Intramyocardial Stem Cell Transplantation during Coronary Artery Bypass Surgery Safely Improves Cardiac Function: Meta-Analysis of 20 Randomized Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Type of Outcome Measurement
2.3. Search Methods and Identification of Studies
2.3.1. Information Sources
2.3.2. Search Protocol
2.4. Data Collection and Analysis
2.5. Data Extraction and Management
2.6. Risk of Bias Analysis
3. Results
3.1. Study Characteristics
3.2. Primary Outcome
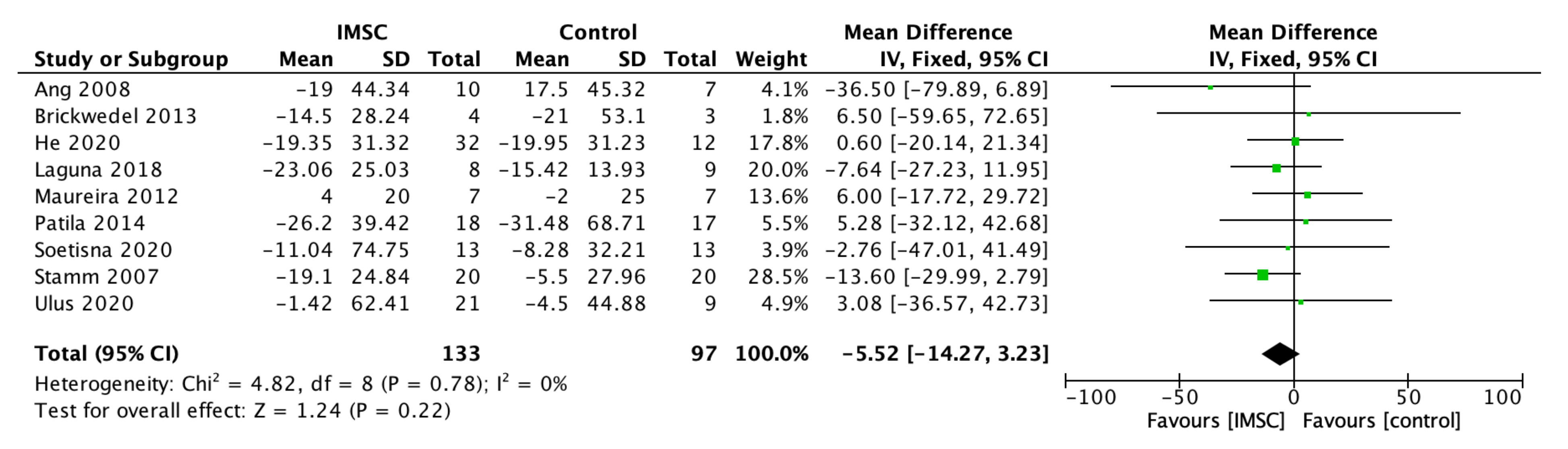
3.2.1. Left Ventricular Function, Contractility, Volume, and Dimension
3.2.2. Functional Outcome
3.2.3. Subgroup Analysis of LVEF
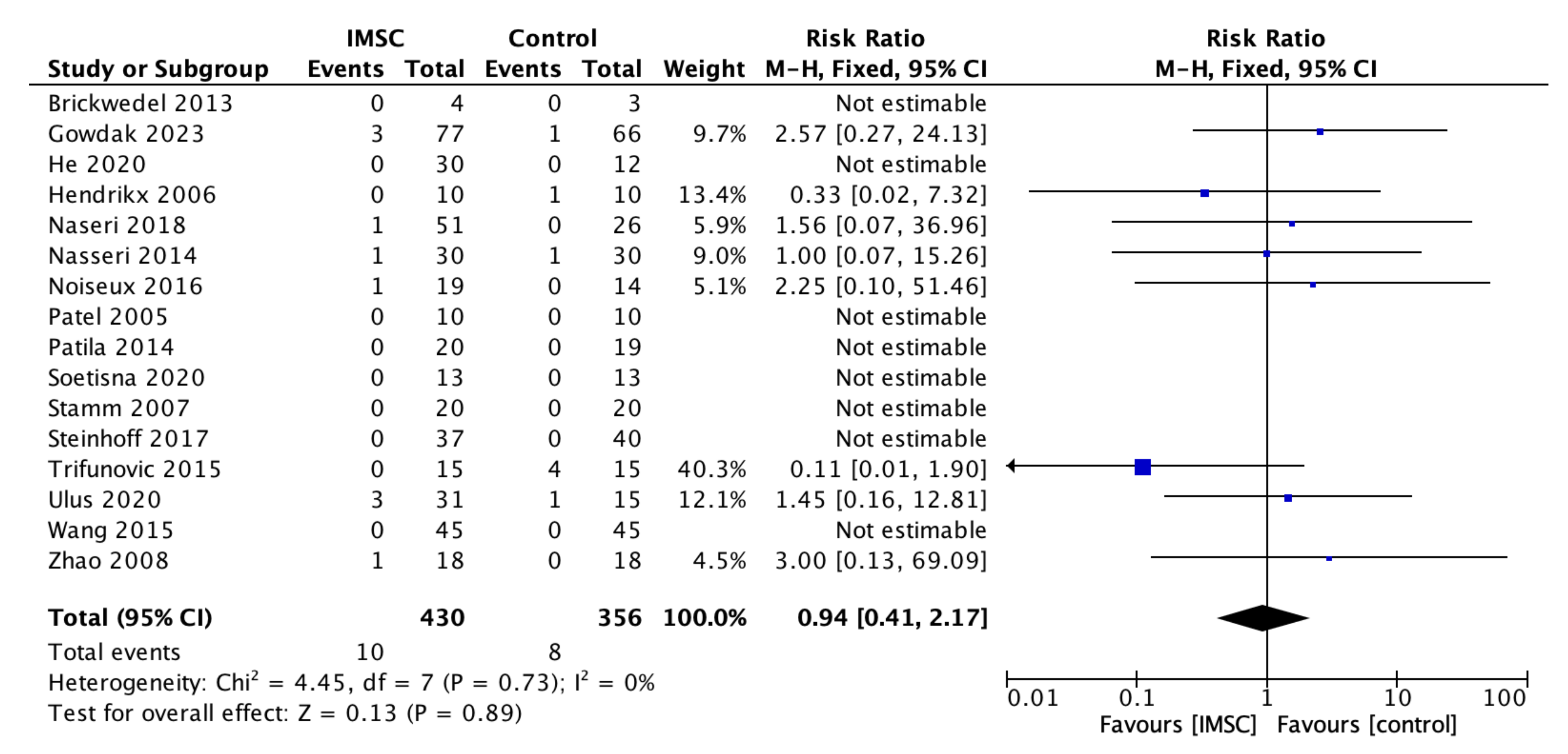
3.3. Secondary Outcome (Safety Measurements)
3.4. Risk of Bias Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yerebakan, C.; Kaminski, A.; Liebold, A.; Steinhoff, G. Safety of Intramyocardial Stem Cell Therapy for the Ischemic Myocardium: Results of the Rostock Trial after 5-Year Follow-Up. Cell Transplant. 2007, 16, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; He, K.; Hou, J. Autologous Bone Marrow Stem Cell Transplantation for Patients Undergoing Coronary Artery Bypass Grafting: A Meta-Analysis of 22 Randomized Controlled Trials. J. Cardiothorac. Surg. 2022, 17, 167. [Google Scholar] [CrossRef] [PubMed]
- Soetisna, T.W.; Sukmawan, R.; Setianto, B.; Mansyur, M.; Murni, T.W.; Listiyaningsih, E.; Santoso, A. Combined Transepicardial and Transseptal Implantation of Autologous CD133+ Bone Marrow Cells during Bypass Grafting Improves Cardiac Function in Patients with Low Ejection Fraction. J. Card. Surg. 2020, 35, 740–746. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, Q.; Zhao, Y.; Zhang, H.; Wang, B.; Pan, J.; Li, J.; Yu, H.; Wang, L.; Dai, J.; et al. Effect of Intramyocardial Grafting Collagen Scaffold With Mesenchymal Stromal Cells in Patients With Chronic Ischemic Heart Disease. JAMA Netw. Open 2020, 3, e2016236. [Google Scholar] [CrossRef]
- Li, Z.; Lin, L.; Wu, H.; Yan, L.; Wang, H.; Yang, H.; Li, H. Global, Regional, and National Death, and Disability-Adjusted Life-Years (DALYs) for Cardiovascular Disease in 2017 and Trends and Risk Analysis From 1990 to 2017 Using the Global Burden of Disease Study and Implications for Prevention. Front. Public Health 2021, 9, 559751. [Google Scholar] [CrossRef]
- Gheorghe, A.; Griffiths, U.; Murphy, A.; Legido-Quigley, H.; Lamptey, P.; Perel, P. The Economic Burden of Cardiovascular Disease and Hypertension in Low- and Middle-Income Countries: A Systematic Review. BMC Public Health 2018, 18, 975. [Google Scholar] [CrossRef]
- Hou, D.; Youssef, E.A.-S.; Brinton, T.J.; Zhang, P.; Rogers, P.; Price, E.T.; Yeung, A.C.; Johnstone, B.H.; Yock, P.G.; March, K.L. Radiolabeled Cell Distribution After Intramyocardial, Intracoronary, and Interstitial Retrograde Coronary Venous Delivery. Circulation 2005, 112, I150–I156. [Google Scholar] [CrossRef]
- Hendrikx, M.; Hensen, K.; Clijsters, C.; Jongen, H.; Koninckx, R.; Bijnens, E.; Ingels, M.; Jacobs, A.; Geukens, R.; Dendale, P.; et al. Recovery of Regional but Not Global Contractile Function by the Direct Intramyocardial Autologous Bone Marrow Transplantation. Circulation 2006, 114, I101–I107. [Google Scholar] [CrossRef]
- Naseri, M.H.; Madani, H.; Tafti, S.H.A.; Farahani, M.M.; Saleh, D.K.; Hosseinnejad, H.; Hosseini, S.; Hekmat, S.; Ahmadi, Z.H.; Dehghani, M.; et al. Erratum: COMPARE CPM-RMI Trial: Intramyocardial Transplantation of Autologous Bone Marrow-Derived CD133+ Cells and MNCs during CABG in Patients with Recent MI: A Phase II/III, Multicenter, Placebo-Controlled, Randomized, Double-Blind Clinical Trial. Cell J. 2018, 20, 449. [Google Scholar] [CrossRef]
- Trifunović, Z.; Obradović, S.; Balint, B.; Ilić, R.; Vukić, Z.; Šišić, M.; Kostić, J.; Rusović, S.; Dobrić, M.; Ostojić, G. Funkcionalni Oporavak Bolesnika Sa Ishemijskom Kardiomiopatijom Lečenih Implantacijom Mononuklearnih Ćelija Koštane Srži Tokom Aortokoronarne Bajpas Hirurgije. Vojnosanit. Pregl. 2015, 72, 225–232. [Google Scholar] [CrossRef]
- Fukushima, S.; Varela-Carver, A.; Coppen, S.R.; Yamahara, K.; Felkin, L.E.; Lee, J.; Barton, P.J.R.; Terracciano, C.M.N.; Yacoub, M.H.; Suzuki, K. Direct Intramyocardial But Not Intracoronary Injection of Bone Marrow Cells Induces Ventricular Arrhythmias in a Rat Chronic Ischemic Heart Failure Model. Circulation 2007, 115, 2254–2261. [Google Scholar] [CrossRef]
- Mozid, A.; Yeo, C.; Arnous, S.; Ako, E.; Saunders, N.; Locca, D.; Brookman, P.; Archbold, R.A.; Rothman, M.; Mills, P.; et al. Safety and Feasibility of Intramyocardial versus Intracoronary Delivery of Autologous Cell Therapy in Advanced Heart Failure: The REGENERATE-IHD Pilot Study. Regen. Med. 2014, 9, 269–278. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (Updated September 2020); Cochrane: Cochrane, AB, Canada, 2020. [Google Scholar]
- RevMan. The Cochrane Collaboration Review Manager; Version 5.4; RevMan: New York, NY, USA, 2020. [Google Scholar]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Lo, C.K.-L.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing Reviewers’ to Authors’ Assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef]
- Brickwedel, J.; Gulbins, H.; Reichenspurner, H. Long-Term Follow-up after Autologous Skeletal Myoblast Transplantation in Ischaemic Heart Disease. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 61–66. [Google Scholar] [CrossRef]
- Ulus, A.T.; Mungan, C.; Kurtoglu, M.; Celikkan, F.T.; Akyol, M.; Sucu, M.; Toru, M.; Gul, S.S.; Cinar, O.; Can, A. Intramyocardial Transplantation of Umbilical Cord Mesenchymal Stromal Cells in Chronic Ischemic Cardiomyopathy: A Controlled, Randomized Clinical Trial (HUC-HEART Trial). Int. J. Stem Cells 2020, 13, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.L.; Chin, D.; Leyva, F.; Foley, P.; Kubal, C.; Chalil, S.; Srinivasan, L.; Bernhardt, L.; Stevens, S.; Shenje, L.T.; et al. Randomized, Controlled Trial of Intramuscular or Intracoronary Injection of Autologous Bone Marrow Cells into Scarred Myocardium during CABG versus CABG Alone. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Gowdak, L.H.W.; Schettert, I.T.; Rochitte, C.E.; de Carvalho, L.P.; Vieira, M.L.C.; Dallan, L.A.O.; de Oliveira, S.A.; César, L.A.M.; Brito, J.O.R.; Guarita-Souza, L.C.; et al. Additional Improvement in Regional Myocardial Ischemia after Intracardiac Injection of Bone Marrow Cells during CABG Surgery. Front. Cardiovasc. Med. 2023, 10, 1040188. [Google Scholar] [CrossRef]
- Komok, V.V.; Bunenkov, N.S.; Beliy, S.A.; Pizin, V.M.; Kondratev, V.M.; Dulaev, A.V.; Kobak, A.E.; Maksimova, T.S.; Sergienko, I.P.; Parusova, E.V.; et al. Evaluation of Effectiveness of Combined Treatment of Coronary Heart Disease—Coronary Artery Bypass Grafting, Transplantation of Autologous Bone Marrow Mononuclear Cells: A Randomized, Blind Placebo-Controlled Study. Vestn. Transpl. Iskusstv. Organov. 2019, 21, 54–66. [Google Scholar] [CrossRef]
- Laguna, G.; Di Stefa No, S.; Maroto, L.; Fulquet, E.; Echevarría, J.R.; Revilla, A.; Urueña, N.; Sevilla, T.; Arnold, R.; Ramos, B.; et al. Effect of Direct Intramyocardial Autologous Stem Cell Grafting in the Sub-Acute Phase after Myocardial Infarction. J. Cardiovasc. Surg. 2018, 59, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Maureira, P.; Tran, N.; Djaballah, W.; Angioï, M.; Bensoussan, D.; Didot, N.; Fay, R.; Sadoul, N.; Villemot, J.P.; Marie, P.Y. Residual Viability Is a Predictor of the Perfusion Enhancement Obtained with the Cell Therapy of Chronic Myocardial Infarction: A Pilot Multimodal Imaging Study. Clin. Nucl. Med. 2012, 37, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Pätilä, T.; Lehtinen, M.; Vento, A.; Schildt, J.; Sinisalo, J.; Laine, M.; Hämmäinen, P.; Nihtinen, A.; Alitalo, R.; Nikkinen, P.; et al. Autologous Bone Marrow Mononuclear Cell Transplantation in Ischemic Heart Failure: A Prospective, Controlled, Randomized, Double-Blind Study of Cell Transplantation Combined with Coronary Bypass. J. Heart Lung Transplant. 2014, 33, 567–574. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Jiang, H.; Ma, D.; Zhou, W.; Zhang, G.; Chen, W.; Huang, J.; Liu, Y. Effect of Autologous Bone Marrow Cell Transplantation Combined with Off-Pump Coronary Artery Bypass Grafting on Cardiac Function in Patients with Chronic Myocardial Infarction. Cardiology 2015, 130, 27–33. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, Y.; Xia, L.; Chen, A.; Wang, Z. Randomized Study of Mononuclear Bone Marrow Cell Transplantation in Patients With Coronary Surgery. Ann. Thorac. Surg. 2008, 86, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, B.A.; Ebell, W.; Dandel, M.; Kukucka, M.; Gebker, R.; Doltra, A.; Knosalla, C.; Choi, Y.H.; Hetzer, R.; Stamm, C. Autologous CD133+ Bone Marrow Cells and Bypass Grafting for Regeneration of Ischaemic Myocardium: The Cardio133 Trial. Eur. Heart J. 2014, 35, 1263–1274. [Google Scholar] [CrossRef]
- Noiseux, N.; Mansour, S.; Weisel, R.; Stevens, L.M.; Der Sarkissian, S.; Tsang, K.; Crean, A.M.; Larose, E.; Li, S.H.; Wintersperger, B.; et al. The IMPACT-CABG Trial: A Multicenter, Randomized Clinical Trial of CD133+ Stem Cell Therapy during Coronary Artery Bypass Grafting for Ischemic Cardiomyopathy. J. Thorac. Cardiovasc. Surg. 2016, 152, 1582–1588.e2. [Google Scholar] [CrossRef]
- Patel, A.N.; Geffner, L.; Vina, R.F.; Saslavsky, J.; Urschel, H.C.; Kormos, R.; Benetti, F. Surgical Treatment for Congestive Heart Failure with Autologous Adult Stem Cell Transplantation: A Prospective Randomized Study. J. Thorac. Cardiovasc. Surg. 2005, 130, 1631–1638.e2. [Google Scholar] [CrossRef]
- Stamm, C.; Kleine, H.D.; Choi, Y.H.; Dunkelmann, S.; Lauffs, J.A.; Lorenzen, B.; David, A.; Liebold, A.; Nienaber, C.; Zurakowski, D.; et al. Intramyocardial Delivery of CD133+ Bone Marrow Cells and Coronary Artery Bypass Grafting for Chronic Ischemic Heart Disease: Safety and Efficacy Studies. J. Thorac. Cardiovasc. Surg. 2007, 133, 717–725.e5. [Google Scholar] [CrossRef]
- Steinhoff, G.; Nesteruk, J.; Wolfien, M.; Kundt, G.; Börgermann, J.; David, R.; Garbade, J.; Große, J.; Haverich, A.; Hennig, H.; et al. Cardiac Function Improvement and Bone Marrow Response: Outcome Analysis of the Randomized PERFECT Phase III Clinical Trial of Intramyocardial CD133+ Application After Myocardial Infarction. EBioMedicine 2017, 22, 208–224. [Google Scholar] [CrossRef]
- Kainuma, S.; Miyagawa, S.; Toda, K.; Yoshikawa, Y.; Hata, H.; Yoshioka, D.; Kawamura, T.; Kawamura, A.; Kashiyama, N.; Ito, Y.; et al. Long-Term Outcomes of Autologous Skeletal Myoblast Cell-Sheet Transplantation for End-Stage Ischemic Cardiomyopathy. Mol. Ther. 2021, 29, 1425–1438. [Google Scholar] [CrossRef] [PubMed]











| Author, Year | Country | Sample | Age (Years, Mean +/− SD) | SC Type | SC Dose (Mean +/− SD) | Control Group Treatment | Follow Up Period (Months) | Outcome Measurement Method | ||
|---|---|---|---|---|---|---|---|---|---|---|
| IMSC (Male, n) | Cont. (Male, n) | IMSC | Cont. | |||||||
| ASM/hUC-MSC | ||||||||||
| Brickwedel et al., 2013 [18] | Germany | 4 | 3 | 56.5 ± 5.1 | 62 ± 6.6 | ASM | High dose: 800 × 106 Low dose: 400 × 106 | CABG + Placebo | 6 | Echo |
| He et al., 2020 [4] | China | 32; 16/16 (25) | 12 (7) | 61.6 8.4 | 65.2 7.9 | hUC-MSC (±collagen hydrogel) | 108 | CABG only | 12 | CMR |
| Ulus et al., 2020 [19] | Turkey | 37; 25/12 (37) | 16 (16) | 60.2 3 | 65.3 1.7 | hUC-MSC/BMMC | 21–26 × 106/70 × 107 | CABG only | 12 | Echo/CMR |
| BMC/BMMC | ||||||||||
| Ang et al., 2008 [20] | United Kingdom | 21 (15) | 20 (18) | 64.7 ± 8.7 | 61.3 ± 8.3 | BMC | BMC 84 ± 56 × 106 CD34+/CD117+ cells 142 ± 166 × 103 | CABG only | 6 | CMR |
| Gowdak et al., 2023 [21] | Brazil | 77 (67) | 66 (51) | 59 11 | 57 11 | BMC | Min 108 | CABG + Placebo | 12 | Echo |
| Hendrikx et al., 2006 [8] | Belgium | 10 (10) | 10 (7) | 63.2 8.5 | 66.8 9.2 | BMC | 60.25 × 106 ± 31.35 × 106 | CABG only | 4 | CMR |
| Komok et al., 2019 [22] | Russia | 25 (6) | 37 (4) | 61 8 | 61.7 6.8 | BMMC | 4.06 x 106 ± 1.78 x 106 | CABG + Placebo | 12 | Echo |
| Laguna et al., 2018 [23] | Spain | 8 (7) | 9 (8) | 62.6 8.4 | 64.8 11.5 | BMMC | 107 | CABG + Placebo | 9 | CMR |
| Maureira et al., 2012 [24] | France | 7 (7) | 7 (6) | 58 10 | 57 10 | BMMC | 30–40 × 107 | CABG only | 6 | CMR |
| Naseri et al., 2018 [9] | Iran | 51; 30/21 (46) | 26 (23) | 52.1 7.9 | 55.5 8.5 | BMMC/CD133+ | BMMC 564.63 ± 69.35 × 106 CD133 + 8.19 ± 4.26 × 106 | CABG + Placebo | 18 | SPECT |
| Patila et al., 2014 [25] | Finland | 20 (19) | 19 (18) | 65 12.8 | 64 9.6 | BMMC | 9.1 ± 6.6 × 108 | CABG + Placebo | 12 | CMR |
| Trifunovic et al., 2015 [10] | Serbia | 15 (14) | 15 (14) | 53.8 10.1 | 60 6.8 | BMMC | 70.7 ± 32.4 × 106 | CABG only | 60 | Echo |
| Wang et al., 2015 [26] | China | 45 (37) | 45 (35) | 61.4 7.4 | 62.9 6.9 | BMC | 5.21 ± 0.44 × 108 | CABG + Placebo | 6 | Echo |
| Zhao et al., 2008 [27] | China | 18 (15) | 18 (15) | 60.3 10.4 | 59.1 15.7 | BMMC | 6.6 ± 5.12 × 108 | CABG + Placebo | 6 | Echo |
| CD34+/CD133+ | ||||||||||
| Nassseri et al., 2014 [28] | Germany | 30 (28) | 30 (29) | 61.9 7.3 | 62.7 10.6 | CD133+ | 5.8 ± 4.7 × 106 | CABG + Placebo | 6 | CMR |
| Noiseux et al., 2016 [29] | Canada | 19 (17) | 14 (13) | 66.4 6.5 | 63.1 7.2 | CD133+ | 6.5 ± 3.1 × 106 | CABG + Placebo | 6 | CMR |
| Patel et al., 2005 [30] | Argentina | 10 (8) | 10 (8) | 64.8 3.9 | 63.6 4.9 | CD34+ | 22 × 106 (median) | CABG only | 6 | Echo |
| Soetisna et al., 2020 [3] | Indonesia | 13 (12) | 13 (12) | 54.6 8.1 | 57.5 8.3 | CD133+ | 5–10 × 106 | CABG only | 6 | CMR |
| Stamm et al., 2007 [31] | Germany | 20 (15) | 20 (16) | 62 10.2 | 63.5 8.4 | CD133+ | 5.5 ± 1.9 × 106 | CABG only | 6 | Echo |
| Steinhoff et al., 2017 [32] | Germany | 37 (33) | 40 (34) | 63.5 8.3 | 62.9 8.5 | CD133+ | 2.3 ± 1.4 × 106 | CABG + Placebo | 6 | CMR |
| Subgroup Variable | No. of Studies | MD (95% CI) | Heterogeneity (I2) (%) | p-Value |
|---|---|---|---|---|
| LVEF Follow-Up Time | ||||
| ≤6 months | 12 | 4.38 (1.18–7.58) | 81 | 0.65 |
| >6 months | 7 | 3.10 (−1.47–7.67) | 84 | |
| LVEF Measurement Method | ||||
| Echo | 8 | 6.47 (2.76–10.19) | 85 | 0.04 |
| CMR/SPECT | 11 | 1.67 (−0.96–4.30) | 58 | |
| Type of Stem Cells | ||||
| BMC/BMMC | 11 | 4.40 (1.19–7.62) | 77 | 0.50 |
| CD133+/CD34+ | 7 | 4.13 (−0.92–9.19) | 89 | |
| ASM/hUC-MSC | 3 | 1.45 (−2.54–5.44) | 0 | |
| Mean Administered Stem Cell Dose | ||||
| <108 | 12 | 4.75 (1.48–8.02) | 79 | 0.49 |
| ≥108 | 9 | 3.05 (−0.51–6.61) | 79 | |
| Mean Baseline LVEF | ||||
| <35% | 7 | 4.37 (−0.52–9.25) | 84 | 0.81 |
| ≥35% | 12 | 3.65 (0.46–6.84) | 82 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soetisna, T.W.; Thamrin, A.M.H.; Permadijana, D.; Ramadhani, A.N.E.; Sugisman; Santoso, A.; Mansyur, M. Intramyocardial Stem Cell Transplantation during Coronary Artery Bypass Surgery Safely Improves Cardiac Function: Meta-Analysis of 20 Randomized Clinical Trials. J. Clin. Med. 2023, 12, 4430. https://doi.org/10.3390/jcm12134430
Soetisna TW, Thamrin AMH, Permadijana D, Ramadhani ANE, Sugisman, Santoso A, Mansyur M. Intramyocardial Stem Cell Transplantation during Coronary Artery Bypass Surgery Safely Improves Cardiac Function: Meta-Analysis of 20 Randomized Clinical Trials. Journal of Clinical Medicine. 2023; 12(13):4430. https://doi.org/10.3390/jcm12134430
Chicago/Turabian StyleSoetisna, Tri Wisesa, Ahmad Muslim Hidayat Thamrin, Diajeng Permadijana, Andi Nurul Erisya Ramadhani, Sugisman, Anwar Santoso, and Muchtaruddin Mansyur. 2023. "Intramyocardial Stem Cell Transplantation during Coronary Artery Bypass Surgery Safely Improves Cardiac Function: Meta-Analysis of 20 Randomized Clinical Trials" Journal of Clinical Medicine 12, no. 13: 4430. https://doi.org/10.3390/jcm12134430
APA StyleSoetisna, T. W., Thamrin, A. M. H., Permadijana, D., Ramadhani, A. N. E., Sugisman, Santoso, A., & Mansyur, M. (2023). Intramyocardial Stem Cell Transplantation during Coronary Artery Bypass Surgery Safely Improves Cardiac Function: Meta-Analysis of 20 Randomized Clinical Trials. Journal of Clinical Medicine, 12(13), 4430. https://doi.org/10.3390/jcm12134430













