Importance of Demographic and Clinical Features in Evaluating the Severity of COVID-19 in Hospitalized Patients: A Serbian Retrospective Study in the First Pandemic Year
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Study Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard; World Health Organization: Geneva, Switzerland, 2022; Available online: https://covid19.who.int/ (accessed on 23 May 2023).
- Xie, J.; Covassin, N.; Fan, Z.; Singh, P.; Gao, W.; Li, G.; Kara, T.; Somers, V.K. Association between Hypoxemia and Mortality in Patients with COVID-19. Mayo Clin. Proc. 2020, 95, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Dhont, S.; Derom, E.; Van Braeckel, E.; Depuydt, P.; Lambrecht, B.N. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir. Res. 2020, 21, 198. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, A.O.K.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Battaglini, D.; Lopes-Pacheco, M.; Castro-Faria-Neto, H.C.; Pelosi, P.; Rocco, P.R.M. Laboratory Biomarkers for Diagnosis and Prognosis in COVID-19. Front. Immunol. 2022, 13, 857573. [Google Scholar] [CrossRef] [PubMed]
- Bal, A.; Brengel-Pesce, K.; Gaymard, A.; Quéromès, G.; Guibert, N.; Frobert, E.; Bouscambert, M.; Trabaud, M.A.; Allantaz-Frager, A.; Oriol, G.; et al. Clinical and laboratory characteristics of symptomatic healthcare workers with suspected COVID-19: A prospective cohort study. Sci. Rep. 2021, 11, 14977. [Google Scholar] [CrossRef] [PubMed]
- Rasmi, Y.; Saavedra, L.P.J.; Cozma, M.A.; El-Nashar, H.; Aly, S.; Fahmy, N.; Eldahshan, O.; El-Shazly, M.; Dobrică, E.C.; Kord-Varkaneh, H.; et al. Laboratory findings in COVID-19—Alterations of hematological, immunological, biochemical, hormonal and other lab panels: A narrative review. J. Mind Med. Sci. 2022, 9, 5. [Google Scholar] [CrossRef]
- Fang, X.; Li, S.; Yu, H.; Wang, P.; Zhang, Y.; Chen, Z.; Li, Y.; Cheng, L.; Li, W.; Jia, H.; et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: A systematic review and meta-analysis. Aging 2020, 12, 12493–12503. [Google Scholar] [CrossRef]
- Gao, Y.D.; Ding, M.; Dong, X.; Zhang, J.J.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Song, C.; Lu, R.; Zhao, Y.; Lin, F.; Han, D.; Chen, L.; Pan, P.; Dai, M. Early Prediction of Disease Progression in Patients with Severe COVID-19 Using C-Reactive Protein to Albumin Ratio. Dis. Markers 2021, 2021, 6304189. [Google Scholar] [CrossRef]
- Hann-Yee, T.; Mathew, Y.; Xin-Ying, T.; Michael, T.; Ranjeev Ooi, K.; Amirah, S.T.; Ubeynarayana, L.; Udhyami, C.; Desmond, M. Predictive performance of emergency department-specific variables on COVID-19 pneumonia. Singap. Med. J. 2022, 63, 715–722. [Google Scholar]
- Al-Shajlawi, M.; Alsayed, A.R.; Abazid, H.; Awajan, D.; Al-Imam, A.; Basheti, I. Using laboratory parameters as predictors for the severity and mortality of COVID-19 in hospitalized patients. Pharm. Pract. 2022, 20, 2721. [Google Scholar] [CrossRef]
- World Health Organization. WHO Living Guidance for Clinical Management of COVID-19; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/ (accessed on 19 November 2022).
- Chung, H.P.; Wu, K.L.; Lin, C.Y.; Tang, Y.H.; Chen, C.H.; Wu, J.C.; Chen, Y.T.; Kuo, K.C.; Chang, W.K. Characteristics and Outcomes of Hospitalized Geriatric Patients with COVID-19 Infection in Taiwan. Int. J. Gerontol. 2022, 16, 207–212. [Google Scholar]
- Zhang, X.; Zheng, J.; Qian, E.; Xue, L.; Liu, X. The association of clinical features and laboratory findings of COVID-19 infection with computed pneumonia volume. Medicine 2022, 101, e28856. [Google Scholar] [CrossRef]
- Amer, F.A.; Saeed, M.A.; Shaltouts, W.; Nofal, H.A.E.; Nafae, R.M.; Arslan, K.; Tanoglu, A.; Nechifor, M.; Luca, C.; Al-kadhim, Z.H.A.; et al. Assessment and outcome of hospitalized patients during delta variant COVID-19 pandemic: A multicenter international study. J. Infect. Dev. Ctries 2022, 16, 1715–1725. [Google Scholar] [CrossRef]
- Eid, R.A.; Attia, A.M.; Hassan, M.; Shaker, M.A.; Kamal, M.A. Demographic, clinical, and laboratory characteristics of patients with COVID-19 during the second and third waves of the pandemic in Egypt. J. Infect. Public Health 2021, 14, 1358–1366. [Google Scholar] [CrossRef]
- Loss, S.H.; Luce, D.C.; Capellari, G. Characteristics and outcomes of COVID-19 patients assisted by intensivists and nonintensivists. Rev. Assoc. Med. Bras. 2022, 68, 1204–1209. [Google Scholar] [CrossRef]
- Stojanović, M.; Miličić, B.; Purić, N.; Jeremić, J.; Jović, M.; Stojčić, M.; Omčikus, M.; Trboljevac, N.; Velickovic, J. Survival of critically ill patients with COVID-19 pneumonia-a single-center experience. J. Infect. Dev. Ctries. 2022, 16, 1424–1431. [Google Scholar] [CrossRef]
- Allwood, B.W.; Koegelenberg, C.F.; Ngah, V.D.; Sigwadhi, L.N.; Irusen, E.M.; Lalla, U.; Yalewb, A.; Tamuzi, J.L.; Allister, M.M.; Zemline, A.; et al. Predicting COVID-19 outcomes from clinical and laboratory parameters in an intensive care facility during the second wave of the pandemic in South Africa. IJID Reg. 2022, 3, 242–247. [Google Scholar] [CrossRef]
- Güven, R.; Çolak, Ş.; Sogut, O.; Yavuz, B.G.; Çalık, M.; Altınbilek, E.; Hokenek, N.M.; Eyüpoğlu, G.; Tayfur, I.; Çakir, A. Predictors of mortality in patients less than 50 years old with coronavirus disease 2019: A multicenter experience in Istanbul. Rev. Assoc. Med. Bras. 2022, 68, 239–244. [Google Scholar] [CrossRef]
- Radonjić, T.; Milićević, O.; Jovanović, I.; Zdravković, M.; Dukić, M.; Mandić, O.M.; Bjekić-Macut, J.; Marković, o.B.; Todorović, Z.; Brajković, M.; et al. Elevated Transaminases as Predictors of COVID-19 Pneumonia Severity. Medicina 2022, 58, 842. [Google Scholar] [CrossRef]
- National Center for Immunization and Respiratory Diseases (U.S.). Division of Viral Diseases. Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). Available online: https://stacks.cdc.gov/view/cdc/88624 (accessed on 2 June 2020).
- Bhimraj, A.; Morgan, R.L.; Shumaker, A.H.; Lavergne, V.; Baden, L.; Cheng, V.C.; Edwards, K.M.; Gandhi, R.; Muller, W.J.; O’Horo, J.C.; et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin. Infect. Dis. 2020, ciaa478. [Google Scholar] [CrossRef] [PubMed]
- Poskurica, M.; Stevanović, Đ.; Zdravković, V.; Čekerevac, I.; Ćupurdija, V.; Zdravković, N.; Nikolić, T.; Marković, M.; Jovanović, M.; Popović, M.; et al. Admission Predictors of Mortality in Hospitalized COVID-19 Patients-A Serbian Cohort Study. J. Clin. Med. 2022, 11, 6109. [Google Scholar] [CrossRef] [PubMed]
- Popovska Jovičić, B.; Raković, I.; Pavković, A.; Marković, V.; Petrović, S.; Gavrilović, J.; Čanović, P.; Radojević Marjanović, R.; Folic, M. Significance of initial clinical laboratory parameters as prognostic factors in patients with COVID-19. Vojnosanit. Pregl. 2022, 79, 849–856. [Google Scholar] [CrossRef]
- Xie, C.; Wang, S.; Zhou, J.; Tong, L.; Shao, C. Albumin level as an independent predictive factor for adverse outcomes in COVID-19 patients: A retrospective cohort study. J. Infect. Dev. Ctries. 2022, 16, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.A.; Charmchi, Z.; Silver, M.; Jacoby, N.; Perk, J.; Anziska, Y. Skeletal Muscle Manifestations and Creatine Kinase in COVID-19. Neurohospitalist 2022, 12, 597–606. [Google Scholar] [CrossRef]
- Leung, T.W.; Wong, K.S.; Hui, A.C.; To, K.F.; Lai, S.T.; Ng, W.F.; Ng, H.K. Myopathic changes associated with severe acute respiratory syndrome: A postmortem case series. Arch Neurol. 2005, 62, 1113–1117. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Li, H.; Qin, Y.Y.; Yan, X.F.; Lu, Y.Q.; Liu, H.L.; Ye, S.K.; Wan, Y.; Zhang, L.; Harypursat, V.; et al. Predictive factors of progression to severe COVID-19. Open Med. 2020, 15, 805–814. [Google Scholar] [CrossRef]
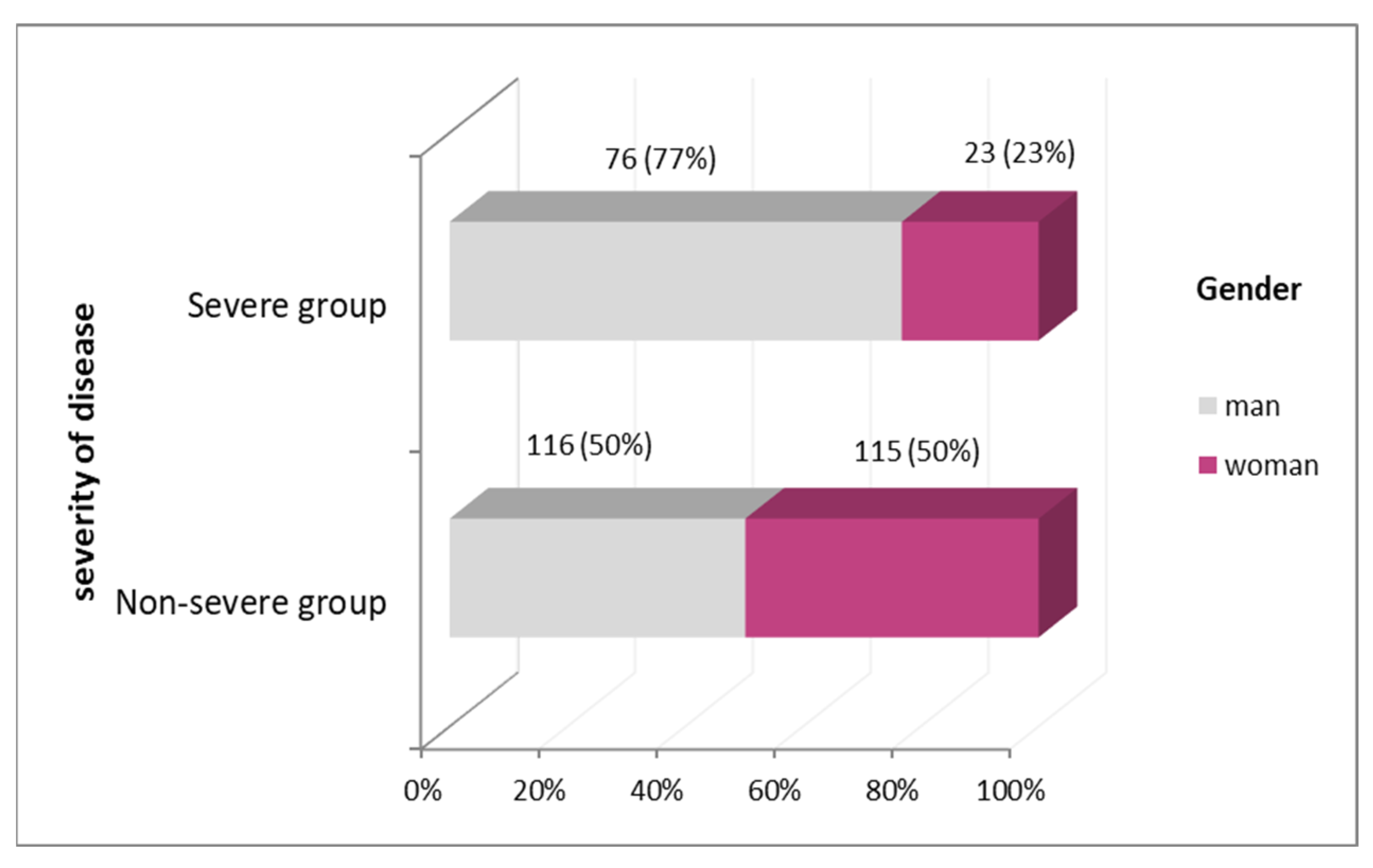
| Characteristics of Patients | Non-Severe Group | Severe Group | Significance of p Value | ||
|---|---|---|---|---|---|
| Number of Patients n (%) | 231 (70.0%) | 99 (30.0%) | |||
| Symptoms | Cough n (%) | 170 (73.6%) | 82 (82.8%) | 0.070 | |
| Fatigue n (%) | 153 (66.2%) | 64 (64.4%) | 0.781 | ||
| Headache n (%) | 62 (26.8%) | 23 (23.2%) | 0.492 | ||
| Gastrointestinal symptoms n (%) | 42 (18.2%) | 19 (19.2%) | 0.829 | ||
| Anosmia n (%) | 27 (11.7%) | 7 (7.1%) | 0.206 | ||
| Comorbidities | Hypertension n (%) | 50 (21.6%) | 33 (33.7%) | 0.022 * | |
| Diabetes mellitus n (%) | 27 (11.7%) | 14 (14.3%) | 0.514 | ||
| Chronical obstructive pulmonary disease n (%) | 5 (2.2%) | 3 (3.1%) | 0.629 | ||
| Radiographic findings | Radiographic finding of pneumonia n (%) | 182 (79.1%) | 91 (94.8%) | 0.001 * | |
| Localization of pneumonia n (%) | Unilateral | 52 (28.6%) | 5 (5.5%) | 0.001 * | |
| Bilateral | 130 (71.4%) | 86 (94.5%) | |||
| Laboratory Parameters | Non-Severe Group | Severe Group | Significance of p Value |
|---|---|---|---|
| Median (Min–Max) | Median (Min–Max) | ||
| Body temperature (°C) | 37 (35–40) | 38 (36–40) | 0.373 |
| Duration of symptoms before admission(days) | 5 (1–30) | 5 (1–30) | 0.841 |
| Leukocytes (×109/L) | 6.30 (2–26) | 6.20 (2–48) | 0.967 |
| Neutrophils (%) | 0.70 (0.23–0.93) | 0.79 (0.10–0.95) | 0.001 * |
| Lymphocytes (%) | 0.26 (0.04–0.61) | 0.18 (0.05–0.84) | 0.001 * |
| Erythrocytes (×1012/L) | 4.5 (2.92–5.98) | 4.49 (2.93–5.70) | 0.591 |
| Hemoglobin (g/L) | 139 (89–185) | 139 (89–176) | 0.907 |
| Hematocrit (L/L) | 0.44 (0.29–0.57) | 0.44 (0.30–0.55) | 0.839 |
| Platelets (×109/L) | 228 (70–989) | 205 (73–442) | 0.123 |
| CRP (IU/mL) | 24 (3–145) | 34 (6–150) | 0.008 * |
| AST (U/L) | 42 (15–241) | 51.5 (21–220) | 0.037 * |
| ALT (U/L) | 42 (10–260) | 44 (12–258) | 0.669 |
| ALP (U/L) | 108 (59–303) | 99 (55–486) | 0.027 * |
| GGT (U/L) | 31.5 (10–256) | 32 (12–258) | 0.288 |
| Amylase (U/L) | 56 (13–262) | 49 (16–150) | 0.286 |
| LDH (U/L) | 415 (194–1399) | 563.50 (247–2081) | 0.001 * |
| CK (U/L) | 92 (16–3285) | 145 (22–1956) | 0.001 * |
| Total proteins (g/L) | 72 (51–98) | 69 (48–86) | 0.001 * |
| Albumin (g/L) | 42 (25–82) | 36.50 (20–80) | 0.001 * |
| Glucose (mmol/L) | 6.64 (4–10) | 7.90 (5–23) | 0.001 * |
| Oxygen saturation (%) | 96 (25–99) | 91 (40–99) | 0.001 * |
| Therapy | Non-Severe Group | Severe Group | Significance of p Value | |
|---|---|---|---|---|
| Number of Patients n (%) | 231 (70.0%) | 99 (30.0%) | ||
| Corticosteroid n (%) | No | 106 (45.9%) | 34 (34.3%) | 0.052 |
| Yes | 125 (54.1%) | 65 (65.7%) | ||
| Oxygen support n (%) | No | 159 (68.8%) | 18 (18.2%) | 0.001 * |
| Yes | 72 (31.2%) | 81 (81.8%) | ||
| Independent Variables | B | P | OR | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Hypertension | 0.394 | 0.413 | 1.483 | 0.577 | 3.807 |
| Radiographic finding of pneumonia | 0.100 | 0.880 | 1.105 | 0.300 | 4.068 |
| Localization of pneumonia | 1.190 | 0.094 | 3.286 | 0.818 | 13.203 |
| Neutrophils | −0.843 | 0.655 | 0.430 | 0.011 | 17.445 |
| Lymphocytes | 0.440 | 0.680 | 1.552 | 0.192 | 12.549 |
| CRP | 0.004 | 0.608 | 1.004 | 0.990 | 1.017 |
| AST | 0.005 | 0.440 | 1.005 | 0.992 | 1.018 |
| ALP | −0.012 | 0.141 | 0.988 | 0.973 | 1.004 |
| LDH | −0.001 | 0.308 | 0.999 | 0.997 | 1.101 |
| CK | 0.002 | 0.032 * | 1.002 | 1.000 | 1.004 |
| Total proteins | −0.052 | 0.171 | 0.949 | 0.881 | 1.023 |
| Albumin | −0.029 | 0.443 | 0.972 | 0.903 | 1.046 |
| Glucose | 0.085 | 0.220 | 1.089 | 0.950 | 1.247 |
| Oxygen saturation | −0.028 | 0.247 | 0.973 | 0.928 | 1.020 |
| Oxygen support | 2.178 | <0.001 * | 8.829 | 3.000 | 25.983 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aritonovic Pribakovic, J.; Peric, M.; Milenkovic, A.; Janicevic, A.; Hadzistevic, S.; Ilic, A.; Stojanovic-Tasic, M.; Bulatovic, K.; Rasic, D.; Mitic, J. Importance of Demographic and Clinical Features in Evaluating the Severity of COVID-19 in Hospitalized Patients: A Serbian Retrospective Study in the First Pandemic Year. J. Clin. Med. 2023, 12, 4638. https://doi.org/10.3390/jcm12144638
Aritonovic Pribakovic J, Peric M, Milenkovic A, Janicevic A, Hadzistevic S, Ilic A, Stojanovic-Tasic M, Bulatovic K, Rasic D, Mitic J. Importance of Demographic and Clinical Features in Evaluating the Severity of COVID-19 in Hospitalized Patients: A Serbian Retrospective Study in the First Pandemic Year. Journal of Clinical Medicine. 2023; 12(14):4638. https://doi.org/10.3390/jcm12144638
Chicago/Turabian StyleAritonovic Pribakovic, Jelena, Milica Peric, Aleksandra Milenkovic, Aleksandra Janicevic, Snezana Hadzistevic, Aleksandra Ilic, Mirjana Stojanovic-Tasic, Kristina Bulatovic, Dragisa Rasic, and Jadranka Mitic. 2023. "Importance of Demographic and Clinical Features in Evaluating the Severity of COVID-19 in Hospitalized Patients: A Serbian Retrospective Study in the First Pandemic Year" Journal of Clinical Medicine 12, no. 14: 4638. https://doi.org/10.3390/jcm12144638
APA StyleAritonovic Pribakovic, J., Peric, M., Milenkovic, A., Janicevic, A., Hadzistevic, S., Ilic, A., Stojanovic-Tasic, M., Bulatovic, K., Rasic, D., & Mitic, J. (2023). Importance of Demographic and Clinical Features in Evaluating the Severity of COVID-19 in Hospitalized Patients: A Serbian Retrospective Study in the First Pandemic Year. Journal of Clinical Medicine, 12(14), 4638. https://doi.org/10.3390/jcm12144638






