Efficacy of Serum Ferritin–Zinc Ratio for Predicting Advanced Liver Fibrosis in Patients with Autoimmune Hepatitis
Abstract
1. Introduction
2. Methods
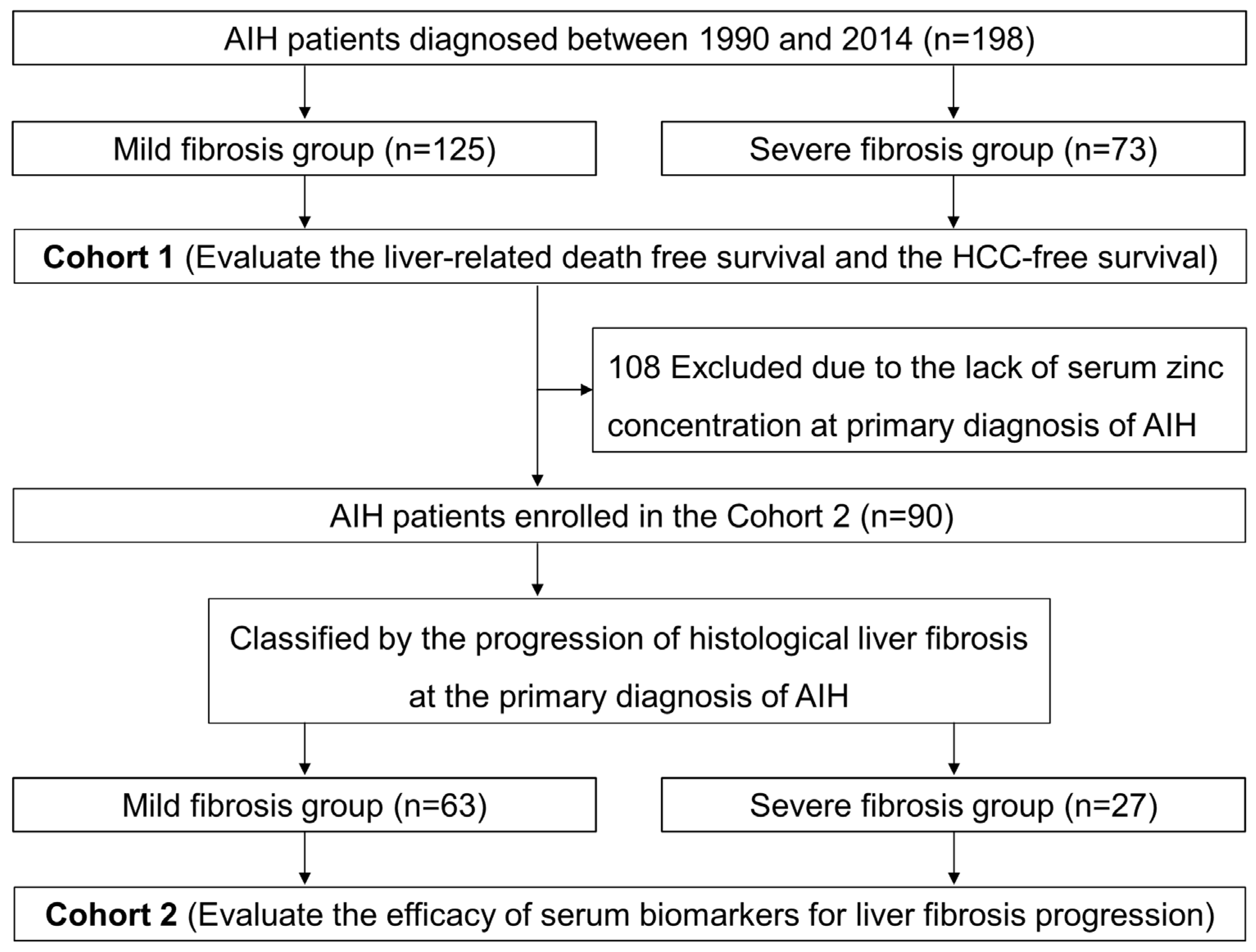
2.1. Patients
2.2. Laboratory Assessments
2.3. Formula of Noninvasive Serum Tests
2.4. Histological Assessments
2.5. HCC Assessments
2.6. Statistical Analyses
2.7. Ethical Issues
3. Results
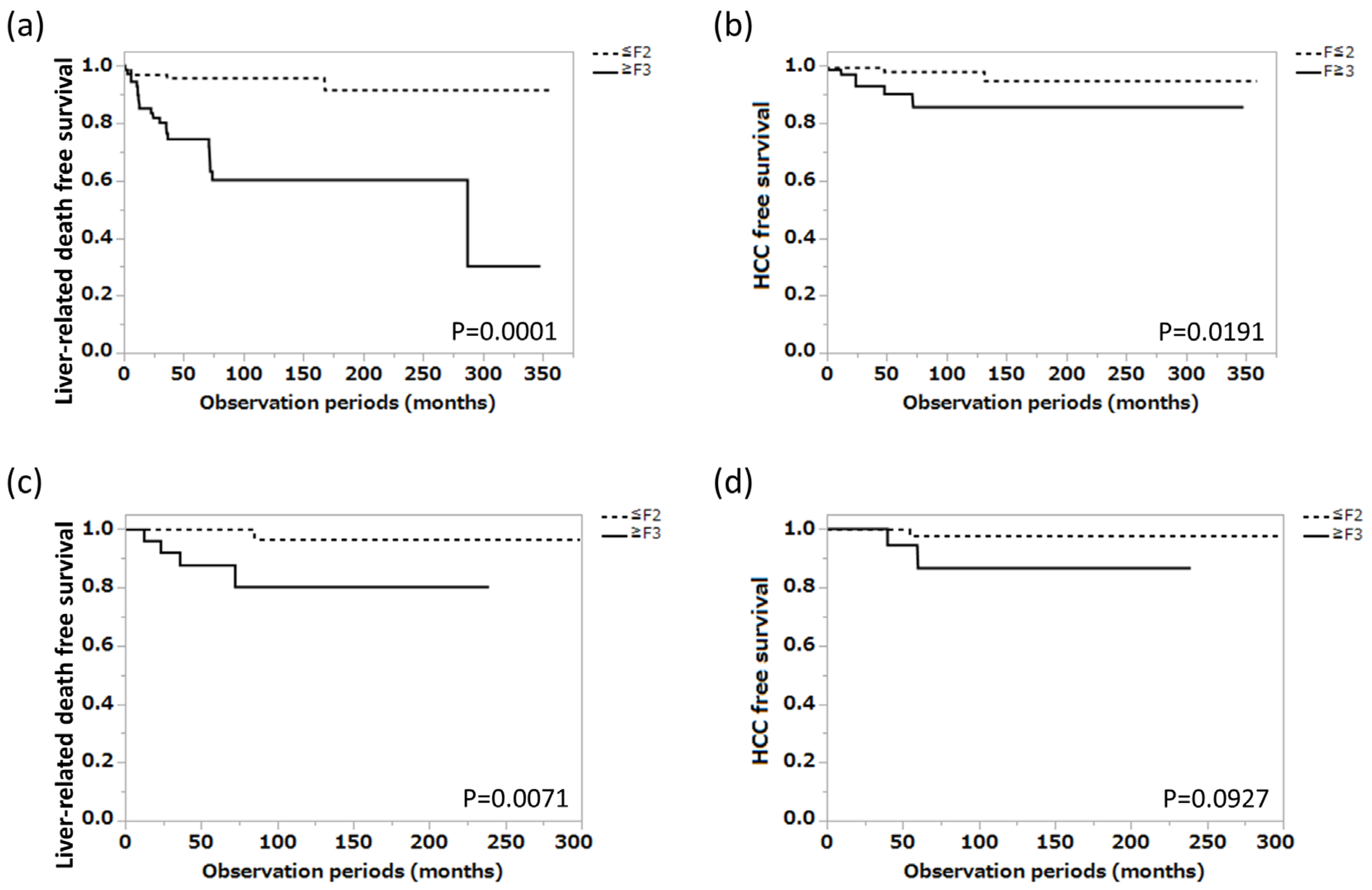
3.1. Long-Term Prognosis of Patients with AIH
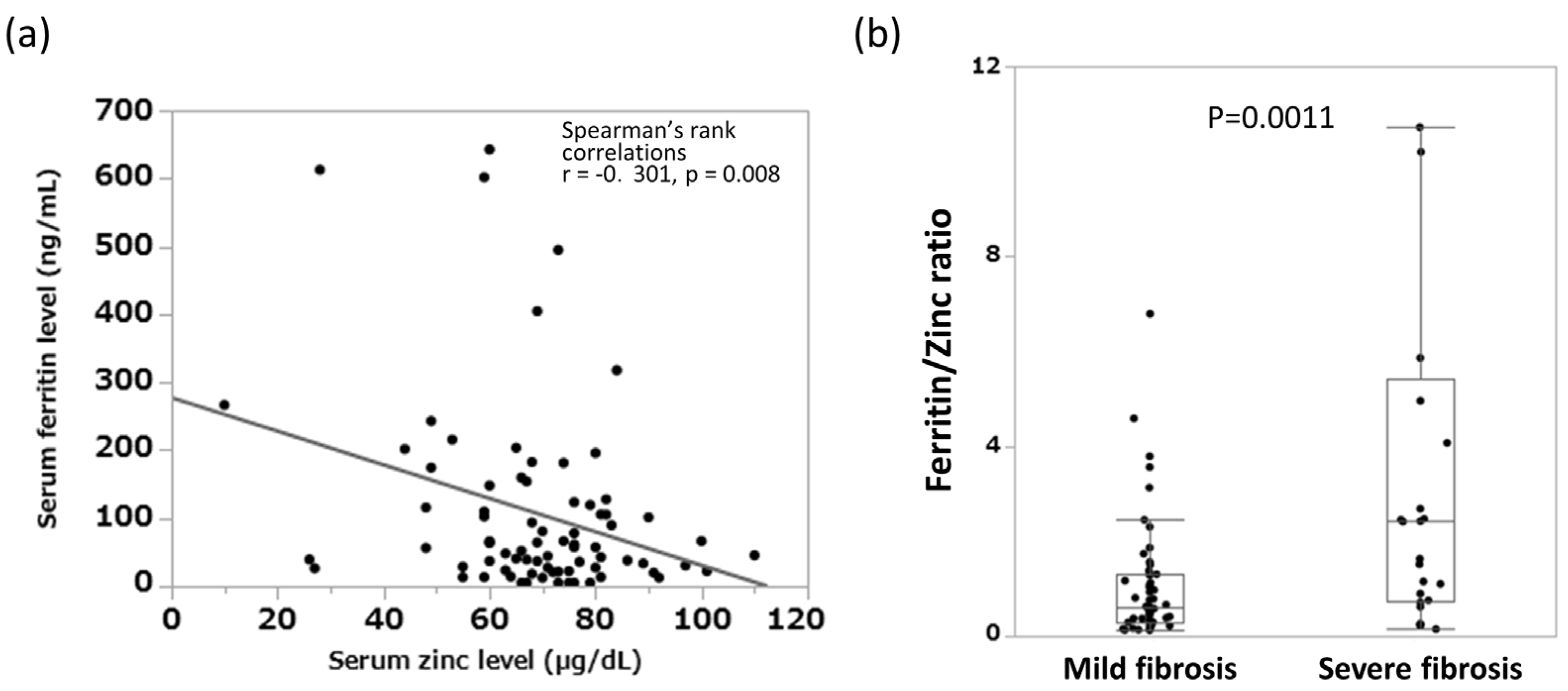
3.2. Relationship between Blood Inorganic Salt Balance and Liver Fibrosis Progression in Patients with AIH
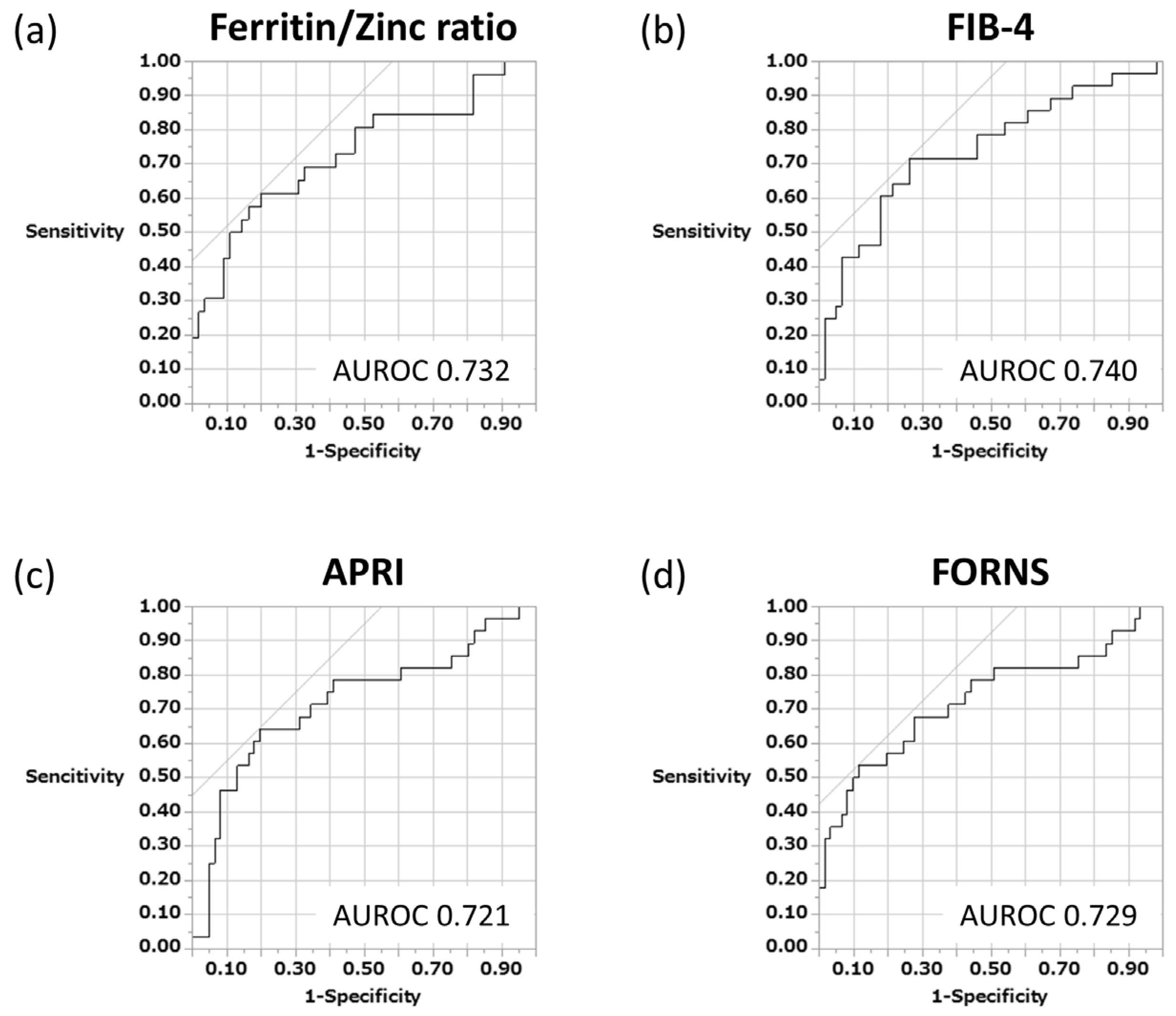
3.3. Measurement and Intercomparison of Various Serum Biomarkers in Predicting Liver Fibrosis Progression That Is Related to the Long-Term Prognosis of Patients with AIH
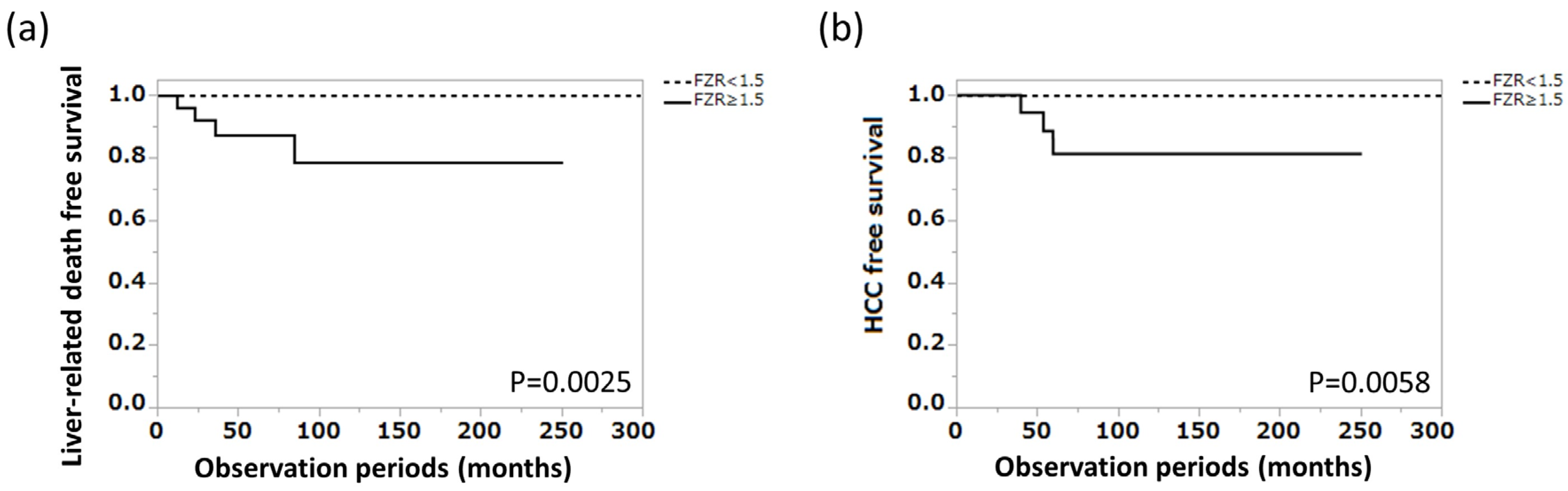
3.4. Ferritin–Zinc Ratio and Long-Term Prognosis of Patients with AIH
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tanaka, A.; Mori, M.; Matsumoto, K.; Ohira, H.; Tazuma, S.; Takikawa, H. Increase trend in the prevalence and Male-to-female ratio of primary biliary cholangitis, autoimmune hepatitis, and primary sclerosing cholangitis in Japan. Hepatol. Res. 2019, 49, 881–889. [Google Scholar] [CrossRef]
- Wong, G.L.; Hui, V.W.; Yip, T.C.; Liang, L.Y.; Zhang, X.; Tse, Y.K.; Lai, J.C.; Chan, H.L.; Wong, V.W. Universal HBV vaccination dramatically reduces the prevalence of HBV infection and incidence of hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2022, 56, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Estirado Gómez, A.; Justo Gil, S.; Limia, A.; Avellón, A.; Arce Arnáez, A.; González-Rubio, R.; Diaz, A.; Del Amo, J.; Working group of the HCV prevalence study in Spain in 2017–2018. Prevalence and undiagnosed fraction of hepatitis C infection in 2018 in Spain: Results from a national population-based survey. Eur. J. Public Health 2021, 31, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015, 149, 389–397.e10. [Google Scholar] [CrossRef] [PubMed]
- Danielsson Borssén, Å.; Marschall, H.U.; Bergquist, A.; Rorsman, F.; Weiland, O.; Kechagias, S.; Nyhlin, N.; Verbaan, H.; Nilsson, E.; Werner, M. Epidemiology and causes of death in a Swedish cohort of patients with autoimmune hepatitis. Scand. J. Gastroenterol. 2017, 52, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- EASL. EASL 2017 Clinical practice guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; SSulkowski, M.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef]
- Forns, X.; Ampurdanes, S.; Llovet, J.M.; Aponte, J.; Quintó, L.; Martınez-Bauer, E.; Bruguera, M.; Sánchez-Tapias, J.M.; Rodés, J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 2002, 36, 986–992. [Google Scholar]
- Efe, C.; Cengiz, M.; Kahramanoğlu-Aksoy, E.; Yılmaz, B.; Özşeker, B.; Beyazıt, Y.; Tanoğlu, A.; Purnak, T.; Kav, T.; Turhan, T.; et al. Angiotensin-converting enzyme for noninvasive assessment of liver fibrosis in autoimmune hepatitis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 649–654. [Google Scholar] [CrossRef]
- Li, J.; Lu, X.; Zhu, Z.; Kalutkiewicz, K.J.; Mounajjed, T.; Terry, M.T.; Venkatesh, S.K.; Sui, Y.; Glaser, K.J.; Hoodeshenas, S.; et al. Head-to-head comparison of MR elastography-based liver stiffness, fat Fraction, and T1 relaxation time in identifying At-Risk NASH. Hepatology, 2023; online ahead of print. [Google Scholar]
- Paranaguá-Vezozzo, D.C.; Benedita Terrabuio, D.R.; Reinoso-Pereira, G.L.; Moutinho, R.; Kioko Ono, S.; Walwyn Salas, V.; Dias França, J.I.; Alves, V.A.; Cançado, E.L.; Carrilho, F.J. Liver elastography can predict degree of advanced fibrosis for autoimmune hepatitis in biochemical remission. JGH Open 2023, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Hartl, J.; Denzer, U.; Ehlken, H.; Zenouzi, R.; Peiseler, M.; Sebode, M.; Hübener, S.; Pannicke, N.; Weiler-Normann, C.; Quaas, A.; et al. Transient elastography in autoimmune hepatitis: Timing determines the impact of inflammation and fibrosis. J. Hepatol. 2016, 65, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, A.; Wessner, C.E.; Guglielmo, F.F.; Wang, S.; Vu, T.; Liu, J.B.; Civan, J.; Lyshchik, A.; Forsberg, F.; Li, H.; et al. Comparison of magnetic resonance-based elastography and ultrasound shear wave elastography in patients with suspicion of nonalcoholic fatty liver disease. Ultrasound Q. 2023, 39, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Luo, Y.; Zheng, Y.; Ji, R.; Zhou, Y. Effect of elevated serum ferritin on the risk of death in patients with decompensated cirrhosis: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2023; online ahead of print. [Google Scholar]
- Stamoulis, I.; Kouraklis, G.; Theocharis, S. Zinc and the liver: An active interaction. Dig. Dis. Sci. 2007, 52, 1595–1612. [Google Scholar] [CrossRef] [PubMed]
- Molenda, M.; Kolmas, J. The Role of Zinc in Bone Tissue Health and Regeneration—A Review. Biol. Trace Elem. Res. 2023; online ahead of print. [Google Scholar]
- Malemud, C.J. Matrix metalloproteinases (MMPs) in health and disease: An overview. Front. Biosci. 2006, 11, 1696–1701. [Google Scholar] [CrossRef]
- Moriya, K.; Nishimura, N.; Namisaki, T.; Takaya, H.; Sawada, Y.; Kawaratani, H.; Kaji, K.; Shimozato, N.; Sato, S.; Furukawa, M.; et al. Zinc administration and improved serum markers of hepatic fibrosis in patients with autoimmune hepatitis. J. Clin. Med. 2021, 10, 2465. [Google Scholar] [CrossRef]
- Moriya, K.; Saeki, K.; Nishimura, N.; Sato, S.; Sawada, Y.; Takaya, H.; Kaji, K.; Kawaratani, H.; Namisaki, T.; Akahane, T.; et al. Zinc supplementation and improved quality of life in patients with autoimmune hepatitis. Intern. Med. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Alvarez, F.; Berg, P.A.; Bianchi, F.B.; Bianchi, L.; Burroughs, A.K.; Cancado, E.L.; Chapman, R.W.; Cooksley, W.G.; Czaja, A.J.; Desmet, V.J.; et al. International Autoimmune Hepatitis Group Report: Review of criteria for diagnosis of autoimmune hepatitis. J. Hepatol. 1999, 31, 929–938. [Google Scholar] [CrossRef]
- Bedossa, P.; Bioulacsage, P.; Callard, P.; Chevallier, M. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996, 24, 289–293. [Google Scholar] [CrossRef]
- Kokudo, N.; Hasegawa, K.; Akahane, M.; Igaki, H.; Izumi, N.; Ichida, T.; Uemoto, S.; Kaneko, S.; Kawasaki, S.; Ku, Y.; et al. Evidence-based clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol. Res. 2015, 45, 123–127. [Google Scholar] [CrossRef]
- Czaja, A.J.; Carpenter, H.A. Progressive fibrosis during corticosteroid therapy of autoimmune hepatitis. Hepatology 2004, 39, 1631–1638. [Google Scholar] [CrossRef]
- Carbone, M.; Neuberger, J.M. Autoimmune liver disease, autoimmunity and liver transplantation. J. Hepatol. 2014, 6, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Vetter, M.; Kremer, A.E.; Agaimy, A.; Konturek, P.C.; Pfeifer, L.; Neurath, M.F.; Siebler, J.; Zopf, S. The amount of liver tissue is essential for accurate histological staging in patients with autoimmune hepatitis. J. Physiol. Pharmacol. 2021, 72, 119–127. [Google Scholar]
- Mack, C.L.; Adams, D.; Assis, D.N.; Kerkar, N.; Manns, M.P.; Mayo, M.J.; Vierling, J.M.; Alsawas, M.; Murad, M.H.; Czaja, A.J. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 Practice guidance and guidelines from the American Association for the Study of Liver Diseases. Hepatology 2020, 72, 671–722. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J. Hepatol. 2015, 63, 971–1004. [Google Scholar] [CrossRef]
- Garrido, M.C.; Hubscher, S.G. Accuracy of staging in primary biliary cirrhosis. J. Clin. Pathol. 1996, 49, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Charlotte, F.; Heurtier, A.; Gombert, S.; Giral, P.; Bruckert, E.; Grimaldi, A.; Capron, F.; Poynard, T.; LIDO Study Group. Sampling variability of liver biopsy in non-alcoholic fatty liver disease. Gastroenterology 2005, 128, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Screening, Care and Treatment of Persons with Chronic Hepatitis C Infection; World Health Organization: Geneva, Switzerland, 2016; Available online: https://www.who.int/publications/i/item/9789241549615 (accessed on 4 June 2023).
- Salomone, F.; Micek, A.; Godos, J. Simple scores of fibrosis and mortality in patients with NAFLD: A systematic review with meta-analysis. J. Clin. Med. 2018, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Yang, J.; Yan, L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis b virus infection: A systemic review and meta-analysis. Hepatology 2015, 61, 292–302. [Google Scholar] [CrossRef]
- Yongpisarn, T.; Thimphitthaya, C.; Laoveeravat, P.; Wongjarupong, N.; Chaiteerakij, R. Non-invasive tests for predicting liver outcomes in chronic hepatitis C patients: A systematic review and meta-analysis. World J. Hepatol. 2021, 13, 949–968. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Chen, Y.; Lyu, G.; Yang, X. Aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in patients with autoimmune hepatitis: A meta-analysis. Front. Immunol. 2022, 13, 892454. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Z.; Zhou, J.; Zeng, N.; He, Z.; Zhan, S.; Jia, J.; You, H. Systematic review: Diagnostic accuracy of non-invasive tests for staging liver fibrosis in autoimmune hepatitis. Hepatol. Int. 2019, 13, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Castera, L. Hepatitis B: Are non-invasive markers of liver fibrosis reliable? Liver Int. 2014, 34, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Duan, S.Z.; Cao, J.; Gao, N.; Xu, J.; Zhang, L. Noninvasive inflammatory markers for assessing liver fibrosis stage in autoimmune hepatitis patients. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Pietrangelo, A. Iron-induced oxidant stress in alcoholic liver fibrogenesis. Alcohol 2003, 30, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Hino, K.; Nishina, S.; Hara, Y. Iron metabolic disorder in chronic hepatitis C: Mechanisms and relevance to hepatocarcinogenesis. J. Gastroenterol. Hepatol. 2013, 28, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Szuster-Ciesielska, A.; Plewka, K.; Daniluk, J.; Kandefer-Szerszeń, M. Zinc supplementation attenuates ethanol- and acetaldehyde-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS) production and by influencing intracellular signaling. Biochem. Pharmacol. 2009, 78, 301–314. [Google Scholar] [CrossRef]
| Observational Study | Cohort 1 | Cohort 2 | |||
|---|---|---|---|---|---|
| Total Patients (n = 198) | Total Patients (n = 90) | Mild Fibrosis (n = 63) | Severe Fibrosis (n = 27) | p | |
| Age (years) | 62.0 [51.0–69.0] | 62.5 [52.8–67.3] | 60.0 [52.0–67.0] | 64.5 [54.8–70.3] | 0.270 |
| Sex (male/female) | 33/165 | 16/74 | 8/55 | 7/20 | 0.135 |
| Fibrosis (F1/F2/F3/F4) | 76/49/40/33 | 39/24/18/9 | 39/24/0/0 | 0/0/18/9 | >0.001 |
| AST (U/L) | 87.0 [48.8–259.8] | 83.5 [52.0–234.0] | 82.5 [54.0–359.5] | 84.0 [45.3–122.0] | 0.013 |
| ALT (U/L) | 81.5 [46.0–323.8] | 86.5 [52.8–229.8] | 98.5 [62.3–329.8] | 70.5 [48.3–103.3] | 0.006 |
| Gamma GT (U/L) | 111.5 [57.0–210.0] | 128.0 [62.0–296.8] | 128.0 [62.3–296.8] | 126.5 [60.0–298.3] | 0.897 |
| Total bilirubin (mg/dL) | 1.1 [0.7–2.3] | 1.0 [0.7–2.0] | 1.0 [0.7–1.4] | 1.2 [1.0–2.1] | 0.079 |
| Albumin (g/dL) | 4.0 [3.6–4.3] | 4.1 [3.8–4.3] | 4.1 [3.9–4.3] | 3.9 [3.2–4.3] | 0.011 |
| Total cholesterol (mg/dL) | 184.0 [154.0–214.0] | 192.5 [160.0–222.8] | 194.0 [162.5–223.8] | 176.0 [134.0–208.3] | 0.437 |
| Ferritin (ng/mL) | 161.4 [80.8–351.7] | 243.0 [93.0–314.5] | 128.4 [64.2–301.9] | 295.3 [255.8–437.1] | 0.063 |
| Zinc (µg/dL) | 69.0 [60.0–79.8] | 69.0 [60.0–79.8] | 73.0 [64.0–81.8] | 63.0 [53.0–71.8] | 0.061 |
| IgG (mg/dL) | 2090.0 [1600.5–2658.5] | 2035.0 [1533.5–2564.5] | 1825.5 [1472.3–2375.3] | 2167.4 [1940.3–3258.0] | 0.007 |
| Platelets (×104/µL) | 18.5 [12.4–23.4] | 19.0 [13.1–23.9] | 21.3 [17.8–25.0] | 13.2 [10.5–16.3] | >0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moriya, K.; Sato, S.; Nishimura, N.; Kawaratani, H.; Takaya, H.; Kaji, K.; Namisaki, T.; Uejima, M.; Nagamatsu, S.; Matsuo, H.; et al. Efficacy of Serum Ferritin–Zinc Ratio for Predicting Advanced Liver Fibrosis in Patients with Autoimmune Hepatitis. J. Clin. Med. 2023, 12, 4463. https://doi.org/10.3390/jcm12134463
Moriya K, Sato S, Nishimura N, Kawaratani H, Takaya H, Kaji K, Namisaki T, Uejima M, Nagamatsu S, Matsuo H, et al. Efficacy of Serum Ferritin–Zinc Ratio for Predicting Advanced Liver Fibrosis in Patients with Autoimmune Hepatitis. Journal of Clinical Medicine. 2023; 12(13):4463. https://doi.org/10.3390/jcm12134463
Chicago/Turabian StyleMoriya, Kei, Shinya Sato, Norihisa Nishimura, Hideto Kawaratani, Hiroaki Takaya, Kosuke Kaji, Tadashi Namisaki, Masakazu Uejima, Shinsaku Nagamatsu, Hideki Matsuo, and et al. 2023. "Efficacy of Serum Ferritin–Zinc Ratio for Predicting Advanced Liver Fibrosis in Patients with Autoimmune Hepatitis" Journal of Clinical Medicine 12, no. 13: 4463. https://doi.org/10.3390/jcm12134463
APA StyleMoriya, K., Sato, S., Nishimura, N., Kawaratani, H., Takaya, H., Kaji, K., Namisaki, T., Uejima, M., Nagamatsu, S., Matsuo, H., & Yoshiji, H. (2023). Efficacy of Serum Ferritin–Zinc Ratio for Predicting Advanced Liver Fibrosis in Patients with Autoimmune Hepatitis. Journal of Clinical Medicine, 12(13), 4463. https://doi.org/10.3390/jcm12134463






