-Prescribing of Proton Pump Inhibitors: Expert Review. Gastroenterology 2022, 162, 1334–1342. [Google Scholar] [CrossRef] [PubMed]

| Group 1 (n = 43,059) | Group 2 (n = 5065) | Group 3 (n = 6779) | p-Value | |
|---|---|---|---|---|
| Age (years) | 59.8 ± 13.1 | 60.2 ± 12.6 | 62.6 ± 12.2 *# | <0.001 |
| Gender (male sex, %) | 26,084 (60.6%) | 2768 (54.6%) | 3932 (58.0%) | <0.001 |
| Hemodialysis vintage (days) | 1593 ± 1714 | 1562 ± 1760 | 1459 ± 1585 *# | <0.001 |
| Underlying cause of ESRD | <0.001 | |||
| Diabetes mellitus | 18,463 (42.9%) | 2257 (44.6%) | 3431 (50.6%) | |
| Hypertension | 11,510 (26.7%) | 1316 (26.0%) | 1583 (23.4%) | |
| Glomerulonephritis | 4657 (10.8%) | 504 (10.0%) | 643 (9.5%) | |
| Others | 3653 (8.5%) | 442 (8.7%) | 486 (7.2%) | |
| Unknown | 4776 (11.1%) | 546 (10.8%) | 636 (9.4%) | |
| CCI score | 7.3 ± 2.9 | 8.0 ± 2.8 * | 8.6 ± 2.8 *# | <0.001 |
| Follow-up duration (days) | 1883 ± 874 | 1813 ± 849 * | 1713 ± 819 *# | <0.001 |
| Vascular access | <0.001 | |||
| Autologous arteriovenous fistula | 36,872 (85.6%) | 4261 (84.1%) | 5676 (83.7%) | |
| Arteriovenous graft | 6187 (14.4%) | 804 (15.9%) | 1103 (16.3%) | |
| Kt/Vurea | 1.53 ± 0.27 | 1.53 ± 0.28 | 1.54 ± 0.27 * | 0.002 |
| UFV (L/session) | 2.29 ± 0.96 | 2.24 ± 0.93 * | 2.20 ± 0.94 *# | <0.001 |
| Hemoglobin (g/dL) | 10.7 ± 0.8 | 10.6 ± 0.8 * | 10.6 ± 0.8 * | <0.001 |
| Serum albumin (g/dL) | 4.00 ± 0.34 | 3.95 ± 0.35 * | 3.93 ± 0.35 *# | <0.001 |
| Serum phosphorus (mg/dL) | 5.0 ± 1.4 | 4.9 ± 1.4 * | 4.6 ± 1.3 *# | <0.001 |
| Serum calcium (mg/dL) | 8.9 ± 0.8 | 8.9 ± 0.8 * | 8.8 ± 0.8 *# | <0.001 |
| Systolic blood pressure (mmHg) | 141 ± 16 | 142 ± 16 * | 141 ± 16 | 0.027 |
| Diastolic blood pressure (mmHg) | 78 ± 9 | 78 ± 10 | 77 ± 10 *# | <0.001 |
| SCr (mg/dL) | 9.6 ± 2.7 | 9.3 ± 2.7 * | 9.1 ± 2.7 *# | <0.001 |
| Use of antihypertensive drug | 28,235 (65.6%) | 3956 (78.1%) | 5456 (80.5%) | <0.001 |
| Use of aspirin | 17,711 (41.1%) | 2464 (48.6%) | 3431 (50.6%) | <0.001 |
| Use of statin | 11,637 (27.0%) | 1826 (36.1%) | 2782 (41.0%) | <0.001 |
| Univariate | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Group | ||||
| Ref: Group 1 | ||||
| Group 2 | 1.07 (1.03–1.13) | 0.003 | 0.99 (0.94–1.05) | 0.846 |
| Group 3 | 1.28 (1.23–1.33) | <0.001 | 1.09 (1.04–1.15) | <0.001 |
| Ref: Group 2 | ||||
| Group 3 | 1.19 (1.12–1.26) | <0.001 | 1.10 (1.03–1.18) | 0.007 |
| Underlying disease of ESRD (Ref: diabetes mellitus) | 0.81 (0.80–0.82) | <0.001 | 0.90 (0.88–0.91) | <0.001 |
| Age (1-year increase) | 1.06 (1.06–1.06) | <0.001 | 1.06 (1.06–1.06) | <0.001 |
| Vascular access type (ref: AVF) | 1.51 (1.46–1.56) | <0.001 | 1.19 (1.14–1.24) | <0.001 |
| CCI score (1-score increase) | 1.14 (1.13–1.14) | <0.001 | 1.07 (1.06–1.08) | <0.001 |
| Gender (Ref: male sex) | 0.87 (0.84–0.89) | <0.001 | 0.97 (0.71–0.77) | <0.001 |
| Hemodialysis vintage (1-day increase) | 1.00 (1.00–1.00) | 0.183 | 1.00 (1.00–1.01) | <0.001 |
| UFV (1 kg/session increase) | 0.92 (0.90–0.93) | <0.001 | 1.07 (1.05–1.09) | <0.001 |
| KtVurea (1-unit increase) | 0.91 (0.87–0.97) | 0.001 | 0.82 (0.76–0.88) | <0.001 |
| Hemoglobin (1 g/dL increase) | 0.86 (0.85–0.88) | <0.001 | 0.90 (0.88–0.92) | <0.001 |
| Serum albumin (1 g/dL increase) | 0.37 (0.36–0.39) | <0.001 | 0.63 (0.59–0.66) | <0.001 |
| SCr (1 mg/dL increase) | 0.87 (0.86–0.87) | <0.001 | 0.94 (0.93–0.94) | <0.001 |
| Serum phosphorus (1 mg/dL increase) | 0.85 (0.84–0.86) | <0.001 | 1.04 (1.03–1.06) | <0.001 |
| Serum calcium (1 mg/dL increase) | 0.94 (0.92–0.95) | <0.001 | 1.07 (1.04–1.09) | <0.001 |
| Systolic blood pressure (1 mmHg increase) | 1.01 (1.01–1.01) | <0.001 | 1.01 (1.00–1.01) | <0.001 |
| Diastolic blood pressure (1 mmHg increase) | 0.98 (0.98–0.99) | <0.001 | 1.00 (1.00–1.01) | 0.018 |
| Use of anti-hypertensive drug | 1.12 (1.09–1.16) | <0.001 | 0.95 (0.91–0.98) | 0.006 |
| Use of aspirin | 1.16 (1.13–1.19) | <0.001 | 0.97 (0.94–1.01) | 0.106 |
| Use of statin | 1.10 (1.07–1.14) | <0.001 | 0.96 (0.92–0.99) | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.H.; Kim, G.O.; Kim, B.Y.; Son, E.J.; Do, J.Y. Effects of Proton Pump Inhibitors on Patient Survival in Patients Undergoing Maintenance Hemodialysis. J. Clin. Med. 2023, 12, 4749. https://doi.org/10.3390/jcm12144749
Kang SH, Kim GO, Kim BY, Son EJ, Do JY. Effects of Proton Pump Inhibitors on Patient Survival in Patients Undergoing Maintenance Hemodialysis. Journal of Clinical Medicine. 2023; 12(14):4749. https://doi.org/10.3390/jcm12144749
Chicago/Turabian StyleKang, Seok Hui, Gui Ok Kim, Bo Yeon Kim, Eun Jung Son, and Jun Young Do. 2023. "Effects of Proton Pump Inhibitors on Patient Survival in Patients Undergoing Maintenance Hemodialysis" Journal of Clinical Medicine 12, no. 14: 4749. https://doi.org/10.3390/jcm12144749
APA StyleKang, S. H., Kim, G. O., Kim, B. Y., Son, E. J., & Do, J. Y. (2023). Effects of Proton Pump Inhibitors on Patient Survival in Patients Undergoing Maintenance Hemodialysis. Journal of Clinical Medicine, 12(14), 4749. https://doi.org/10.3390/jcm12144749






