Safety and Healthcare Resource Utilization in Patients Undergoing Left Atrial Appendage Closure—A Nationwide Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
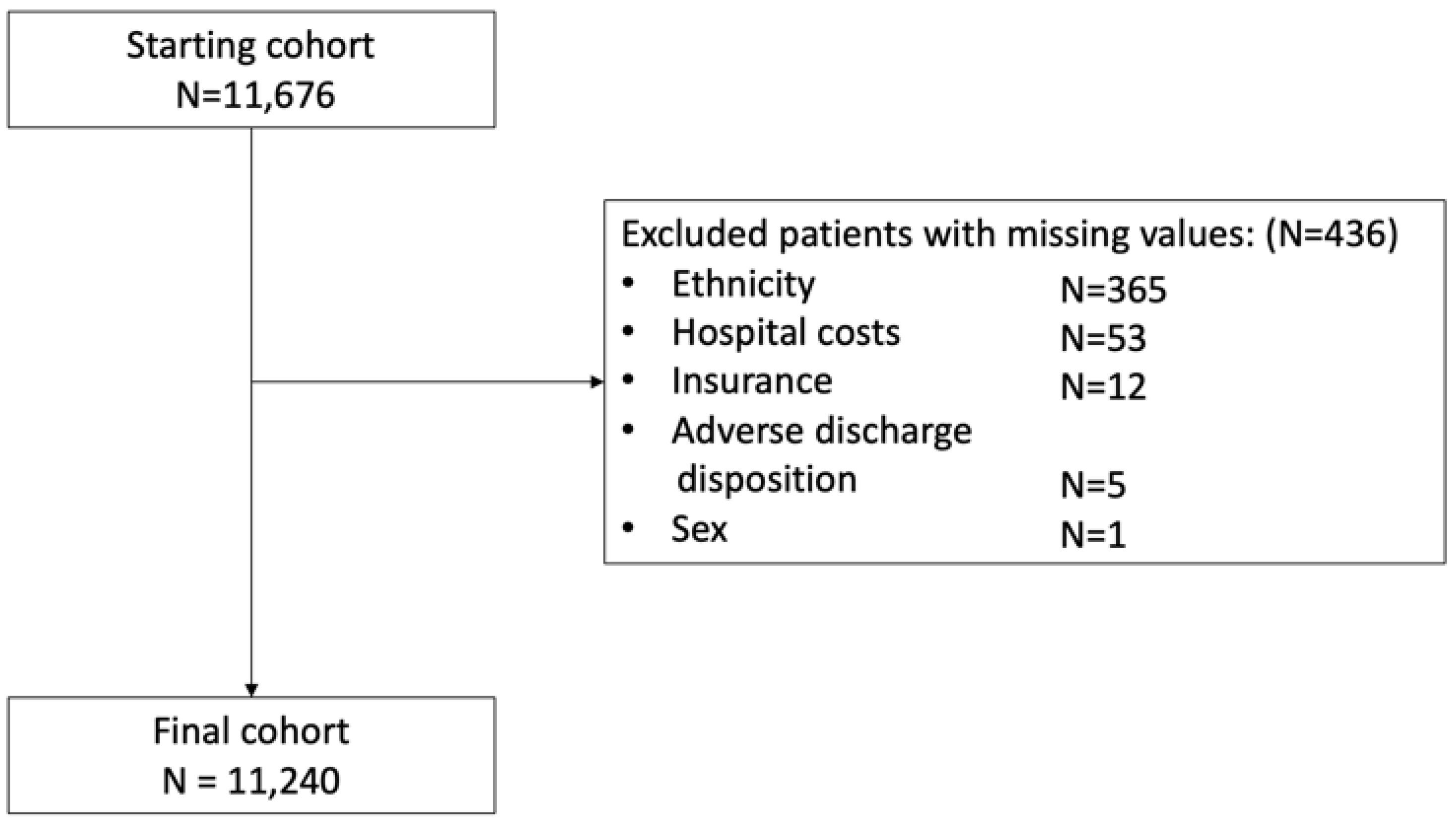
2.2. Study Population
2.3. Outcomes
2.4. Statistical Analysis
2.5. Sensitivity Analyses
2.6. Geographic Analyses
3. Results
3.1. Patient Characteristics
3.2. Primary Outcome: Safety
3.3. Secondary Outcomes: Healthcare Resource Utilization
3.4. Subgroup Analyses
3.5. Geographic Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Colilla, S.; Crow, A.; Petkun, W.; Singer, D.E.; Simon, T.; Liu, X. Estimates of Current and Future Incidence and Prevalence of Atrial Fibrillation in the U.S. Adult Population. Am. J. Cardiol. 2013, 112, 1142–1147. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS)The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Developed with the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, E254–E743. [Google Scholar] [CrossRef] [PubMed]
- Buck, J.; Fromings Hill, J.; Martin, A.; Springate, C.; Ghosh, B.; Ashton, R.; Lee, G.; Orlowski, A. Reasons for Discontinuing Oral Anticoagulation Therapy for Atrial Fibrillation: A Systematic Review. Age Ageing 2021, 50, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, I.M.; Newton, N.; Welner, S.A.; Cowell, W.; Lip, G.Y.H. Underuse of Oral Anticoagulants in Atrial Fibrillation: A Systematic Review. Am. J. Med. 2010, 123, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Collado, F.M.S.; von Buchwald, C.M.L.; Anderson, C.K.; Madan, N.; Suradi, H.S.; Huang, H.D.; Jneid, H.; Kavinsky, C.J. Left Atrial Appendage Occlusion for Stroke Prevention in Nonvalvular Atrial Fibrillation. J. Am. Heart Assoc. 2021, 10, 22274. [Google Scholar] [CrossRef]
- Yaghi, S.; Song, C.; Gray, W.A.; Furie, K.L.; Elkind, M.S.V.; Kamel, H. Left Atrial Appendage Function and Stroke Risk. Stroke 2015, 46, 3554. [Google Scholar] [CrossRef]
- Landmesser, U.; Holmes, D.R. Left Atrial Appendage Closure: A Percutaneous Transcatheter Approach for Stroke Prevention in Atrial Fibrillation. Eur. Heart J. 2012, 33, 698–704. [Google Scholar] [CrossRef]
- Holmes, D.R.; Reddy, V.Y.; Turi, Z.G.; Doshi, S.K.; Sievert, H.; Buchbinder, M.; Mullin, C.M.; Sick, P. Percutaneous Closure of the Left Atrial Appendage versus Warfarin Therapy for Prevention of Stroke in Patients with Atrial Fibrillation: A Randomised Non-Inferiority Trial. Lancet 2009, 374, 534–542. [Google Scholar] [CrossRef]
- Holmes, D.R.; Kar, S.; Price, M.J.; Whisenant, B.; Sievert, H.; Doshi, S.K.; Huber, K.; Reddy, V.Y. Prospective Randomized Evaluation of the Watchman Left Atrial Appendage Closure Device in Patients with Atrial Fibrillation Versus Long-Term Warfarin Therapy: The PREVAIL Trial. J. Am. Coll. Cardiol. 2014, 64, 1–12. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Doshi, S.K.; Kar, S.; Gibson, D.N.; Price, M.J.; Huber, K.; Horton, R.P.; Buchbinder, M.; Neuzil, P.; Gordon, N.T.; et al. 5-Year Outcomes After Left Atrial Appendage Closure: From the PREVAIL and PROTECT AF Trials. J. Am. Coll. Cardiol. 2017, 70, 2964–2975. [Google Scholar] [CrossRef] [PubMed]
- Lakkireddy, D.; Thaler, D.; Ellis, C.R.; Swarup, V.; Sondergaard, L.; Carroll, J.; Gold, M.R.; Hermiller, J.; Diener, H.C.; Schmidt, B.; et al. Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE): A Randomized, Controlled Trial. Circulation 2021, 144, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- OmegaTM Left Atrial Appendage (LAA) Occluder in Patients with Non-Valvular Atrial Fibrillation—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03526471 (accessed on 14 November 2022).
- Lifetech LAmbreTM Left Atrial Appendage (LAA) Closure System Post-Market Clinical Follow-up—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03666780 (accessed on 14 November 2022).
- CHAMPION-AF Clinical Trial—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04394546 (accessed on 14 November 2022).
- Comparison of Anticoagulation with Left Atrial Appendage Closure After AF Ablation—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03795298 (accessed on 14 November 2022).
- Amplatzer Amulet LAAO, vs. NOAC—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04226547 (accessed on 3 October 2022).
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. 4-Year Outcomes After Left Atrial Appendage Closure Versus Nonwarfarin Oral Anticoagulation for Atrial Fibrillation. J. Am. Coll. Cardiol. 2022, 79, 1–14. [Google Scholar] [CrossRef]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. Left Atrial Appendage Closure Versus Direct Oral Anticoagulants in High-Risk Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 75, 3122–3135. [Google Scholar] [CrossRef]
- Boersma, L.V.; Schmidt, B.; Betts, T.R.; Sievert, H.; Tamburino, C.; Teiger, E.; Pokushalov, E.; Kische, S.; Schmitz, T.; EWOLUTION Investigators; et al. Implant Success and Safety of Left Atrial Appendage Closure with the WATCHMAN Device: Peri-Procedural Outcomes from the EWOLUTION Registry. Eur. Heart J. 2016, 37, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Tondo, C.; Camm, J.; Diener, H.C.; Paul, V.; Schmidt, B.; Settergren, M.; Teiger, E.; Nielsen-Kudsk, J.E.; Hildick-Smith, D. Left Atrial Appendage Occlusion with the AMPLATZER Amulet Device: One-Year Follow-up from the Prospective Global Amulet Observational Registry. EuroIntervention 2018, 14, e590–e597. [Google Scholar] [CrossRef]
- Jules Mesnier, M.; Ignacio Cruz-González, M.P.; Dabit Arzamendi, M.; Xavier Freixa, M.P.; Luis Nombela-Franco, M.P.; Vicente Peral, M.P.; Berenice Caneiro-Queija, M.; Antonio Mangieri, M.; Blanca Trejo-Velasco, M.; Lluis Asmarats, M.; et al. Incidence and Predictors of Early Death in Patients Undergoing Percutaneous Left Atrial Appendage Closure. Clin. Electrophysiol. 2022, 8, 1093–1102. [Google Scholar] [CrossRef]
- Kawamura, I.; Kuno, T.; Sahashi, Y.; Tanaka, Y.; Passman, R.; Briasoulis, A.; Malik, A.H. Thirty-Day Readmission Rate of Same-Day Discharge Protocol after Left Atrial Appendage Occlusion: A Propensity Score–Matched Analysis from the National Readmission Database. Heart Rhythm 2022, 19, 1819–1825. [Google Scholar] [CrossRef]
- Isogai, T.; Agrawal, A.; Saad, A.M.; Kuroda, S.; Shekhar, S.; Abushouk, A.I.; Wazni, O.M.; Hussein, A.A.; Krishnaswamy, A.; Kapadia, S.R. Periprocedural and Short-Term Outcomes of Percutaneous Left Atrial Appendage Closure According to Type of Atrial Fibrillation. J. Am. Heart Assoc. 2021, 10, e022124. [Google Scholar] [CrossRef]
- Vaughan Sarrazin, M.; Kabra, R.; Girotra, S. Clinical Outcomes of Mortality, Readmissions, and Ischemic Stroke Among Medicare Patients Undergoing Left Atrial Appendage Closure via Implanted Device. JAMA Netw. Open 2019, 2, e1914268. [Google Scholar] [CrossRef]
- Vuddanda, V.L.K.; Turagam, M.K.; Umale, N.A.; Shah, Z.; Lakkireddy, D.R.; Bartus, K.; McCausland, F.R.; Velagapudi, P.; Mansour, M.; Heist, E.K. Incidence and Causes of In-Hospital Outcomes and 30-Day Readmissions after Percutaneous Left Atrial Appendage Closure: A US Nationwide Retrospective Cohort Study Using Claims Data. Heart Rhythm 2020, 17, 374–382. [Google Scholar] [CrossRef] [PubMed]
- HCUP-US NIS Overview. Available online: https://www.hcup-us.ahrq.gov/nisoverview.jsp (accessed on 4 December 2022).
- Farwati, M.; Amin, M.; Isogai, T.; Saad, A.M.; Abushouk, A.I.; Krishnaswamy, A.; Wazni, O.; Kapadia, S.R. Short-Term Outcomes Following Left Atrial Appendage Closure in the Very Elderly: A Population-Based Analysis. J. Am. Heart Assoc. 2022, 11, 24574. [Google Scholar] [CrossRef] [PubMed]
- Glasheen, W.P.; Cordier, T.; Gumpina, R.; Haugh, G.; Davis, J.; Renda, A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am. Health Drug Benefits 2019, 12, 188. [Google Scholar]
- Kim, T.H.; Yang, P.S.; Kim, D.; Yu, H.T.; Uhm, J.S.; Kim, J.Y.; Pak, H.N.; Lee, M.H.; Joung, B.; Lip, G.Y.H. CHA2DS2-VASc Score for Identifying Truly Low-Risk Atrial Fibrillation for Stroke: A Korean Nationwide Cohort Study. Stroke 2017, 48, 2984–2990. [Google Scholar] [CrossRef]
- Gage, B.F.; Waterman, A.D.; Shannon, W.; Boechler, M.; Rich, M.W.; Radford, M.J. Validation of Clinical Classification Schemes for Predicting Stroke: Results from the National Registry of Atrial Fibrillation. JAMA 2001, 285, 2864–2870. [Google Scholar] [CrossRef]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; De Vos, C.B.; Crijns, H.J.G.M.; Lip, G.Y.H.; Andresen, D.; Camm, A.J.; Davies, W.; Capucci, A.; et al. A Novel User-Friendly Score (HAS-BLED) to Assess 1-Year Risk of Major Bleeding in Patients with Atrial Fibrillation: The Euro Heart Survey. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef]
- Frost, P.H.; Davis, B.R.; Burlando, A.J.; Curb, J.D.; Guthrie, G.P.; Isaacsohn, J.L.; Wassertheil-Smoller, S.; Wilson, A.C.; Stamler, J. Coronary Heart Disease Risk Factors in Men and Women Aged 60 Years and Older. Circulation 1996, 94, 26–34. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; MacH, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Jun, M.; James, M.T.; Manns, B.J.; Quinn, R.R.; Ravani, P.; Tonelli, M.; Perkovic, V.; Winkelmayer, W.C.; Ma, Z.; Hemmelgarn, B.R. The Association between Kidney Function and Major Bleeding in Older Adults with Atrial Fibrillation Starting Warfarin Treatment: Population Based Observational Study. BMJ 2015, 350, h246. [Google Scholar] [CrossRef]
- Fuchs, G.; Thevathasan, T.; Chretien, Y.R.; Mario, J.; Piriyapatsom, A.; Schmidt, U.; Eikermann, M.; Fintelmann, F.J. Lumbar Skeletal Muscle Index Derived from Routine Computed Tomography Exams Predict Adverse Post-Extubation Outcomes in Critically Ill Patients. J. Crit. Care 2018, 44, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.; Holmes, D.R.; Cavalcante, J.L.; Freeman, J.V.; Goldsweig, A.M.; Kavinsky, C.J.; Moussa, I.D.; Munger, T.M.; Price, M.J.; Reisman, M.; et al. SCAI/HRS Expert Consensus Statement on Transcatheter Left Atrial Appendage Closure. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 100577. [Google Scholar] [CrossRef]
- Holmes, D.R.; Reddy, V.Y.; Gordon, N.T.; Delurgio, D.; Doshi, S.K.; Desai, A.J.; Stone, J.E.; Kar, S. Long-Term Safety and Efficacy in Continued Access Left Atrial Appendage Closure Registries. J. Am. Coll. Cardiol. 2019, 74, 2878–2889. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Holmes, D.; Doshi, S.K.; Neuzil, P.; Kar, S. Safety of Percutaneous Left Atrial Appendage Closure. Circulation 2011, 123, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Tzikas, A.; Gafoor, S.; Meerkin, D.; Freixa, X.; Cruz-Gonzalez, I.; Lewalter, T.; Saw, J.; Berti, S.; Nielsen-Kudsk, J.E.; Ibrahim, R.; et al. Left Atrial Appendage Occlusion with the AMPLATZER Amulet Device: An Expert Consensus Step-by-Step Approach. EuroIntervention 2016, 11, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.; Russo, A.M.; Thaler, D.; Windecker, S.; Anderson, J.A.; Gage, R.; Lakkireddy, D. Sex Differences in Safety and Effectiveness of LAAO: Insights from the Amulet IDE Trial. JACC Cardiovasc. Interv. 2022, 15, 2143–2155. [Google Scholar] [CrossRef]
- Darden, D.; Duong, T.; Du, C.; Munir, M.B.; Han, F.T.; Reeves, R.; Saw, J.; Zeitler, E.P.; Al-Khatib, S.M.; Russo, A.M.; et al. Sex Differences in Procedural Outcomes Among Patients Undergoing Left Atrial Appendage Occlusion: Insights from the NCDR LAAO Registry. JAMA Cardiol. 2021, 6, 1275–1284. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Jin, Q.; Kong, D.; Zhang, Y.; Li, M.; Zhang, L.; Chen, S.; Pan, W.; Zhou, D.; et al. Pericardial Effusion During the Perioperative Period for Left Atrial Appendage Closure. Front. Cardiovasc. Med. 2021, 8, 678460. [Google Scholar] [CrossRef]
- Munir, M.B.; Khan, M.Z.; Darden, D.; Pasupula, D.K.; Balla, S.; Han, F.T.; Reeves, R.; Hsu, J.C. Pericardial Effusion Requiring Intervention in Patients Undergoing Percutaneous Left Atrial Appendage Occlusion: Prevalence, Predictors, and Associated in-Hospital Adverse Events from 17,700 Procedures in the United States. Heart Rhythm 2021, 18, 1508–1515. [Google Scholar] [CrossRef]
- Michowitz, Y.; Rahkovich, M.; Oral, H.; Zado, E.S.; Tilz, R.; John, S.; Denis, A.; Di Biase, L.; Winkle, R.A.; Mikhaylov, E.N.; et al. Effects of Sex on the Incidence of Cardiac Tamponade after Catheter Ablation of Atrial Fibrillation Results from a Worldwide Survey in 34 943 Atrial Fibrillation Ablation Procedures. Circ. Arrhythmia Electrophysiol. 2014, 7, 274–280. [Google Scholar] [CrossRef]
- Cheng, X.; Hu, Q.; Gao, L.; Liu, J.; Qin, S.; Zhang, D. Sex-Related Differences in Catheter Ablation of Atrial Fibrillation: A Systematic Review and Meta-Analysis. Europace 2019, 21, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, R.; Kosiński, A.; Brala, M.; Piwko, G.; Lewicka, E.; Dąbrowska-Kugacka, A.; Raczak, G.; Kozłowski, D.; Grzybiak, M. Variability of the Left Atrial Appendage in Human Hearts. PLoS ONE 2015, 10, e0141901. [Google Scholar] [CrossRef] [PubMed]
- Akoum, N.; Mahnkopf, C.; Kholmovski, E.G.; Brachmann, J.; Marrouche, N.F. Age and Sex Differences in Atrial Fibrosis among Patients with Atrial Fibrillation. EP Eur. 2018, 20, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Abusnina, W.; Latif, A.; Al-abdouh, A.; Mostafa, M.R.; Radaideh, Q.; Alshebani, Y.; Aboeata, A.; Ben-Dor, I.; Michos, E.D.; Dahal, K. Sex Differences in the Clinical Outcomes After Left Atrial Appendage Closure: A Systematic Review and Meta-Analysis. Cardiovasc. Revasc. Med. 2022, 41, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Vlastra, W.; Chandrasekhar, J.; García Del Blanco, B.; Tchétché, D.; de Brito, F.S.; Barbanti, M.; Kornowski, R.; Latib, A.; D’Onofrio, A.; Ribichini, F.; et al. Sex Differences in Transfemoral Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 74, 2758–2767. [Google Scholar] [CrossRef]
- Lansky, A.; Baron, S.J.; Grines, C.L.; Tremmel, J.A.; Al-Lamee, R.; Angiolillo, D.J.; Chieffo, A.; Croce, K.; Jacobs, A.K.; Madan, M.; et al. SCAI Expert Consensus Statement on Sex-Specific Considerations in Myocardial Revascularization. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100016. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Gibson, D.N.; Kar, S.; O’Neill, W.; Doshi, S.K.; Horton, R.P.; Buchbinder, M.; Gordon, N.T.; Holmes, D.R. Post-Approval U.S. Experience with Left Atrial Appendage Closure for Stroke Prevention in Atrial Fibrillation. J. Am. Coll. Cardiol. 2017, 69, 253–261. [Google Scholar] [CrossRef]
- Tzikas, A.; Shakir, S.; Gafoor, S.; Omran, H.; Berti, S.; Santoro, G.; Kefer, J.; Landmesser, U.; Nielsen-Kudsk, J.E.; Cruz-Gonzalez, I.; et al. Left Atrial Appendage Occlusion for Stroke Prevention in Atrial Fibrillation: Multicentre Experience with the AMPLATZER Cardiac Plug. EuroIntervention 2016, 11, 1170–1179. [Google Scholar] [CrossRef]
- Turagam, M.K.; Osmancik, P.; Neuzil, P.; Dukkipati, S.R.; Reddy, V.Y. Left Atrial Appendage Closure Versus Oral Anticoagulants in Atrial Fibrillation: A Meta-Analysis of Randomized Trials. J. Am. Coll. Cardiol. 2020, 76, 2795–2797. [Google Scholar] [CrossRef]
- Cabin, H.S.; Clubb, K.S.; Hall, C.; Perlmutter, R.A.; Feinstein, A.R. Risk for Systemic Embolization of Atrial Fibrillation without Mitral Stenosis. Am. J. Cardiol. 1990, 65, 1112–1116. [Google Scholar] [CrossRef]
- Lan, D.H.; Jiang, C.; Du, X.; He, L.; Guo, X.Y.; Zuo, S.; Xia, S.J.; Chang, S.S.; Wen, S.N.; Wu, J.H.; et al. Female Sex as a Risk Factor for Ischemic Stroke and Systemic Embolism in Chinese Patients with Atrial Fibrillation: A Report from the China-AF Study. J. Am. Heart Assoc. 2018, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Boersma, L.V.; Ince, H.; Kische, S.; Pokushalov, E.; Schmitz, T.; Schmidt, B.; Gori, T.; Meincke, F.; Protopopov, A.V.; Betts, T.; et al. Efficacy and Safety of Left Atrial Appendage Closure with WATCHMAN in Patients with or without Contraindication to Oral Anticoagulation: 1-Year Follow-up Outcome Data of the EWOLUTION Trial. Heart Rhythm 2017, 14, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Tsuboko, Y.; Iwasaki, K. Latest Outcomes of Transcatheter Left Atrial Appendage Closure Devices and Direct Oral Anticoagulant Therapy in Patients with Atrial Fibrillation over the Past 5 Years: A Systematic Review and Meta-Analysis. Cardiovasc. Interv. Ther. 2022, 37, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Pedro Luis Cepas-Guillén, M.D.; Boris Schmidt, M.D.; Sergio Berti, M.D.; Sven Fischer, M.D.; Kai Magnusson, M.D.; Carsten Skurk, M.D.; Zeus, T.; Freixa, X. Left Atrial Appendage Occlusion in Nonagenarians. J. Invasive Cardiol. 2022, 34, E296–E298. [Google Scholar]
- Piayda, K.; Afzal, S.; Nielsen-Kudsk, J.E.; Schmidt, B.; Mazzone, P.; Berti, S.; Fischer, S.; Lund, J.; Montorfano, M.; Hildick-Smith, D.; et al. Length of Stay Following Percutaneous Left Atrial Appendage Occlusion: Data from the Prospective, Multicenter Amplatzer Amulet Occluder Observational Study. PLoS ONE 2021, 16, e0255721. [Google Scholar] [CrossRef]
- Tan, B.E.X.; Boppana, L.K.T.; Abdullah, A.S.; Chuprun, D.; Shah, A.; Rao, M.; Bhatt, D.L.; Depta, J.P. Safety and Feasibility of Same-Day Discharge after Left Atrial Appendage Closure with the WATCHMAN Device. Circ. Cardiovasc. Interv. 2021, 14, 73–83. [Google Scholar] [CrossRef]
- Sanjoy, S.S.; Choi, Y.H.; Sparrow, R.T.; Jneid, H.; Abbott, J.D.; Nombela-Franco, L.; Azzalini, L.; Holmes, D.R.; Alraies, M.C.; Elgendy, I.Y.; et al. Outcomes of Elderly Patients Undergoing Left Atrial Appendage Closure. J. Am. Heart Assoc. 2021, 10, 21973. [Google Scholar] [CrossRef]
- Badheka, A.O.; Chothani, A.; Mehta, K.; Patel, N.J.; Deshmukh, A.; Hoosien, M.; Shah, N.; Singh, V.; Grover, P.; Savani, G.T.; et al. Utilization and Adverse Outcomes of Percutaneous Left Atrial Appendage Closure for Stroke Prevention in Atrial Fibrillation in the United States. Circ. Arrhythmia Electrophysiol. 2015, 8, 42–48. [Google Scholar] [CrossRef]
- Freeman, J.V.; Varosy, P.; Price, M.J.; Slotwiner, D.; Kusumoto, F.M.; Rammohan, C.; Kavinsky, C.J.; Turi, Z.G.; Akar, J.; Koutras, C.; et al. The NCDR Left Atrial Appendage Occlusion Registry. J. Am. Coll. Cardiol. 2020, 75, 1503. [Google Scholar] [CrossRef]


| Characteristics | Value |
|---|---|
Year of LAAC
| 1026 (9.1) 2054 (18.3) 3287 (29.2) 4873 (43.4) |
| 77 [71–82] 834 (7.4) 3572 (31.8) 6834 (60.8) |
Sex
| 4706 (41.9) 6534 (58.1) |
Ethnicity
| 9840 (87.5) 557 (5.0) 460(4.1) 157 (1.4) 38 (0.3) 188 (1.7) |
Hospital size
| 7531 (67.0) 2578 (22.9) 1131 (10.1) |
Insurance
| 10,100 (89.9) 910 (8.1) 52 (0.5) 178 (1.6) |
CCI
| 2.00 [1.0–3.0] 2511 (22.3) 8729 (77.7) |
| Heart failure | 3835 (34.1) |
| Renal failure | 2730 (24.3) |
| Cardiovascular risk burden | 4 [3–5] |
Cardiovascular risk factors
| 6427 (57.2) 4435 (39.5) 9715 (86.4) 6764 (60.2) 4078 (36.3) 1402 (12.5) 125 (1.1) 4078 (36.3) 3878 (34.5) |
Hospital region
| 350 (3.1) 1551 (13.8) 1606 (14.3) 759 (6.8) 2444 (21.7) 617 (5.5%) 1443 (12.8) 1081 (9.6) 1389 (12.4) |
| CHA2DS2-VASc score | 4 [3–4] |
| Simplified HAS-BLED score | 2 [2–3] |
Hospital location and teaching status
| 212 (1.9) 1057 (9.4) 9971 (88.7) |
| Characteristics | Stroke (n = 54) | Systemic Embolism (n = 9) | Pericardial Effusion (n = 85) | Major Bleeding (n = 608) | In-Hospital Mortality (n = 16) |
|---|---|---|---|---|---|
| Year of LAAC | |||||
| 1 | 1 | 1 | 1 | 1 |
| 0.70 (0.27–1.89) | 0.21 (0.03–1.11) | 2.19 (0.89–6.59) | 1.32 (0.95–1.86) | 0.49 (0.09–2.71) |
| 0.58 (0.24–1.51) | 0.05 (0.00–0.38) | 1.34 (0.55–4.02) | 1.09 (0.80–1.52) | 0.29 (0.05–1.59) |
| 0.56 (0.25–1.39) | 0.03 (0.00–0.24) | 1.28 (0.54–3.78) | 0.99 (0.73–1.36) | 0.47 (0.13–2.24) |
| Age groups | |||||
| 1 | 1 | 1 | 1 | 1 |
| 0.74 (0.39–1.35) | 0.31 (0.02–1.80) | 0.76 (0.46–1.24) | 0.93 (0.77–1.12) | 0.52 (0.12–1.66) |
| 0.37 (0.06–1.33) | 1.21 (0.03–15.2) | 0.52 (0.15–1.40) | 0.86 (0.60–1.20) | 0.73 (0.04–4.09) |
Sex
| 1 1.36 (0.78–2.37) | 1 6.00 (1.28–43.6) | 1 3.86 (2.41–6.39) | 1 0.99 (0.83–1.17) | 1 2.57 (0.92–7.80) |
| Insurance | |||||
| 1 | 1 | 1 | 1 | 1 |
| 1.03 (0.28-2.84) | 1.91 (0.06-22.5) | 1.81 (0.79-3.67) | 1.26 (0.92-1.70) | - |
| - | - | - | 0.36 (0.02-1.65) | - |
| - | - | 1.26 (0.07-5.94) | 0.92 (0.43-1.73) | - |
| Ethnicity | |||||
| 1 | 1 | 1 | 1 | 1 |
| 0.70 (0.11–2.30) | - | 0.66 (0.16–1.79) | 0.85 (0.55–1.25) | - |
| 0.39 (0.02–1.83) | - | 0.96(0.29–2.36) | 1.34 (0.92–1.91) | - |
| 0.97 (0.05–5.20) | - | 0.82 (0.05–3.85) | 1.19 (0.58–2.17) | 3.96 (0.21–21.7) |
| - | - | - | 2.07 (0.61–5.26) | |
| 0.91 (0.05–4.34) | - | 2.82 (0.84–7.03) | 1.19 (0.62–2.06) | 3.32 (0.18–18.0) |
| Hospital size | |||||
| 1 | 1 | 1 | 1 | 1 |
| 1.13 (0.57–2.11) | 1.69 (0.33–7.18) | 1.02 (0.59–1.70) | 1.00 (0.81–1.21) | 0.44 (0.07–1.63) |
| 0.85 (0.29–2.03) | - | 1.42 (0.70–2.64) | 0.86 (0.63–1.14) | 0.53 (0.03–2,75) |
| CCI | |||||
| 1 | 1 | 1 | 1 | 1 |
| - | 0.75 (0.05–19.3) | 0.84 (0.39–1.79) | 1.05 (0.79–1.38) | 1.45 (0.05–38.4) |
| - | 0.86 (0.05–25.2) | 0.99 (0.44–2.21) | 1.22 (0.91–1.65) | 4.66 (0.38–109) |
| - | 0.20 (0.00–12.2) | 0.81 (0.29–2.16) | 1.58 (1.11–2.23) | 15.9 (1.45–376) |
| - | - | 0.59 (0.18–1.83) | 1.86 (1.25–2.76) | 18.6 (1.37–482) |
| - | 0.15(0.00–18.8) | 0.55 (0.14–1.99) | 1.82 (1.12–2.91) | 19.2 (0.97–591) |
| - | - | 0.82 (0.20–3.10) | 2.98 (1.78–4.92) | 32.8(1.39–1,144) |
| - | - | 0.63 (0.08–3.29) | 3.18 (1.68–5.83) | 36.0(0.82–1,498) |
| - | - | 4.58 (0.79–20.8) | 1.46 (0.34–4.28) | - |
| - | - | - | 3.69 (0.85–11.2) | - |
| - | - | - | 21.2 (4.01–101) | - |
| - | 1035 (-) | - | 9.87 (1.36–48.5) | - |
| - | 0.03 (-) | - | - | - |
| - | - | - | 10.1 (0.49–82.5) | - |
| - | - | - | - | - |
| - | - | - | - | - |
| - | - | - | - | - |
| - | 1.23 (-) | - | - | - |
| Heart failure | 0.76 (0.42–1.35) | 6.30 (1.07–54.0) | 1.64 (0.97–2.80) | 1.02 (0.84–1.25) | 1.31 (0.43–4.47) |
| Renal failure | 0.31 (0.14–0.66) | 5.06 (0.28–66.9) | 2.23 (1.10–4.52) | 0.83 (0.64–1.09) | 1.17 (0.32–4.76) |
| Cardiovascular | |||||
| risk burden | |||||
| 1 | 1 | 1 | 1 | 1 |
| 0.16 (0.01–3.80) | - | 0.32 (0.04–6.68) | 1.02 (0.28–6.53) | - |
| 0.04 (0.00–0.89) | - | 0.16 (0.02–3.37) | 0.85 (0.24–5.36) | - |
| 0.04 (0.00–0.95) | - | 0.16 (0.02–3.38) | 0.83 (0.24–5.27) | - |
| 0.03 (0.00–0.83) | - | 0.27 (0.03–5.51) | 0.77 (0.22–4.87) | - |
| 0.02 (0.00–0.57) | 3.83 (-) | 0.25 (0.03–5.29) | 0.68 (0.19–4.87) | - |
| 0.01 (0.00–0.32) | - | 0.24 (0.03–5.32) | 0.60 (0.16–3.84) | - |
| 0.04 (0.00–1.10) | 0.90 (-) | 0.00 (0–429) | 0.45 (0.11–3.11) | - |
| - | 163 (-) | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thevathasan, T.; Degbeon, S.; Paul, J.; Wendelburg, D.-K.; Füreder, L.; Gaul, A.L.; Scheitz, J.F.; Stadler, G.; Rroku, A.; Lech, S.; et al. Safety and Healthcare Resource Utilization in Patients Undergoing Left Atrial Appendage Closure—A Nationwide Analysis. J. Clin. Med. 2023, 12, 4573. https://doi.org/10.3390/jcm12144573
Thevathasan T, Degbeon S, Paul J, Wendelburg D-K, Füreder L, Gaul AL, Scheitz JF, Stadler G, Rroku A, Lech S, et al. Safety and Healthcare Resource Utilization in Patients Undergoing Left Atrial Appendage Closure—A Nationwide Analysis. Journal of Clinical Medicine. 2023; 12(14):4573. https://doi.org/10.3390/jcm12144573
Chicago/Turabian StyleThevathasan, Tharusan, Sêhnou Degbeon, Julia Paul, Darius-Konstantin Wendelburg, Lisa Füreder, Anna Leonie Gaul, Jan F. Scheitz, Gertraud Stadler, Andi Rroku, Sonia Lech, and et al. 2023. "Safety and Healthcare Resource Utilization in Patients Undergoing Left Atrial Appendage Closure—A Nationwide Analysis" Journal of Clinical Medicine 12, no. 14: 4573. https://doi.org/10.3390/jcm12144573
APA StyleThevathasan, T., Degbeon, S., Paul, J., Wendelburg, D.-K., Füreder, L., Gaul, A. L., Scheitz, J. F., Stadler, G., Rroku, A., Lech, S., Buspavanich, P., Huemer, M., Attanasio, P., Nagel, P., Reinthaler, M., Landmesser, U., & Skurk, C. (2023). Safety and Healthcare Resource Utilization in Patients Undergoing Left Atrial Appendage Closure—A Nationwide Analysis. Journal of Clinical Medicine, 12(14), 4573. https://doi.org/10.3390/jcm12144573






