Evolution of National Guidelines on Medicines Used to Treat COVID-19 in Pregnancy in 2020–2022: A Scoping Review
Abstract
1. Introduction
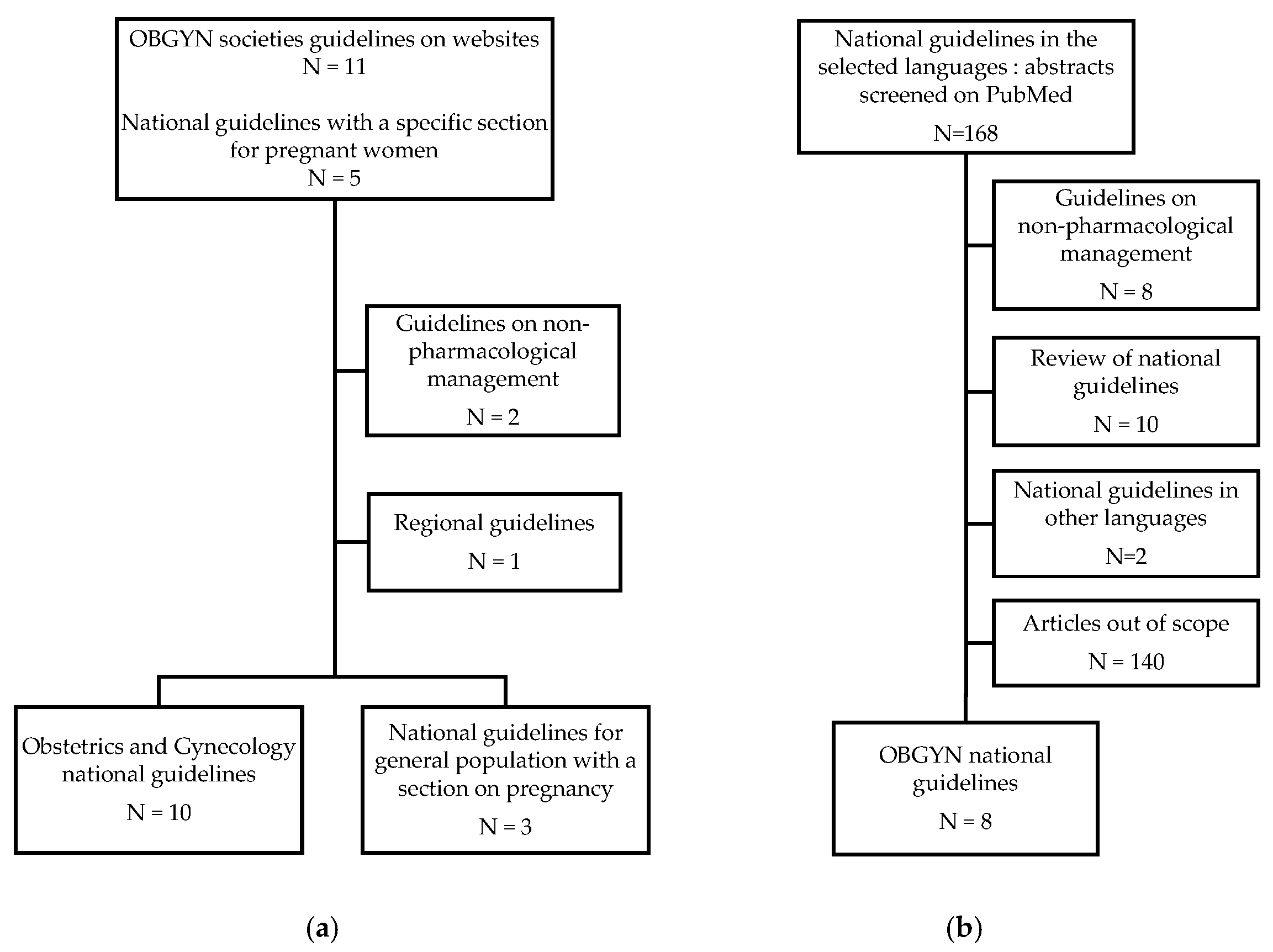
2. Materials and Methods
2.1. Selection Criteria
2.2. Documents of Interest and Research Process
2.3. Outcomes of Interest
2.4. Recommendation Grading
3. Results
3.1. Antibiotics
3.2. Antimalarials
3.3. Antivirals
- Remdesivir
- Lopinavir–ritonavir
- Nirmatrelvir–ritonavir
3.4. Corticosteroids
3.5. Tocilizumab
3.6. Anti-SARS-CoV-2 Monoclonal Antibodies
3.7. Convalescent Plasma
3.8. Intravenous Immunoglobulins
3.9. Thromboprophylactic Agents
| Medicine | Year | 2020 | 2021 | 2022 | Ref | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | March | April | May | June | July | August | September | October | November | December | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | ||
| Azithromycin | Australia | [32] | ||||||||||||||||||
| Belgium | [71] | |||||||||||||||||||
| Canada | ||||||||||||||||||||
| France | [26] | |||||||||||||||||||
| India | [72] | |||||||||||||||||||
| Italy | § | [21] | ||||||||||||||||||
| Norway | [37] | |||||||||||||||||||
| S. Arabia | ||||||||||||||||||||
| Spain | ||||||||||||||||||||
| Switzerland | ||||||||||||||||||||
| The Netherlands | [38] | |||||||||||||||||||
| UK | [45] | |||||||||||||||||||
| USA | [40] | |||||||||||||||||||
| WHO | ||||||||||||||||||||
| Hydroxychloroquine | Australia | [32] | ||||||||||||||||||
| Belgium | [73] | |||||||||||||||||||
| Canada | ||||||||||||||||||||
| France | [26] | |||||||||||||||||||
| India | [36] | |||||||||||||||||||
| Italy | [47] | |||||||||||||||||||
| Norway | [37] | |||||||||||||||||||
| S. Arabia | [34] | |||||||||||||||||||
| Spain | ||||||||||||||||||||
| Switzerland | ||||||||||||||||||||
| The Netherlands | [38] | |||||||||||||||||||
| UK | [45] | |||||||||||||||||||
| USA | [74] | |||||||||||||||||||
| WHO | [75] | |||||||||||||||||||
| Remdesivir | Australia | [32] | ||||||||||||||||||
| Belgium | [76] | |||||||||||||||||||
| Canada | ||||||||||||||||||||
| France | [26] | |||||||||||||||||||
| India | [72] | |||||||||||||||||||
| Italy | [21] | |||||||||||||||||||
| Norway | † | [37] | ||||||||||||||||||
| S. Arabia | [17] | |||||||||||||||||||
| Spain | ||||||||||||||||||||
| Switzerland | ||||||||||||||||||||
| The Netherlands | †† | [38] | ||||||||||||||||||
| UK | [45] | |||||||||||||||||||
| USA | [74] | |||||||||||||||||||
| WHO | ††† | [77] | ||||||||||||||||||
| Lopinavir-Ritonavir | Australia | [32] | ||||||||||||||||||
| Belgium | [76] | |||||||||||||||||||
| Canada | ||||||||||||||||||||
| France | [26] | |||||||||||||||||||
| India | [36] | |||||||||||||||||||
| Italy | [21] | |||||||||||||||||||
| Norway | † | [37] | ||||||||||||||||||
| S. Arabia | [17] | |||||||||||||||||||
| Spain | ||||||||||||||||||||
| Switzerland | ||||||||||||||||||||
| The Netherlands | [38] | |||||||||||||||||||
| UK | [45] | |||||||||||||||||||
| USA | [64] | |||||||||||||||||||
| WHO | [75] | |||||||||||||||||||
| Nirmatrelvir-Ritonavir | Australia | [32] | ||||||||||||||||||
| Belgium | [78] | |||||||||||||||||||
| Canada | ||||||||||||||||||||
| France | ||||||||||||||||||||
| India | ||||||||||||||||||||
| Italy | [21] | |||||||||||||||||||
| Norway | [37] | |||||||||||||||||||
| S. Arabia | ||||||||||||||||||||
| Spain | ||||||||||||||||||||
| Switzerland | ||||||||||||||||||||
| The Netherlands | [38] | |||||||||||||||||||
| UK | [39] | |||||||||||||||||||
| USA | [64] | |||||||||||||||||||
| WHO | [75] | |||||||||||||||||||
| Corticosteroids (systemic) | Australia | * | [32] | |||||||||||||||||
| Belgium | * αβ | χ | [79] | |||||||||||||||||
| Canada | * | [80] | ||||||||||||||||||
| France | * | [26] | ||||||||||||||||||
| India | * αβ | χ | [72] | |||||||||||||||||
| Italy | * αβ | φ | [21] | |||||||||||||||||
| Norway | * | λ | [37] | |||||||||||||||||
| S. Arabia | * δε | [17] | ||||||||||||||||||
| Spain | * γ | [27] | ||||||||||||||||||
| Switzerland | ||||||||||||||||||||
| The Netherlands | * | ζ | [38] | |||||||||||||||||
| UK | * δε | [81] | ||||||||||||||||||
| USA | η | * | [64] | |||||||||||||||||
| WHO | * | δεζ | [75] | |||||||||||||||||
| Tocilizumab | Australia | [32] | ||||||||||||||||||
| Belgium | [79] | |||||||||||||||||||
| Canada | ||||||||||||||||||||
| France | [26] | |||||||||||||||||||
| India | [72] | |||||||||||||||||||
| Italy | [21] | |||||||||||||||||||
| Norway | † | [37] | ||||||||||||||||||
| S. Arabia | ||||||||||||||||||||
| Spain | ||||||||||||||||||||
| Switzerland | ||||||||||||||||||||
| The Netherlands | ± | [38] | ||||||||||||||||||
| UK | [45] | |||||||||||||||||||
| USA | [64] | |||||||||||||||||||
| WHO | [77] | |||||||||||||||||||
| Anti-IL 1 | Australia | [32] | ||||||||||||||||||
| Belgium | ||||||||||||||||||||
| Canada | ||||||||||||||||||||
| France | ||||||||||||||||||||
| India | ||||||||||||||||||||
| Italy | [21] | |||||||||||||||||||
| Norway | ||||||||||||||||||||
| S. Arabia | ||||||||||||||||||||
| Spain | ||||||||||||||||||||
| Switzerland | ||||||||||||||||||||
| The Netherlands | [38] | |||||||||||||||||||
| UK | ||||||||||||||||||||
| USA | [64] | |||||||||||||||||||
| WHO | ||||||||||||||||||||
| Anti-SARS-CoV-2 monoclonal antibodies | Australia | Σ | $ | [32] | ||||||||||||||||
| Belgium | [79] | |||||||||||||||||||
| Canada | ||||||||||||||||||||
| France | [26] | |||||||||||||||||||
| India | [72] | |||||||||||||||||||
| Italy | ≈ | [21] | ||||||||||||||||||
| Norway | † | [37] | ||||||||||||||||||
| S. Arabia | ||||||||||||||||||||
| Spain | ||||||||||||||||||||
| Switzerland | Σ | [66] | ||||||||||||||||||
| The Netherlands | [38] | |||||||||||||||||||
| UK | ∞ | ∞ | [45] | |||||||||||||||||
| USA | [64] | |||||||||||||||||||
| WHO | [77] | |||||||||||||||||||
| Convalescent plasma | Australia | [32] | ||||||||||||||||||
| Belgium | ||||||||||||||||||||
| Canada | ||||||||||||||||||||
| France | [26] | |||||||||||||||||||
| India | [72] | |||||||||||||||||||
| Italy | ||||||||||||||||||||
| Norway | [37] | |||||||||||||||||||
| S. Arabia | [17] | |||||||||||||||||||
| Spain | ||||||||||||||||||||
| Switzerland | ||||||||||||||||||||
| The Netherlands | [38] | |||||||||||||||||||
| UK | [45] | |||||||||||||||||||
| USA | √ | [64] | ||||||||||||||||||
| WHO | [77] | |||||||||||||||||||
| IVIg | Australia | [32] | ||||||||||||||||||
| Belgium | ||||||||||||||||||||
| Canada | ||||||||||||||||||||
| France | ||||||||||||||||||||
| India | ||||||||||||||||||||
| Italy | ||||||||||||||||||||
| Norway | [37] | |||||||||||||||||||
| S. Arabia | [34] | |||||||||||||||||||
| Spain | ||||||||||||||||||||
| Switzerland | ||||||||||||||||||||
| The Netherlands | [23] | |||||||||||||||||||
| UK | ||||||||||||||||||||
| USA | [64] | |||||||||||||||||||
| WHO | ||||||||||||||||||||
| LMWH | Australia | ** & | ** | [32] | ||||||||||||||||
| Belgium | * | ** | [70] | |||||||||||||||||
| Canada | ||||||||||||||||||||
| France | & | [26] | ||||||||||||||||||
| India | [72] | |||||||||||||||||||
| Italy | [21] | |||||||||||||||||||
| Norway | [37] | |||||||||||||||||||
| S. Arabia | ||||||||||||||||||||
| Spain | [27] | |||||||||||||||||||
| Switzerland | ** | [28] | ||||||||||||||||||
| The Netherlands | [23] | |||||||||||||||||||
| UK | ** | [81] | ||||||||||||||||||
| USA | ** | [64] | ||||||||||||||||||
| WHO | ** | [75] | ||||||||||||||||||
| Color code | Strong recommendation for | Only in research settings | ||||||||||||||||||
| “Consider” or “can be offered” | Conditional recommendation against | |||||||||||||||||||
| “Under study/assessment” or unknown | Compassionate use | |||||||||||||||||||
| Conditional recommendation | Strong recommendation against | |||||||||||||||||||
4. Discussion
4.1. Main Findings
4.2. Difficulties Encountered in Developing Guidelines to Treat COVID-19 in Pregnant Women
4.3. Response through Constantly Updated Online Treatment Guidelines
4.4. Strength
4.5. Limitations
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamilton, S.; Goldman, I.; van der Vos, K.; Yu, S. Two years of pandemic: A wealth of data and many remaining questions. Cell Rep. Med. 2022, 3, 100581. [Google Scholar] [CrossRef]
- Recovery-Collaborative-Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Recovery-Collaborative-Group; Horby, P.; Mafham, M.; Linsell, L.; Bell, J.L.; Staplin, N.; Emberson, J.R.; Wiselka, M.; Ustianowski, A.; Elmahi, E.; et al. Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2020, 383, 2030–2040. [Google Scholar] [CrossRef] [PubMed]
- Recovery-Collaborative-Group. Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Recovery-Collaborative-Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- Recovery-Collaborative-Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2020, 396, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Investigators Remap-Cap; Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Annane, D.; Beane, A.; van Bentum-Puijk, W.; et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [CrossRef]
- WHO Solidarity Trial Consortium; Pan, H.; Peto, R.; Henao-Restrepo, A.M.; Preziosi, M.P.; Sathiyamoorthy, V.; Abdool Karim, Q.; Alejandria, M.M.; Hernandez Garcia, C.; Kieny, M.P.; et al. Repurposed Antiviral Drugs for COVID-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The COVID-NMA Initiative—A Living Mapping and Living Systematic Review of COVID-19 Trials. Available online: https://www.covid-nma.com/dataviz/ (accessed on 15 April 2023).
- Taylor, M.M.; Kobeissi, L.; Kim, C.; Amin, A.; Thorson, A.E.; Bellare, N.B.; Brizuela, V.; Bonet, M.; Kara, E.; Thwin, S.S.; et al. Inclusion of pregnant women in COVID-19 treatment trials: A review and global call to action. Lancet Glob. Health 2021, 9, e366–e371. [Google Scholar] [CrossRef]
- Dashraath, P.; Nielsen-Saines, K.; Madhi, S.A.; Baud, D. COVID-19 vaccines and neglected pregnancy. Lancet 2020, 396, e22. [Google Scholar] [CrossRef]
- Krubiner, C.B.; Faden, R.R.; Karron, R.A.; Little, M.O.; Lyerly, A.D.; Abramson, J.S.; Beigi, R.H.; Cravioto, A.R.; Durbin, A.P.; Gellin, B.G.; et al. Pregnant women & vaccines against emerging epidemic threats: Ethics guidance for preparedness, research, and response. Vaccine 2021, 39, 85–120. [Google Scholar] [CrossRef] [PubMed]
- Badr, D.A.; Mattern, J.; Carlin, A.; Cordier, A.G.; Maillart, E.; El Hachem, L.; El Kenz, H.; Andronikof, M.; De Bels, D.; Damoisel, C.; et al. Are clinical outcomes worse for pregnant women at >/=20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case-control study with propensity score matching. Am. J. Obstet. Gynecol. 2020, 223, 764–768. [Google Scholar] [CrossRef]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef] [PubMed]
- DeSisto, C.L.; Wallace, B.; Simeone, R.M.; Polen, K.; Ko, J.Y.; Meaney-Delman, D.; Ellington, S.R. Risk for Stillbirth Among Women with and without COVID-19 at Delivery Hospitalization—United States, March 2020–September 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1640–1645. [Google Scholar] [CrossRef]
- Kowalski, S.C.; Morgan, R.L.; Falavigna, M.; Florez, I.D.; Etxeandia-Ikobaltzeta, I.; Wiercioch, W.; Zhang, Y.; Sakhia, F.; Ivanova, L.; Santesso, N.; et al. Development of rapid guidelines: 1. Systematic survey of current practices and methods. Health Res. Policy Syst. 2018, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Saudi Ministry of Health. COVID-19 in Pregnancy, Rapid Response Guidelines Version 1.1, Saudi Ministry of Health. Available online: https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Documents/COVID-19-IN-PREGNANCY-MOH-Protocol.pdf (accessed on 15 April 2023).
- Elwood, E.; Raeside, A.; Watson, H.; Boucoiran, I.; Money, D.; Yudin, M.; VanSchalkwyk, J.; Castillo, E.; Poliquin, V. Committee Opinion No. 400: COVID-19 and Pregnancy. (Original: March 13th, 2020 Updated: July 27th, 2020). Available online: https://www.sogc.org/common/Uploaded%20files/Media%20Updates/EN_Statement-COVID_Pregnancy.pdf (accessed on 15 April 2023).
- Royal College of Obstetricians and Gynaecologists. Coronavirus (COVID-19) Infection in Pregnancy—Information for Healthcare Professionals Version 10: Published Thursday 4 June 2020. Available online: https://www.rcm.org.uk/media/4113/2020-06-04-coronavirus-covid-19-infection-in-pregnancy.pdf (accessed on 15 April 2023).
- Society for Maternal Fetal Medicine (SMFM). Society for Maternal-Fetal Medicine—Management Considerations for Pregnant Patients with COVID-19—Developed with Guidance from Torre Halscott, MD, MS.; Jason Vaught, MD; and the SMFM COVID-19 Task Force. Available online: SMFM_COVID_Management_of_COVID_pos_preg_patients_2-2-21_(final) (accessed on 15 April 2023).
- Agencia Italiana del Farmaco (AIFA). Medicines Usable for Treatment of COVID-19 Disease—AIFA (Italian Medicines Agency). Available online: https://www.aifa.gov.it/web/guest/aggiornamento-sui-farmaci-utilizzabili-per-il-trattamento-della-malattia-covid19 (accessed on 15 April 2023).
- Vogel, J.P.; Tendal, B.; Giles, M.; Whitehead, C.; Burton, W.; Chakraborty, S.; Cheyne, S.; Downton, T.; Fraile Navarro, D.; Gleeson, G.; et al. Clinical care of pregnant and postpartum women with COVID-19: Living recommendations from the National COVID-19 Clinical Evidence Taskforce. Aust. New Zealand J. Obstet. Gynaecol. 2020, 60, 840–851. [Google Scholar] [CrossRef]
- Federatie Medisch Specialisten (FMS); Nederlandse Vereniging voor Obstetrie and Gynaecologie (NVOG). Standpunt COVID-19 en Zwangerschap, Bevalling en Kraambed—23 April 2021. Available online: https://www.nvog.nl/wp-content/uploads/2021/04/standpunt-COVID-19-en-zwangerschap-en-bevalling-versie-23-april-2021-2.pdf (accessed on 15 April 2023).
- Agence Fédérale des Médicaments et des Produits de Santé (AFMPS-FAGG); Sciensano. Interim Clinical Guidance for Adults with Suspected or Confirmed COVID-19 in Belgium. Available online: https://COVID-19.sciensano.be/sites/default/files/Covid19/COVID-19_InterimGuidelines_Treatment_ENG.pdf (accessed on 15 April 2023).
- Peyronnet, V.; Sibiude, J.; Deruelle, P.; Huissoud, C.; Lescure, X.; Lucet, J.C.; Mandelbrot, L.; Nisand, I.; Vayssiere, C.; Yazpandanah, Y.; et al. SARS-CoV-2 infection during pregnancy. Information and proposal of management care. CNGOF. Gynecol. Obstet. Fertil. Senol. 2020, 48, 436–443. [Google Scholar] [CrossRef]
- Peyronnet, V.; Sibiude, J.; Huissoud, C.; Lescure, F.X.; Lucet, J.C.; Mandelbrot, L.; Nisand, I.; Belaish-Allart, J.; Vayssiere, C.; Yazpandanah, Y.; et al. Infection with SARS-CoV-2 in pregnancy. Update of Information and proposed care. CNGOF. Gynecol. Obstet. Fertil. Senol. 2020, 48, 858–870. [Google Scholar] [CrossRef]
- Spanish Ministry of Health. Manejo de la Mujer Embarazada y el Recién Nacido con COVID-19—Version de 17 Junio de 2020. Available online: https://www.COVID-19.seth.es/wp-content/uploads/2020/06/2020-06-17_Documento-manejo-embarazo-y-recien-nacido-COVID19.pdf (accessed on 15 April 2023).
- Swiss Society in Gynecology & Obstetrics. Recommandation of the Swiss Society of Gynecologists and Obstetricians on COVID-19 mRNA and Pregnancy, Lettre d’experts SSGO gynécologie suisse: Infection à coronavirus COVID-19, Grossesse et accouchement (Etat: 05.08.2020). Available online: https://www.sggg.ch/fileadmin/user_upload/Dokumente/1_Ueber_uns/Empfehlung_Coronavirusinfektion_COVID-19_05.08.2020_FR.pdf (accessed on 15 April 2023).
- Norsk Gynekologisk Forening. Koronavirus ved Svangerskap og Fødsel—Norsk Gynekologisk Forening—Versjon 5. Available online: https://www.legeforeningen.no/foreningsledd/fagmed/norsk-gynekologisk-forening/veiledere/koronavirus-ved-svangerskap-og-fodsel/ (accessed on 15 April 2023).
- Girolamo, R.D.; Khalil, A.; Rizzo, G.; Capannolo, G.; Buca, D.; Liberati, M.; Acharya, G.; Odibo, A.O.; D’Antonio, F. Systematic review and critical evaluation of quality of clinical practice guidelines on the management of SARS-CoV-2 infection in pregnancy. Am. J. Obstet. Gynecol. MFM 2022, 4, 100654. [Google Scholar] [CrossRef]
- Akinosoglou, K.; Schinas, G.; Rigopoulos, E.A.; Polyzou, E.; Tzouvelekis, A.; Adonakis, G.; Gogos, C. COVID-19 Pharmacotherapy in Pregnancy: A Literature Review of Current Therapeutic Choices. Viruses 2023, 15, 787. [Google Scholar] [CrossRef]
- National COVID-19 Clinical Evidence Taskforce. Australian Guidelines for the Clinical Care of People with COVID-19. Available online: https://app.magicapp.org/#/guideline/L4Q5An (accessed on 15 April 2023).
- World Health Organization. Therapeutics and COVID-19—Living Guideline 24 September 2021—World Health Organization. Available online: https://apps.who.int/iris/handle/10665/345356 (accessed on 15 April 2023).
- Faden, Y.A.; Alghilan, N.A.; Alawami, S.H.; Alsulmi, E.S.; Alsum, H.A.; Katib, Y.A.; Sabr, Y.S.; Tahir, F.H.; Bondagji, N.S. Saudi Society of Maternal-Fetal Medicine guidance on pregnancy and coronavirus disease 2019. Saudi Med. J. 2020, 41, 779–790. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health; COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health—Guidelines Archive. Available online: https://www.covid19treatmentguidelines.nih.gov/about-the-guidelines/guidelines-archive/ (accessed on 15 April 2023).
- Federation of Obstetric and Gynaecological Societies of India. FOGSI Good Clinical Recommendation Practice on Pregnancy with COVID-19. Available online: https://www.fogsi.org/wp-content/uploads/covid19/fogsi_gcpr_on_pregnancy_with_COVID_19_version_1.pdf (accessed on 15 April 2023).
- Norsk Gynekologisk Forening; Norwegian Society of Gynecology and Obstetrics. Coronavirus during Pregnancy and Delivery (In Norwegian: Koronavirus ved Svangerskap og Fødsel—Norsk Gynekologisk Forening). Version 7. Available online: https://www.legeforeningen.no/foreningsledd/fagmed/norsk-gynekologisk-forening/veiledere/veileder-i-fodselshjelp/koronavirus-ved-svangerskap-og-fodsel/ (accessed on 15 April 2023).
- Federatie Medisch Specialisten. Samenvatting van de Adviezen over Medicamenteuze Behandeling van COVID-19. Available online: https://richtlijnendatabase.nl/gerelateerde_documenten/f/25968/Samenvatting%20richtlijn%20medicamenteuze%20behandeling%20van%20COVID.pdf (accessed on 15 April 2023).
- Royal College of Obstetricians and Gynaecologists. Coronavirus (COVID-19) Infection in Pregnancy—Information for Healthcare Professionals—Version 16: Published 15 December 2022. Available online: https://www.rcog.org.uk/media/ftzilsfj/2022-12-15-coronavirus-COVID-19-infection-in-pregnancy-v16.pdf (accessed on 15 April 2023).
- National Institutes of Health; COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health—11 February 2021. Available online: https://files.covid19treatmentguidelines.nih.gov/guidelines/archive/covid19treatmentguidelines-02-11-2021.pdf (accessed on 15 April 2023).
- World Health Organization. Therapeutics and COVID-19 Guidelines. Available online: https://www.who.int/teams/health-care-readiness-clinical-unit/COVID-19/therapeutics (accessed on 15 April 2023).
- Sieswerda, E.; de Boer, M.G.J.; Bonten, M.M.J.; Boersma, W.G.; Jonkers, R.E.; Aleva, R.M.; Kullberg, B.J.; Schouten, J.A.; van de Garde, E.M.W.; Verheij, T.J.; et al. Recommendations for antibacterial therapy in adults with COVID-19—An evidence based guideline. Clin. Microbiol. Infect. 2021, 27, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, C.E.; Doan, T. Azithromycin for severe COVID-19. Lancet 2020, 396, 936–937. [Google Scholar] [CrossRef] [PubMed]
- Berard, A.; Sheehy, O.; Zhao, J.P.; Nordeng, H. Use of macrolides during pregnancy and the risk of birth defects: A population-based study. Pharmacoepidemiol. Drug. Saf. 2015, 24, 1241–1248. [Google Scholar] [CrossRef]
- Royal College of Obstetricians and Gynaecologists. Coronavirus (COVID-19) Infection in Pregnancy—Information for Healthcare Professionals—Version 14: Published Wednesday 25 August 2021. Available online: https://www.rcm.org.uk/media/5255/2021-08-25-coronavirus-COVID-19-infection-in-pregnancy-v14.pdf (accessed on 15 April 2023).
- Louchet, M.; Sibiude, J.; Peytavin, G.; Picone, O.; Treluyer, J.M.; Mandelbrot, L. Placental transfer and safety in pregnancy of medications under investigation to treat coronavirus disease 2019. Am. J. Obstet. Gynecol. MFM 2020, 2, 100159. [Google Scholar] [CrossRef]
- Agencia Italiana del Farmaco (AIFA). Hydroxychloroquine in the Treatment of Adult Patients with COVID-19—Update: 22 December 2020. Available online: https://www.aifa.gov.it/documents/20142/1267737/Hydroxychloroquine_EN_22.12.2020.pdf (accessed on 15 April 2023).
- Vollaard, A.; Gieling, E.M.; van der Linden, P.D.; Sinha, B.; de Boer, M.G.J. Hydroxychloroquine and chloroquine for COVID-19: No evidence of effectiveness. Ned. Tijdschr. Geneeskd. 2020, 164, D5141. [Google Scholar]
- Gonzalez, R.; Garcia-Otero, L.; Pons-Duran, C.; Marban-Castro, E.; Gonce, A.; Llurba, E.; Gil, M.D.M.; Rodriguez-Zambrano, M.A.; Chen, H.; Ramirez, M.; et al. Hydroxychloroquine efficacy and safety in preventing SARS-CoV-2 infection and COVID-19 disease severity during pregnancy (COVID-Preg): A structured summary of a study protocol for a randomised placebo controlled trial. Trials 2020, 21, 607. [Google Scholar] [CrossRef]
- Belgian Centre for Phamacotherapeutic Information (BCFi). Hydroxychloroquine of Chloroquine Niet Voorschrijven ter Preventie van COVID-19, Noch Voor Thuisbehandeling van COVID-19-Patiënten—Bericht van 18/03/20. Available online: https://www.bcfi.be/nl/gows/3306 (accessed on 15 April 2023).
- Chawla, D.; Chirla, D.; Dalwai, S.; Deorari, A.K.; Ganatra, A.; Gandhi, A.; Kabra, N.S.; Kumar, P.; Mittal, P.; Parekh, B.J.; et al. Perinatal-Neonatal Management of COVID-19 Infection—Guidelines of the Federation of Obstetric and Gynaecological Societies of India (FOGSI), National Neonatology Forum of India (NNF), and Indian Academy of Pediatrics (IAP). Indian Pediatr. 2020, 57, 536–548. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Burwick, R.M.; Yawetz, S.; Stephenson, K.E.; Collier, A.Y.; Sen, P.; Blackburn, B.G.; Kojic, E.M.; Hirshberg, A.; Suarez, J.F.; Sobieszczyk, M.E.; et al. Compassionate Use of Remdesivir in Pregnant Women with Severe Coronavirus Disease 2019. Clin. Infect. Dis. 2021, 73, e3996–e4004. [Google Scholar] [CrossRef]
- Stiching Werkgroep Antibioticabeleid (SWAB). Medicamenteuze Behandeling Voor Patiënten Met COVID-19 (Infectie Met SARS–CoV-2). Available online: https://swab.nl/nl/COVID-19 (accessed on 15 April 2023).
- Society for Maternal Fetal Medicine (SMFM). FDA Issues EUA for the Treatment of Mild-to-Moderate COVID-19—Maternal-Fetal Medicine Subspecialists Support Use in Pregnant Patients. Available online: https://s3.amazonaws.com/cdn.smfm.org/media/3287/Treatment_1.10.pdf (accessed on 15 April 2023).
- Zhuang, W.; Xu, J.; Wu, Y.; Yang, J.; Lin, X.; Liao, Y.; Wan, J.; Weng, L.; Lin, W. Post-marketing safety concerns with nirmatrelvir: A disproportionality analysis of spontaneous reports submitted to the FDA Adverse Event Reporting System. Br. J. Clin. Pharmacol. 2023. [Google Scholar] [CrossRef]
- Benski, C.; Di Filippo, D.; Taraschi, G.; Reich, M.R. Guidelines for Pregnancy Management During the COVID-19 Pandemic: A Public Health Conundrum. Int. J. Environ. Res. Public. Health 2020, 17, 8277. [Google Scholar] [CrossRef] [PubMed]
- Vidaeff, A.C.; Aagaard, K.M.; Belfort, M.A. Antenatal corticosteroids in COVID-19 perspective. World J. Exp. Med. 2021, 11, 37–43. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, J.J. Corticosteroid Guidance for Pregnancy during COVID-19 Pandemic. Am. J. Perinatol. 2020, 37, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Magala Ssekandi, A.; Sserwanja, Q.; Olal, E.; Kawuki, J.; Bashir Adam, M. Corticosteroids Use in Pregnant Women with COVID-19: Recommendations from Available Evidence. J. Multidiscip. Healthc. 2021, 14, 659–663. [Google Scholar] [CrossRef]
- Khan, F.A.; Stewart, I.; Fabbri, L.; Moss, S.; Robinson, K.; Smyth, A.R.; Jenkins, G. Systematic review and meta-analysis of anakinra, sarilumab, siltuximab and tocilizumab for COVID-19. Thorax 2021, 76, 907–919. [Google Scholar] [CrossRef]
- Royal College of Obstetricains and Gynecologists; Royal College of Midwives; UK Teratolgy Information Service; MacDonald Obstetric Medicine Society (MOMS). Treatment of COVID-19 in Pregnant Patients—Version 1: Tuesday 7 December 2021. Available online: https://www.rcog.org.uk/media/4skdxeiu/2021-12-07-treatment-COVID-19-pregnant-patients-v1.pdf (accessed on 15 April 2023).
- Agence Fédérale des Médicaments et des Produits de Santé (AFMPS-FAGG). Tocilizumab Safety Profile—30 April 2020. Available online: https://www.bvikm.org/media/docs/COVID-19/TOCILIZUMAB%20safety%20profile.pdf (accessed on 15 April 2023).
- National Institutes of Health; COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health—28 December 2022. Available online: https://files.covid19treatmentguidelines.nih.gov/guidelines/archive/covid19treatmentguidelines-12-28-2022.pdf (accessed on 15 April 2023).
- Conte, E.; Di Girolamo, R.; D’Antonio, F.; Raffone, A.; Neola, D.; Saccone, G.; Dell’Aquila, M.; Sarno, L.; Miceli, M.; Carbone, L.; et al. Do Anti-SARS-CoV-2 Monoclonal Antibodies Have an Impact on Pregnancy Outcome? A Systematic Review and Meta-Analysis. Vaccines 2023, 11, 344. [Google Scholar] [CrossRef]
- Swiss Society in Gynecology & Obstetrics. Use of Monoclonal Antibodies against Coronavirus in Pregnant Women in Switzerland. 7 January 2022. Available online: https://www.sggg.ch/fileadmin/user_upload/Dokumente/1_Ueber_uns/AC_MoAb_in_pregnancy_07.01.2022__002_.pdf (accessed on 15 April 2023).
- Franchini, M.; Prefumo, F.; Grisolia, G.; Bergamini, V.; Glingani, C.; Pisello, M.; Presti, F.; Zaffanello, M. Convalescent Plasma for Pregnant Women with COVID-19: A Systematic Literature Review. Viruses 2021, 13, 1194. [Google Scholar] [CrossRef]
- Neunert, C.; Lim, W.; Crowther, M.; Cohen, A.; Solberg, L., Jr.; Crowther, M.A.; The American Society of Hematology. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011, 117, 4190–4207. [Google Scholar] [CrossRef]
- Milross, L.; Majo, J.; Cooper, N.; Kaye, P.M.; Bayraktar, O.; Filby, A.; Fisher, A.J. Post-mortem lung tissue: The fossil record of the pathophysiology and immunopathology of severe COVID-19. Lancet Respir. Med. 2022, 10, 95–106. [Google Scholar] [CrossRef]
- Belgian Society for Thrombosis and Haemostasis (BSTH). Anticoagulation Management in COVID-19 Positive Patients—Belgian Society for Thrombosis and Haemostasis Consensus Guideline. Available online: https://COVID-19.sciensano.be/sites/default/files/Covid19/COVID-19_Anticoagulation_Management_1.pdf (accessed on 15 April 2023).
- Centre Belge d’information Pharmacothérapeutique (CBiP). COVID-19: Pas de Place Pour L’azithromycine Dans le Traitement du COVID-19, Que ce Soit en Première Ligne ou à L’hôpital. Available online: https://www.cbip.be/fr/gows/3337 (accessed on 15 April 2023).
- Federation of Obstetric and Gynaecological Societies of India. Clinical Practice Guidelines—Perinatal-Neonatal Management of COVID-19—Version 3.0—16 June 2021. Available online: https://www.fogsi.org/wp-content/uploads/gcpr/perinatal-neonatal-management-of-covid-19.pdf (accessed on 15 April 2023).
- Centre Belge d’information Pharmacothérapeutique (CBiP). COVID-19: L’initiation Précoce D’hydroxychloroquine Chez des Patients Ambulatoires N’avait Aucun Impact Sur L’évolution des Symptômes. Available online: https://www.cbip.be/fr/gows/3425 (accessed on 15 April 2023).
- National Institutes of Health; COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health—21 April 2020. Available online: https://files.covid19treatmentguidelines.nih.gov/guidelines/archive/covid19treatmentguidelines-04-21-2020.pdf (accessed on 15 April 2023).
- World Health Organization. Therapeutics and COVID-19—Living Guideline 14 January 2022—World Health Organization. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.1 (accessed on 15 April 2023).
- Centre Belge d’information Pharmacothérapeutique (CBiP). COVID-19: L’étude Solidarity sur le Remdésivir, L’hydroxychloroquine, le Lopinavir et L’interféron Bêta-1a—14 December 2020. Available online: https://www.cbip.be/fr/gows/3499 (accessed on 15 April 2023).
- World Health Organization. Therapeutics and COVID-19: Living Guideline—Tenth Version—Published 22 April 22—Updated 15 July 22. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.4 (accessed on 15 April 2023).
- Centre Belge d’information Pharmacothérapeutique (CBiP). COVID-19: Concernant les Antiviraux Oraux Molnupiravir et PF-07321332 + Ritonavir: Aucune Étude Publiée Pour Le moment—1 December 2021. Available online: https://www.cbip.be/fr/gows/3730 (accessed on 15 April 2023).
- Agence Fédérale des Médicaments et des Produits de Santé (AFMPS-FAGG); Sciensano. Interim Clinical Guidance for Adults with Confirmed COVID-19. in Belgium—March 2023—Version 35. Available online: https://kce.fgov.be/sites/default/files/2023-03/COVID-19_InterimGuidelines_Treatment_ENG.pdf (accessed on 15 April 2023).
- Elwood, E.; Raeside, A.; Watson, H.; Boucoiran, I.; Money, D.; Yudin, M.; VanSchalkwyk, J.; Castillo, E.; Poliquin, V. Committee Opinion No. 400: COVID-19 and Pregnancy.(Original: March 13, 2020 Reaffirmed: February 15, 2021). Available online: https://sogc.org/common/Uploaded%20files/Latest%20News/Committee%20Opinion%20No.%20400%20COVID-19%20and%20Pregnancy.pdf (accessed on 15 April 2023).
- Royal College of Obstetricians and Gynaecologists. Coronavirus (COVID-19) Infection in Pregnancy—Information for Healthcare Professionals Version 4: Published Saturday 21 March 2020. Available online: https://www.rcm.org.uk/media/3800/2020-03-21-covid19-pregnancy-guidance.pdf (accessed on 15 April 2023).
- Whitehead, C.L.; Walker, S.P. Consider pregnancy in COVID-19 therapeutic drug and vaccine trials. Lancet 2020, 395, e92. [Google Scholar] [CrossRef]
- World Health Organization. Public Health Emergency Solidarity Trial of Treatments for COVID-19 Infection in Hospitalized Patients. Available online: https://www.isrctn.com/ISRCTN83971151 (accessed on 15 April 2023).
- Giesbers, S.; Goh, E.; Kew, T.; Allotey, J.; Brizuela, V.; Kara, E.; Kunst, H.; Bonet, M.; Thangaratinam, S.; Preg, C.O.V.G. Treatment of COVID-19 in pregnant women: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 267, 120–128. [Google Scholar] [CrossRef]
- Jirjees, F.; Saad, A.K.; Al Hano, Z.; Hatahet, T.; Al Obaidi, H.; Dallal Bashi, Y.H. COVID-19 Treatment Guidelines: Do They Really Reflect Best Medical Practices to Manage the Pandemic? Infect. Dis. Rep. 2021, 13, 259–284. [Google Scholar] [CrossRef]
- Swiss National COVID-19 Science Task Force. Available online: https://sciencetaskforce.ch/en/organisation-expert-groups/ (accessed on 15 April 2023).
- Putman, M.S.; Ruderman, E.M.; Niforatos, J.D. Publication Rate and Journal Review Time of COVID-19-Related Research. Mayo Clin. Proc. 2020, 95, 2290–2291. [Google Scholar] [CrossRef]
- Elwood, C.; Boucoiran, I.; VanSchalkwyk, J.; Money, D.; Yudin, M.; Poliquin, V. SOGC Committee Opinion—COVID-19 in Pregnancy. J. Obstet. Gynaecol. Can. 2020; online ahead of print. [Google Scholar] [CrossRef]
- Florez, I.D.; Morgan, R.L.; Falavigna, M.; Kowalski, S.C.; Zhang, Y.; Etxeandia-Ikobaltzeta, I.; Santesso, N.; Wiercioch, W.; Schunemann, H.J. Development of rapid guidelines: 2. A qualitative study with WHO guideline developers. Health Res. Policy Syst. 2018, 16, 62. [Google Scholar] [CrossRef]
- Homer, C.S.; Roach, V.; Cusack, L.; Giles, M.L.; Whitehead, C.; Burton, W.; Downton, T.; Gleeson, G.; Gordon, A.; Hose, K.; et al. The National COVID-19 Clinical Evidence Taskforce: Pregnancy and perinatal guidelines. Med. J. Aust. 2022, 217 (Suppl. S9), S14–S19. [Google Scholar] [CrossRef]
- Favre, G.; Gerbier, E.; Maisonneuve, E.; Pomar, L.; Winterfeld, U.; Lepigeon, K.; Bloemenkamp, K.W.M.; De Bruin, O.; Eimir, H.; Nordeng, H.; et al. COVID-19 related medicine utilization study in pregnancy—The COVI-PREG cohort. Br. J. Clin. Pharmacol. 2022, 89, 1560–1574. [Google Scholar] [CrossRef]
- Gajbhiye, R.K.; Sawant, M.S.; Kuppusamy, P.; Surve, S.; Pasi, A.; Prusty, R.K.; Mahale, S.D.; Modi, D.N. Differential impact of COVID-19 in pregnant women from high-income countries and low- to middle-income countries: A systematic review and meta-analysis. Int. J. Gynaecol. Obstet. 2021, 155, 48–56. [Google Scholar] [CrossRef]
- D’Souza, R.S.; D’Souza, S.; Strand, N.; Anderson, A.; Vogt, M.N.P.; Olatoye, O. YouTube as a source of medical information on the novel coronavirus 2019 disease (COVID-19) pandemic. Glob. Public. Health 2020, 15, 935–942. [Google Scholar] [CrossRef]
- BCNatal. Protocolo: Coronavirus (COVID-19) Y Gestación (V18 1 February 2023). Available online: https://portal.medicinafetalbarcelona.org/protocolos/es/patologia-materna-obstetrica/covid19-embarazo.html (accessed on 15 April 2023).
- Costantine, M.M.; Landon, M.B.; Saade, G.R. Protection by Exclusion: Another Missed Opportunity to Include Pregnant Women in Research during the Coronavirus Disease 2019 (COVID-19) Pandemic. Obstet. Gynecol. 2020, 136, 26–28. [Google Scholar] [CrossRef]
- Prasad, S.; Kalafat, E.; Blakeway, H.; Townsend, R.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; Le Doare, K.; Ladhani, S.; et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat. Commun. 2022, 13, 2414. [Google Scholar] [CrossRef]
- National Institutes of Health. Special Considerations During Pregnancy and After Delivery Updated 20 April 2023. Available online: https://www.covid19treatmentguidelines.nih.gov/special-populations/pregnancy/ (accessed on 15 June 2023).
| Color | Grading | Color Code Meaning in WHO Guidelines | Color Code Meaning in Australian Guidelines |
|---|---|---|---|
| Strong recommendation for | It is better to prescribe based on strong evidence. The benefits outweigh the harms for almost everyone. All or nearly all informed patients would likely want this option. | There is high-certainty evidence showing that the overall benefits of the intervention are clearly greater than the disadvantages. This means that all, or nearly all, patients will want the recommended intervention. | |
| Conditional recommendation for | The medication is indicated in some categories of patients, such as patients with comorbidities, or patients with seronegative status for COVID-19. Benefits outweigh harms for the majority, but not for everyone. The majority of patients would likely want this option. | A conditional recommendation is given when it is considered that the benefits of the intervention are greater than the disadvantages, or the available evidence cannot rule out a significant benefit of the intervention while assessing that the adverse effects are few or absent. | |
| “under study” or “under assessment” or unknown | The national societies are not able to conclude at the time of their recommendations because of insufficient evidence in the literature. | ||
| Only in research settings | The experts recommend not to use the medication, except in the context of a clinical trial. | The panel recommends that the intervention should only be considered in a randomized clinical trial with appropriate ethical approval. In any other circumstance, the intervention is not recommended. | |
| Conditional recommendation against | It is not recommended to prescribe a drug in some categories of patients, such as patients who do not require oxygen therapy, because it has been shown to be ineffective. A majority would likely decline the intervention. | A conditional recommendation is given against the intervention when it is judged that the disadvantages of the intervention are greater than the benefits, but where this is not substantiated by strong evidence. This recommendation is also used where there is strong evidence of both beneficial and harmful effects, but where the balance between them is difficult to determine. | |
| Strong recommendation against | It is not recommended to prescribe a drug because it has been shown to be ineffective, or even potentially harmful. All or nearly all would likely decline the intervention. | There is high-certainty evidence showing that the overall disadvantages of the intervention are clearly greater than the benefits. A strong recommendation is also used when the examination of the evidence shows that an intervention is not safe. |
| Guidelines in Pregnant Women or General Population with Specific Section for Pregnancy | Date of First and Latest Version until 31 December 2022 | Number of Versions | Guidelines Methodology (Number of References) | Other National Societies (Involved and/or Collaborating) | |
|---|---|---|---|---|---|
| Australia | National COVID-19 Clinical Evidence Taskforce [22] | 14 May 2020 19 December 2022 | 9 | Living guideline, GRADE methodology (29 ref.) | RANZCOG: Royal Australian and New Zealand College of OBGYNs ACM: Australian College of Midwives |
| Belgium | AFMPS (Agence fédérale des Médicaments et Produits de Santé)–FAGG [24] | 17 April 2020 December 2022 | 33 | Living guideline (256 ref.) | SBIMC: Société Belge d’Infectiologie et de Microbiologie Clinique Sciensano: National Institute of Public Health CBiP: Centre Belge d’information Pharmacothérapeutique |
| Canada | SOGC (Society of Obstetricians and Gynecologists of Canada) [18] | 17 March 2002 15 February 2021 | 4 | Expert opinion (70 ref.) | - |
| France | CNGOF (Collège National des Gynécologues-Obstétriciens français) [26] | 19 March 2002 5 October 2020 | 2 | Expert opinion (98 ref.) | DGS: General Direction of Health HCSP: French High Council for Public Health HAS: French National Authority for Health CARO: Club of Anesthetists in Obstetrics |
| India | FOGSI (Federation of Obstetric and Gynaecological Societies of India) [36] | 28 March 2020 16 June 2021 | 3 | CPG, GRADE approach (168 ref.) | NNF: National Neonatology Forum of India IAP: Indian Academy of Pediatrics ICMR: National Institute for Research in Reproductive Health |
| Italy | AIFA (Italian Medicines Agency) [21] | Information sheets on individual drugs | - | ||
| Norway | Norsk Gynekologisk Forening (Norwegian Society of Gynecology and Obstetrics) [37] | 24 March 2020 22 March 2022 | 7 | Expert opinion (85 ref.) | OBGYNs, infectious disease specialists, microbiologists, epidemiologists and pharmacologists involved |
| Saudi Arabia | Saudi Society of Maternal-Fetal Medicine [34] | 1 August 2020 - | 1 | Expert opinion (6 ref.) | Saudi Ministry of Health [17] |
| Spain | SEGO (Sociedad Española de Ginecología y Obstetricia) [27] | 13 May 2020 17 June 2020 | 2 | Expert opinion (90 ref.) | SENEO: Sociedad Española de Neonatología SEDAR: Sociedad Española de Anestesiología, Reanimación y Terapéutica del Dolor SETH: Sociedad Española de Trombosis y Hemostasia SEEN: Sociedad Española de Enfermería Neonatal AEM: Asociación Española de Matronas FAME: Federación de Asociaciones de Matronas de España IHAN: Iniciativa para la Humanización de la Asistencia al Nacimiento y Lactancia CGCOM: Consejo General de Colegios Oficiales de Médicos CGE: Consejo General de Enfermería |
| Switzerland | SSGO (Swiss Society in Gynecology & Obstetrics) [28] | 5 August 2020 7 January 2022 | 2 | Expert opinion (28 ref.) | - |
| the Netherlands | FMS (Federatie Medisch Specialisten) and NVOG (Nederlandse Vereniging voor Obstetrie en Gynaecologie) [38] | 1 June 2020 8 November 2022 | 7 | Expert opinion (40 ref.) | RIVM: Rijksinstituur voor Volksgezondheid en Milieu SWAB: Stichting Werkgroep AntibioticaBeleid KNOV: Koninklijke Nederlandse Organisatie van Verloskundigen NVA: Nederlandse Vereniging voor Anesthesiologie NVK: Nederlandse Vereniging voor Kindergeneeskunde NVMM: Nederlandse Vereniging voor Medische Microbiologie K&Z: Stichting Kind en Ziekenhuis NVZ: Nederlandse Vereniging van Ziekenhuizen |
| UK | RCOG (Royal College of Obstetricians and Gynaecologists) and Royal College of Midwives [39] | 9 March 2020 15 December 2022 | 16 | Expert opinion (199 ref.) | Royal College of Paediatrics and Child health, Royal College of Anaesthetists NICE: National Institute for Health and Care Excellence |
| USA | SMFM (Society for Maternal Fetal Medicine) [20] * | 17 March 2020 21 June 2022 | 10 | Expert opinion (48 ref.) | ACOG: American College of OBGYN |
| NIH (National Institutes of Health) [40] | 21 April 2020 28 December 2022 | 62 | Living guideline (around 30 ref. per section) | CDC: Center for disease Control and Prevention | |
| “World” | WHO (World Health Organization) [41] | 2 September 2020 14 July 2022 | 10 | Living guideline, GRADE methodology (168 ref.) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maisonneuve, E.; de Bruin, O.; Favre, G.; Goncé, A.; Donati, S.; Engjom, H.; Hurley, E.; Al-Fadel, N.; Siiskonen, S.; Bloemenkamp, K.; et al. Evolution of National Guidelines on Medicines Used to Treat COVID-19 in Pregnancy in 2020–2022: A Scoping Review. J. Clin. Med. 2023, 12, 4519. https://doi.org/10.3390/jcm12134519
Maisonneuve E, de Bruin O, Favre G, Goncé A, Donati S, Engjom H, Hurley E, Al-Fadel N, Siiskonen S, Bloemenkamp K, et al. Evolution of National Guidelines on Medicines Used to Treat COVID-19 in Pregnancy in 2020–2022: A Scoping Review. Journal of Clinical Medicine. 2023; 12(13):4519. https://doi.org/10.3390/jcm12134519
Chicago/Turabian StyleMaisonneuve, Emeline, Odette de Bruin, Guillaume Favre, Anna Goncé, Serena Donati, Hilde Engjom, Eimir Hurley, Nouf Al-Fadel, Satu Siiskonen, Kitty Bloemenkamp, and et al. 2023. "Evolution of National Guidelines on Medicines Used to Treat COVID-19 in Pregnancy in 2020–2022: A Scoping Review" Journal of Clinical Medicine 12, no. 13: 4519. https://doi.org/10.3390/jcm12134519
APA StyleMaisonneuve, E., de Bruin, O., Favre, G., Goncé, A., Donati, S., Engjom, H., Hurley, E., Al-Fadel, N., Siiskonen, S., Bloemenkamp, K., Nordeng, H., Sturkenboom, M., Baud, D., & Panchaud, A. (2023). Evolution of National Guidelines on Medicines Used to Treat COVID-19 in Pregnancy in 2020–2022: A Scoping Review. Journal of Clinical Medicine, 12(13), 4519. https://doi.org/10.3390/jcm12134519








