Fulminant Liver Failure after Treatment with a Checkpoint Inhibitor for Gastric Cancer: A Case Report and Review of the Literature
Abstract
1. Introduction
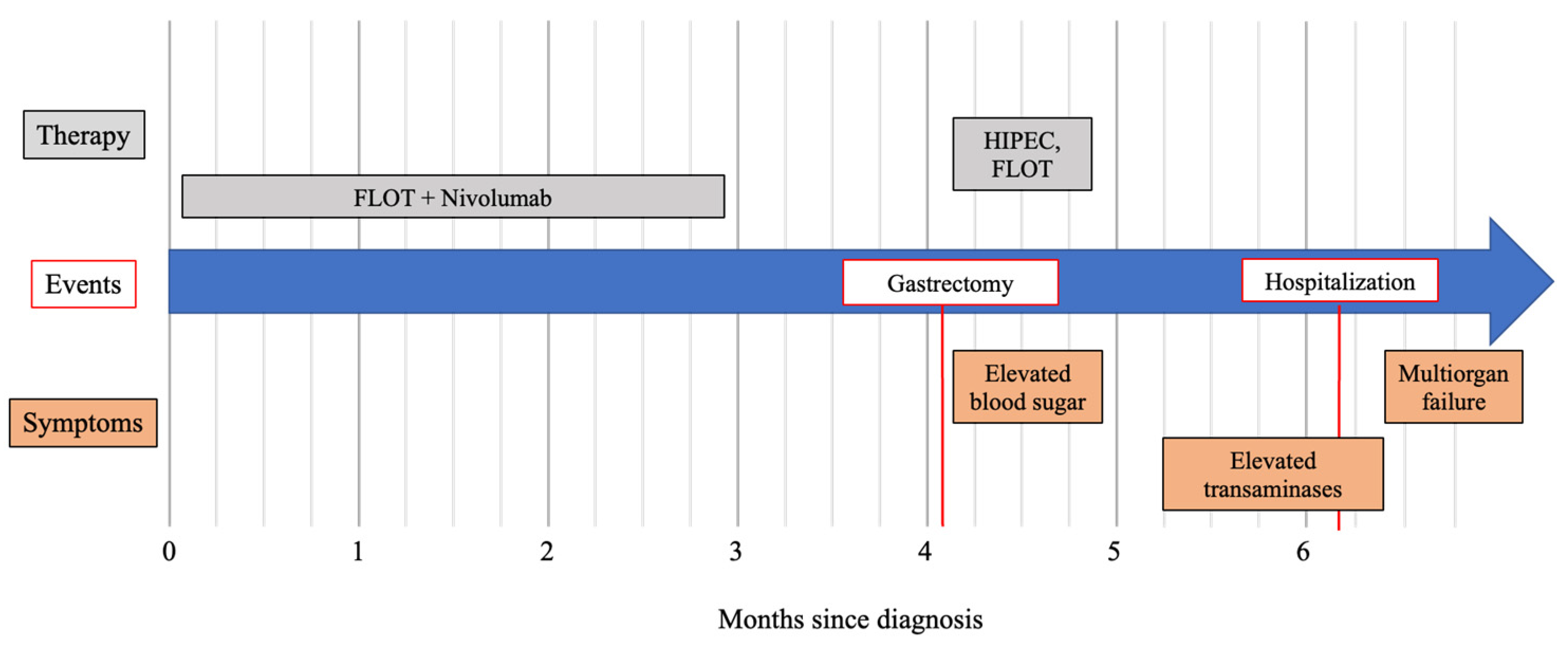
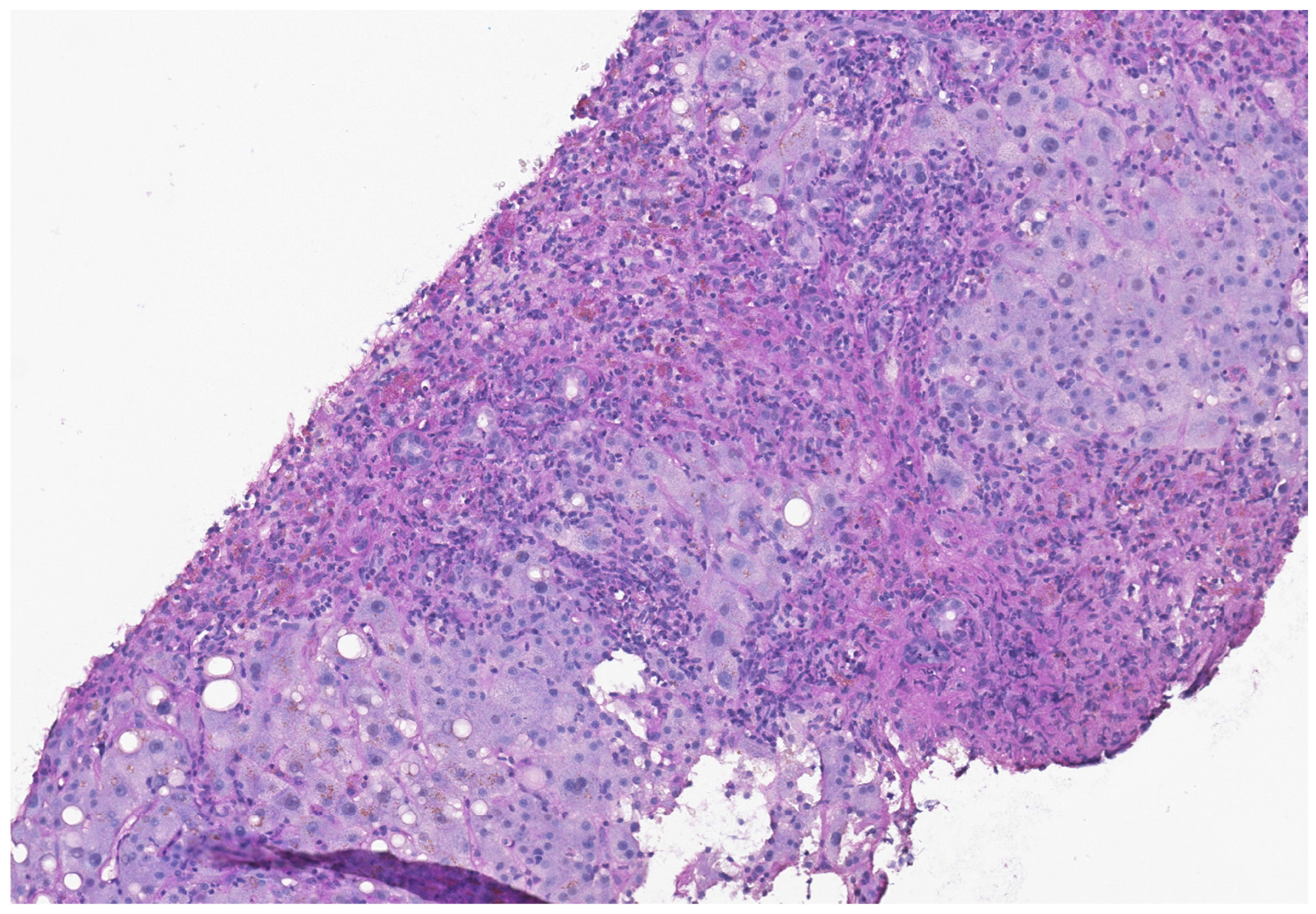
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernal, W.; Wendon, J. Acute Liver Failure. NEJM 2013, 369, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Hosack, T.; Damry, D.; Biswas, S. Drug-Induced Liver Injury: A Comprehensive Review. Ther. Adv. Gastroenterol. 2023, 16, 175628482311634. [Google Scholar] [CrossRef] [PubMed]
- Karvellas, C.J.; Leventhal, T.M.; Rakela, J.L.; Zhang, J.; Durkalski, V.; Reddy, K.R.; Fontana, R.J.; Stravitz, R.T.; Lake, J.R.; Lee, W.M.; et al. Outcomes of Patients with Acute Liver Failure Listed for Liver Transplantation: A Multicenter Prospective Cohort Analysis. Liver Transplant. 2023, 29, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Chayanupatkul, M.; Schiano, T.D. Acute Liver Failure Secondary to Drug-Induced Liver Injury. Clin. Liver Dis. 2020, 24, 75–87. [Google Scholar] [CrossRef]
- Koch, D.G.; Tillman, H.; Durkalski, V.; Lee, W.M.; Reuben, A. Development of a Model to Predict Transplant-Free Survival of Patients With Acute Liver Failure. CGH 2016, 14, 1199–1206.e2. [Google Scholar] [CrossRef]
- Kang, Y.K.; Boku, N.; Satoh, T.; Ryu, M.H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.S.; Muro, K.; Kang, W.K.; et al. Nivolumab in Patients with Advanced Gastric or Gastro-Oesophageal Junction Cancer Refractory to, or Intolerant of, at Least Two Previous Chemotherapy Regimens (ONO-4538-12, ATTRACTION-2): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef]
- Finkelmeier, F.; Waidmann, O.; Trojan, J. Nivolumab for the Treatment of Hepatocellular Carcinoma. Expert. Rev. Anticancer 2018, 18, 1169–1175. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer Immunoediting: From Immunosurveillance to Tumor Escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Möhn, N.; Beutel, G.; Gutzmer, R.; Ivanyi, P.; Satzger, I.; Skripuletz, T. Neurological Immune Related Adverse Events Associated with Nivolumab, Ipilimumab, and Pembrolizumab Therapy—Review of the Literature and Future Outlook. J. Clin. Med. 2019, 8, 1777. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Vera Aguilera, J.; Chintakuntlawar, A.; et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-Analysis. JAMA Oncol. 2019, 5, 1008–1019. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse Effects of Immune-Checkpoint Inhibitors: Epidemiology, Management and Surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.J.; Van Cutsem, E.; Limon, M.L.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/ Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. 2022, 40, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. NEJM 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Darnell, E.P.; Mooradian, M.J.; Baruch, E.N.; Yilmaz, M.; Reynolds, K.L. Immune-Related Adverse Events (IrAEs): Diagnosis, Management, and Clinical Pearls. Curr. Oncol. Rep. 2020, 22, 39. [Google Scholar] [CrossRef]
- Cuzzubbo, S.; Javeri, F.; Tissier, M.; Roumi, A.; Barlog, C.; Doridam, J.; Lebbe, C.; Belin, C.; Ursu, R.; Carpentier, A.F. Neurological Adverse Events Associated with Immune Checkpoint Inhibitors: Review of the Literature. Eur. J. Cancer 2017, 73, 1–8. [Google Scholar] [CrossRef]
- Mandiga, P.; Foris, L.A.; Bollu, P.C. Hepatic Encephalopathy; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Larkin, J.; Chmielowski, B.; Lao, C.D.; Hodi, F.S.; Sharfman, W.; Weber, J.; Suijkerbuijk, K.P.M.; Azevedo, S.; Li, H.; Reshef, D.; et al. Neurologic Serious Adverse Events Associated with Nivolumab Plus Ipilimumab or Nivolumab Alone in Advanced Melanoma, Including a Case Series of Encephalitis. Oncologist 2017, 22, 709–718. [Google Scholar] [CrossRef]
- Haugh, A.M.; Probasco, J.C.; Johnson, D.B. Neurologic Complications of Immune Checkpoint Inhibitors. Expert. Opin. Drug. Saf. 2020, 19, 479. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Elhalawani, H.; Fouad, M. Risk of Elevated Transaminases in Cancer Patients Treated with Immune Checkpoint Inhibitors: A Meta-Analysis. Expert. Opin. Drug. Saf. 2015, 14, 1507–1518. [Google Scholar] [CrossRef]
- Grover, S.; Rahma, O.E.; Hashemi, N.; Lim, R.M. Gastrointestinal and Hepatic Toxicities of Checkpoint Inhibitors: Algorithms for Management; ASCO: Alexandria, VA, USA, 2018; pp. 13–19. [Google Scholar] [CrossRef]
- McGregor, B.; Mortazavi, A.; Cordes, L.; Salabao, C.; Vandlik, S.; Apolo, A.B. Management of Adverse Events Associated with Cabozantinib plus Nivolumab in Renal Cell Carcinoma: A Review. Cancer Treat. Rev. 2022, 103, 102333. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Plimack, E.R.; Procopio, G.; McDermott, D.F.; et al. Nivolumab versus Everolimus in Patients with Advanced Renal Cell Carcinoma: Updated Results with Long-Term Follow-up of the Randomized, Open-Label, Phase 3 CheckMate 025 Trial. Cancer 2020, 126, 4156–4167. [Google Scholar] [CrossRef]
- Spain, L.; Diem, S.; Larkin, J. Management of Toxicities of Immune Checkpoint Inhibitors. Cancer Treat. Rev. 2016, 44, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yan, C.; Zhao, Y.; Li, D.; Guo, M.; Cui, X. Hepatic Failure Associated with Immune Checkpoint Inhibitors: An Analysis of the Food and Drug Administration Adverse Event Reporting System Database. Cancer Med. 2023, 12, 9167–9174. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef]
- De Martin, E.; Michot, J.M.; Papouin, B.; Champiat, S.; Mateus, C.; Lambotte, O.; Roche, B.; Antonini, T.M.; Coilly, A.; Laghouati, S.; et al. Characterization of Liver Injury Induced by Cancer Immunotherapy Using Immune Checkpoint Inhibitors. J. Hepatol. 2018, 68, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Zen, Y.; Yeh, M.M. Hepatotoxicity of Immune Checkpoint Inhibitors: A Histology Study of Seven Cases in Comparison with Autoimmune Hepatitis and Idiosyncratic Drug-Induced Liver Injury. Mod. Pathol. 2018, 31, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Tanaka, Y.; Nishio, R.; Mitsuiye, T.; Mizoguchi, A.; Wang, J.; Ishida, M.; Hiai, H.; Matsumori, A.; Minato, N.; et al. Autoantibodies against Cardiac Troponin I Are Responsible for Dilated Cardiomyopathy in PD-1-Deficient Mice. Nat. Med. 2003, 9, 1477–1483. [Google Scholar] [CrossRef]
- Mohty, M. Mechanisms of Action of Antithymocyte Globulin: T-Cell Depletion and Beyond. Leukemia 2007, 21, 1387–1394. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dibos, M.; Dumoulin, J.; Mogler, C.; Wunderlich, S.; Reichert, M.; Rasch, S.; Schmid, R.M.; Ringelhan, M.; Ehmer, U.; Lahmer, T. Fulminant Liver Failure after Treatment with a Checkpoint Inhibitor for Gastric Cancer: A Case Report and Review of the Literature. J. Clin. Med. 2023, 12, 4641. https://doi.org/10.3390/jcm12144641
Dibos M, Dumoulin J, Mogler C, Wunderlich S, Reichert M, Rasch S, Schmid RM, Ringelhan M, Ehmer U, Lahmer T. Fulminant Liver Failure after Treatment with a Checkpoint Inhibitor for Gastric Cancer: A Case Report and Review of the Literature. Journal of Clinical Medicine. 2023; 12(14):4641. https://doi.org/10.3390/jcm12144641
Chicago/Turabian StyleDibos, Miriam, Johanna Dumoulin, Carolin Mogler, Silke Wunderlich, Maximilian Reichert, Sebastian Rasch, Roland M. Schmid, Marc Ringelhan, Ursula Ehmer, and Tobias Lahmer. 2023. "Fulminant Liver Failure after Treatment with a Checkpoint Inhibitor for Gastric Cancer: A Case Report and Review of the Literature" Journal of Clinical Medicine 12, no. 14: 4641. https://doi.org/10.3390/jcm12144641
APA StyleDibos, M., Dumoulin, J., Mogler, C., Wunderlich, S., Reichert, M., Rasch, S., Schmid, R. M., Ringelhan, M., Ehmer, U., & Lahmer, T. (2023). Fulminant Liver Failure after Treatment with a Checkpoint Inhibitor for Gastric Cancer: A Case Report and Review of the Literature. Journal of Clinical Medicine, 12(14), 4641. https://doi.org/10.3390/jcm12144641





