MRI-Conditional Breast Tissue Expander: First In-Human Multi-Case Assessment of MRI-Related Complications and Image Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
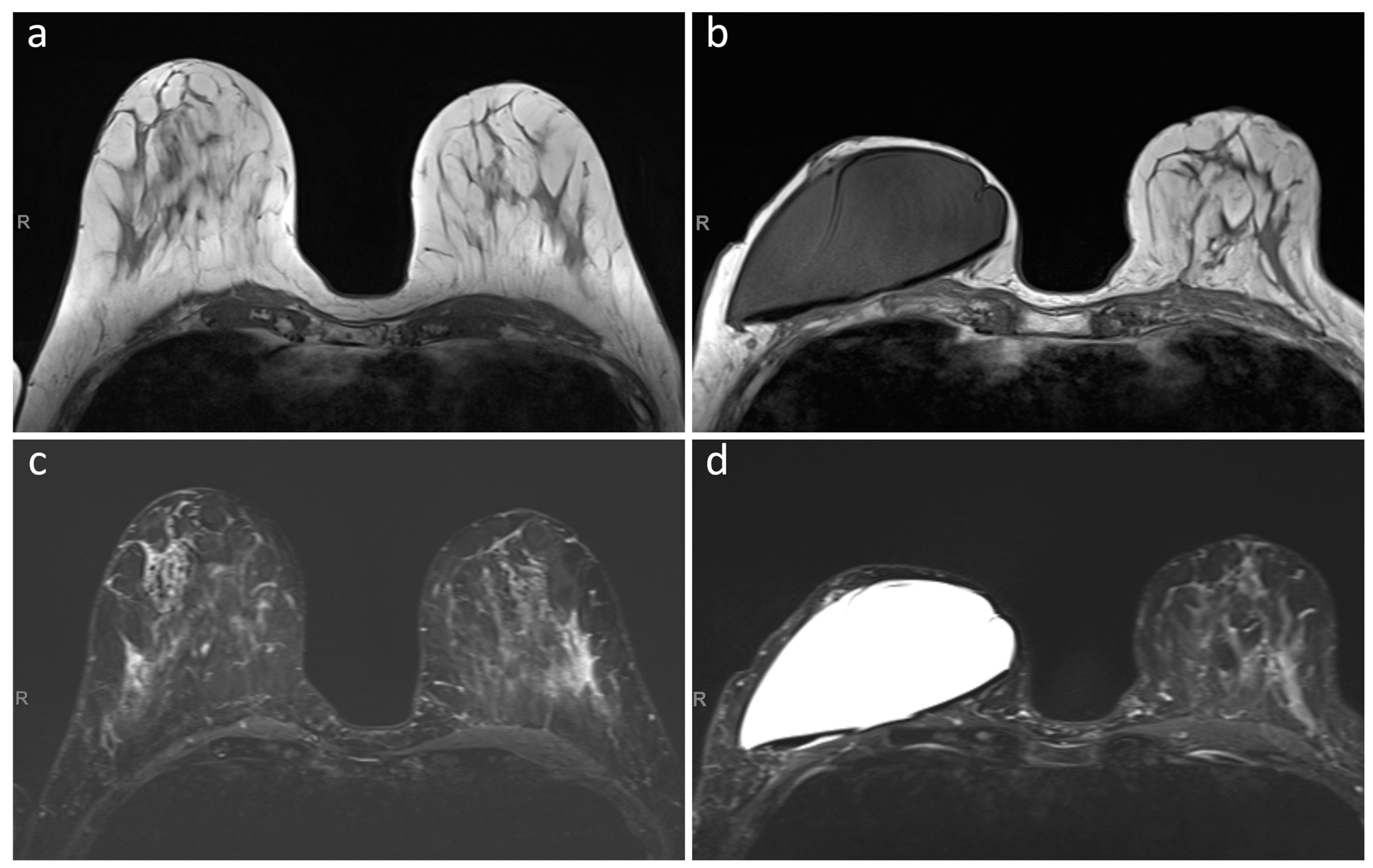
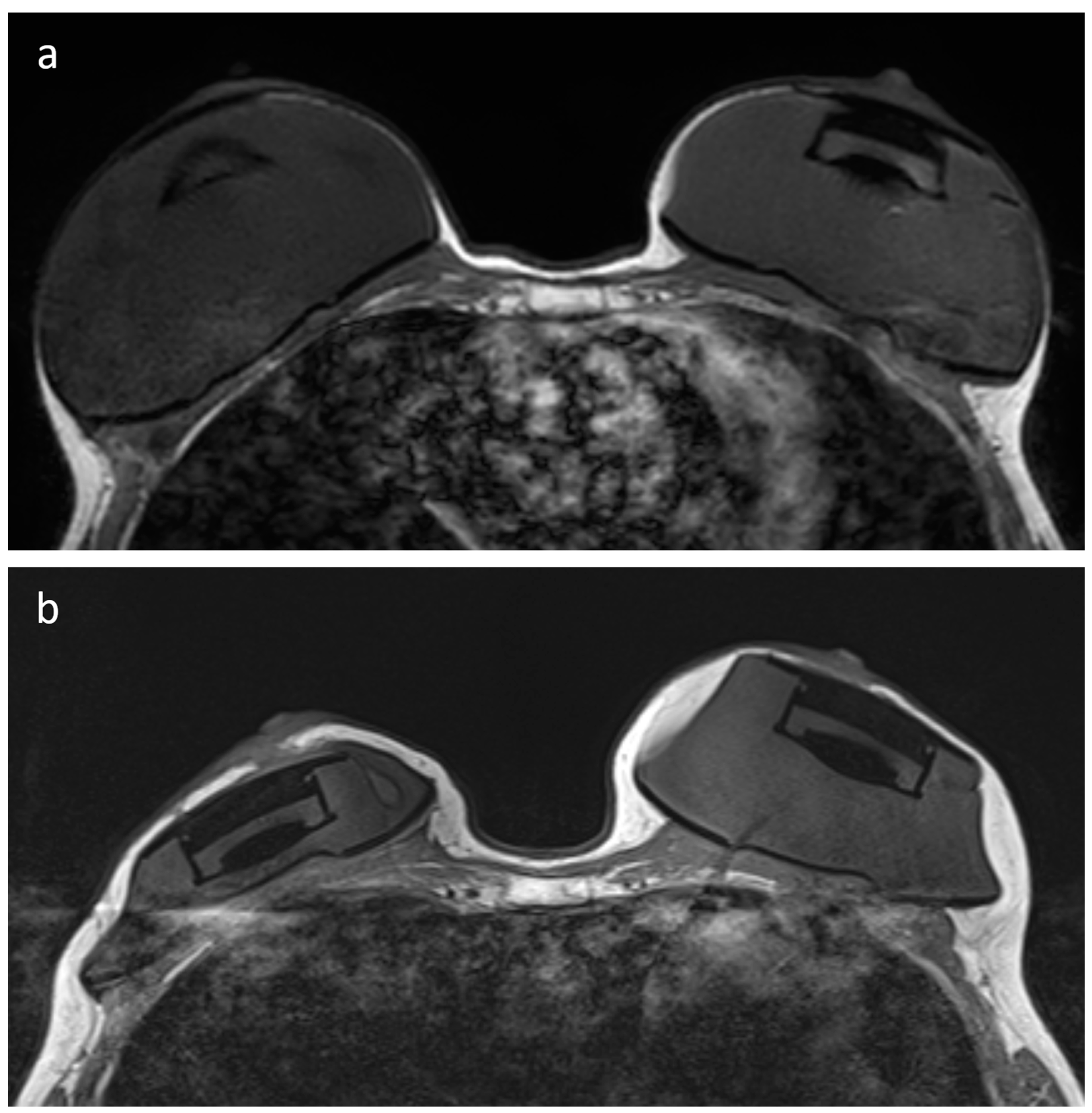
2.2. Image Quality Assessment
- Overall image quality for diagnostic purposes, on a qualitative four-level Likert scale (“Poor”, “Sufficient”, “Good”, and “Excellent”);
- Field homogeneity, with dichotomic assessment (“Affected” and “Not affected”);
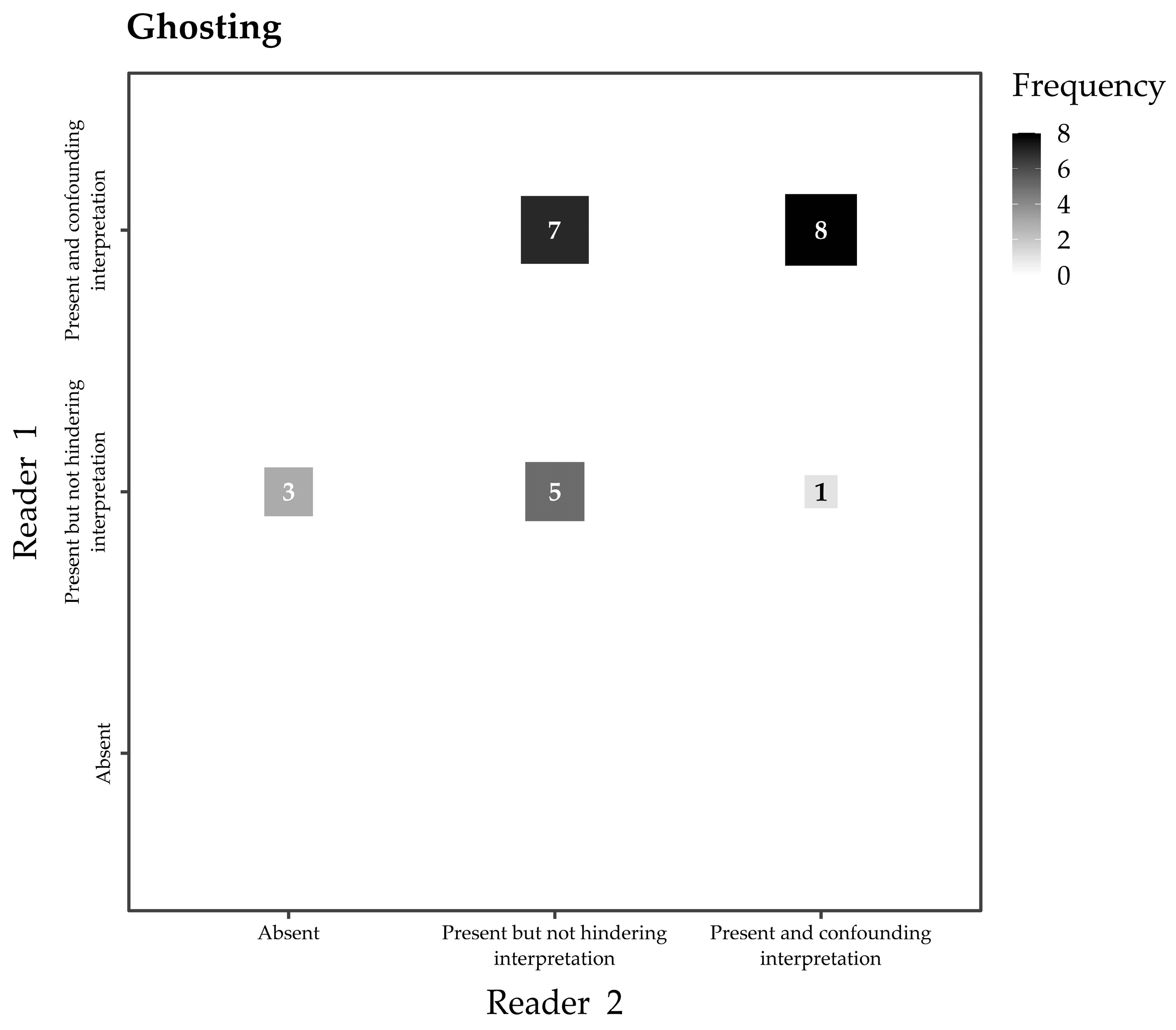
- Image noise, on a three-level ordinal scale (“Present and confounding interpretation”, “Present but not hindering interpretation”, and “Absent”);
- Maximum diameter of the artifact caused by the RFID port on axial T1-weighted images (on the side with largest artifact in patients with bilateral expanders) and maximum diameter of the expander on the same axial plane, with calculation of their ratio.
- 5.
- Overall image quality for diagnostic purposes, again on a four-level scale (“Poor”, “Sufficient”, “Good”, and “Excellent”);
- 6.
- Ability to distinguish thoracic anatomical structures (chest wall and sternum), with dichotomic assessment;
- 7.
- Quality of fat suppression, on a three-level ordinal scale (“Insufficient”, “Inhomogeneous”, and “Homogeneous”);
- 8.
- Presence of ghosting artifacts, on a three-level ordinal scale (“Present and confounding interpretation”, “Present but not hindering interpretation”, and “Absent”)
- 9.
- Maximum diameter of the RFID-related artifact.
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fairbairn, K.; Cervantes, A.; Rayhrer, C.; Steen, S. Trends in Contralateral Prophylactic Mastectomy. Aesthetic Plast. Surg. 2020, 44, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Carbine, N.E.; Lostumbo, L.; Wallace, J.; Ko, H. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst. Rev. 2018, 4, CD002748. [Google Scholar] [CrossRef] [PubMed]
- Teoh, V.; Tasoulis, M.-K.; Gui, G. Contralateral Prophylactic Mastectomy in Women with Unilateral Breast Cancer Who Are Genetic Carriers, Have a Strong Family History or Are just Young at Presentation. Cancers 2020, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Crispin, M.; Udovicich, C.; Chan, S.; Wong, S.; Pitcher, M. Trends in genetic screening referral in breast cancer patients under the age of forty: 2001–2016. Breast J. 2018, 24, 1109–1111. [Google Scholar] [CrossRef]
- Manahan, E.R.; Kuerer, H.M.; Sebastian, M.; Hughes, K.S.; Boughey, J.C.; Euhus, D.M.; Boolbol, S.K.; Taylor, W.A. Consensus Guidelines on Genetic’ Testing for Hereditary Breast Cancer from the American Society of Breast Surgeons. Ann. Surg. Oncol. 2019, 26, 3025–3031. [Google Scholar] [CrossRef]
- Hartmann, L.C.; Lindor, N.M. The Role of Risk-Reducing Surgery in Hereditary Breast and Ovarian Cancer. N. Engl. J. Med. 2016, 374, 454–468. [Google Scholar] [CrossRef]
- Krontiras, H.; Farmer, M.; Whatley, J. Breast Cancer Genetics and Indications for Prophylactic Mastectomy. Surg. Clin. N. Am. 2018, 98, 677–685. [Google Scholar] [CrossRef]
- Heemskerk-Gerritsen, B.A.M.; Jager, A.; Koppert, L.B.; Obdeijn, A.I.-M.; Collée, M.; Meijers-Heijboer, H.E.J.; Jenner, D.J.; Oldenburg, H.S.A.; van Engelen, K.; de Vries, J.; et al. Survival after bilateral risk-reducing mastectomy in healthy BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2019, 177, 723–733. [Google Scholar] [CrossRef]
- Colwell, A.S.; Taylor, E.M. Recent Advances in Implant-Based Breast Reconstruction. Plast. Reconstr. Surg. 2020, 145, 421e–432e. [Google Scholar] [CrossRef]
- Kappos, E.A.; Schulz, A.; Regan, M.M.; Moffa, G.; Harder, Y.; Ribi, K.; Potter, S.; Pusic, A.L.; Fehr, M.K.; Hemkens, L.G.; et al. Prepectoral versus subpectoral implant-based breast reconstruction after skin-sparing mastectomy or nipple-sparing mastectomy (OPBC-02/PREPEC): A pragmatic, multicentre, randomised, superiority trial. BMJ Open 2021, 11, e045239. [Google Scholar] [CrossRef]
- Bedrosian, I.; Parker, P.A.; Brewster, A.M. Who should get a contralateral prophylactic mastectomy for breast cancer? Cancer 2019, 125, 1400–1403. [Google Scholar] [CrossRef]
- Scheepens, J.C.C.; van’t Veer, L.; Esserman, L.; Belkora, J.; Mukhtar, R.A. Contralateral prophylactic mastectomy: A narrative review of the evidence and acceptability. Breast 2021, 56, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Schmauss, D.; Machens, H.-G.; Harder, Y. Breast Reconstruction after Mastectomy. Front. Surg. 2016, 2, 71. [Google Scholar] [CrossRef]
- Nahabedian, M.Y.; Hammer, J. Use of Magnetic Resonance Imaging in Patients with Breast Tissue Expanders. Plast. Reconstr. Surg. 2022, 150, 963–968. [Google Scholar] [CrossRef]
- Bayasgalan, M.; Munhoz, A.M.; Shellock, F.G. Breast Tissue Expander With Radiofrequency Identification Port: Assessment of MRI Issues. Am. J. Roentgenol. 2020, 215, 159–164. [Google Scholar] [CrossRef]
- ASPS National Clearinghouse of Plastic Surgery Procedural Statistics Plastic Surgery Statistics Report. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf (accessed on 12 June 2023).
- Elmore, L.C.; Dietz, J.R.; Myckatyn, T.M.; Margenthaler, J.A. The Landmark Series: Mastectomy Trials (Skin-Sparing and Nipple-Sparing and Reconstruction Landmark Trials). Ann. Surg. Oncol. 2021, 28, 273–280. [Google Scholar] [CrossRef]
- Galimberti, V.; Vicini, E.; Corso, G.; Morigi, C.; Fontana, S.; Sacchini, V.; Veronesi, P. Nipple-sparing and skin-sparing mastectomy: Review of aims, oncological safety and contraindications. Breast 2017, 34, S82–S84. [Google Scholar] [CrossRef]
- Bellini, E.; Pesce, M.; Santi, P.; Raposio, E. Two-Stage Tissue-Expander Breast Reconstruction: A Focus on the Surgical Technique. BioMed Res. Int. 2017, 2017, 1791546. [Google Scholar] [CrossRef] [PubMed]
- Shellock, F.G.; Woods, T.O.; Crues, J.V. MR Labeling Information for Implants and Devices: Explanation of Terminology. Radiology 2009, 253, 26–30. [Google Scholar] [CrossRef]
- Nazarian, S.; Beinart, R.; Halperin, H.R. Magnetic Resonance Imaging and Implantable Devices. Circ. Arrhythmia Electrophysiol. 2013, 6, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Morrison, K.A.; Ascherman, B.M.; Ascherman, J.A. Evolving Approaches to Tissue Expander Design and Application. Plast. Reconstr. Surg. 2017, 140, 23S–29S. [Google Scholar] [CrossRef] [PubMed]
- Stillaert, F.B.J.L.; Lannau, B.; Van Landuyt, K.; Blondeel, P.N. The Prepectoral, Hybrid Breast Reconstruction: The Synergy of Lipofilling and Breast Implants. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2966. [Google Scholar] [CrossRef] [PubMed]
- Sardanelli, F.; Boetes, C.; Borisch, B.; Decker, T.; Federico, M.; Gilbert, F.J.; Helbich, T.; Heywang-Köbrunner, S.H.; Kaiser, W.A.; Kerin, M.J.; et al. Magnetic resonance imaging of the breast: Recommendations from the EUSOMA working group. Eur. J. Cancer 2010, 46, 1296–1316. [Google Scholar] [CrossRef]
- Linnemeyer, H.; Shellock, F.G.; Ahn, C.Y. In vitro assessment of MRI issues at 3-Tesla for a breast tissue expander with a remote port. Magn. Reson. Imaging 2014, 32, 297–302. [Google Scholar] [CrossRef]
- Nava, M.B.; Bertoldi, S.; Forti, M.; Catanuto, G.; Vergnaghi, D.; Altomare, L.; Tanzi, M.C.; Farè, S. Effects of the Magnetic Resonance Field on Breast Tissue Expanders. Aesthetic Plast. Surg. 2012, 36, 901–907. [Google Scholar] [CrossRef]
- Thimmappa, N.D.; Prince, M.R.; Colen, K.L.; Ahn, C.Y.; Dutruel, S.P.; Boddu, S.R.; Greenspun, D.T.; Vasile, J.V.; Chen, C.M.; Usal, H.; et al. Breast Tissue Expanders with Magnetic Ports. Plast. Reconstr. Surg. 2016, 138, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Fausto, A.; Bernini, M.; Giacomo, L.D.; Schivartche, V.; Marcasciano, M.; Casella, D.; Volterrani, L.; Mazzei, M.A. Diagnostic value and safety of dynamic MRI of contralateral breast and axilla in subjects with tissue expander. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 1282–1285. [Google Scholar] [CrossRef]
- Dibbs, R.; Culo, B.; Tandon, R.; Hilaire, H.S.; Shellock, F.G.; Lau, F.H. Reconsidering the “MR Unsafe” breast tissue expander with magnetic infusion port: A case report and literature review. Arch. Plast. Surg. 2019, 46, 375–380. [Google Scholar] [CrossRef]
- Marano, A.A.; Henderson, P.W.; Prince, M.R.; Dashnaw, S.M.; Rohde, C.H. Effect of MRI on breast tissue expanders and recommendations for safe use. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 1702–1707. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, M.K. Research Methods in Political Science, 8th ed.; Cengage Learning: Boston, MA, USA, 2012. [Google Scholar]
- Otte, M.; Nestle-Krämling, C.; Fertsch, S.; Hagouan, M.; Munder, B.; Richrath, P.; Stambera, P.; Abu-Ghazaleh, A.; Andree, C. Conservative mastectomies and Immediate-DElayed AutoLogous (IDEAL) breast reconstruction: The DIEP flap. Gland Surg. 2016, 5, 24–31. [Google Scholar] [CrossRef]
- Fabiocchi, L.; Lucattelli, E.; Cattin, F.; Cipriani, F.; Dellachiesa, L.; Fogacci, T.; Frisoni, G.; Semprini, G.; Samorani, D. Reverse Expansion for Breast Reconstruction after Skin-sparing and Nipple-sparing Mastectomy: Our First 100 Cases. Plast. Reconstr. Surg. Glob. Open 2023, 11, e4915. [Google Scholar] [CrossRef]
- Pusic, A.L.; Matros, E.; Fine, N.; Buchel, E.; Gordillo, G.M.; Hamill, J.B.; Kim, H.M.; Qi, J.; Albornoz, C.; Klassen, A.F.; et al. Patient-Reported Outcomes 1 Year After Immediate Breast Reconstruction: Results of the Mastectomy Reconstruction Outcomes Consortium Study. J. Clin. Oncol. 2017, 35, 2499–2506. [Google Scholar] [CrossRef]
- Santosa, K.B.; Qi, J.; Kim, H.M.; Hamill, J.B.; Wilkins, E.G.; Pusic, A.L. Long-term Patient-Reported Outcomes in Postmastectomy Breast Reconstruction. JAMA Surg. 2018, 153, 891. [Google Scholar] [CrossRef]
- Panchal, H.; Shukla, D.; Razdan, S.N.; El-Tamer, M.; Matros, E.; Henderson, P.W. American trends in oncoplastic breast surgery for 2006–2015: A retrospective analysis of NSQIP database. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 644–710. [Google Scholar] [CrossRef]
- Ribuffo, D.; Berna, G.; De Vita, R.; Di Benedetto, G.; Cigna, E.; Greco, M.; Valdatta, L.; Onesti, M.G.; Lo Torto, F.; Marcasciano, M.; et al. Dual-Plane Retro-pectoral Versus Pre-pectoral DTI Breast Reconstruction: An Italian Multicenter Experience. Aesthetic Plast. Surg. 2021, 45, 51–60. [Google Scholar] [CrossRef]
- Weinzierl, A.; Schmauss, D.; Harder, Y. Der Stellenwert von synthetischen Netzen und biologischen Matrices in der Implantat-basierten Brustrekonstruktion. Handchir. Mikrochir. Plast. Chir. 2022, 54, 269–278. [Google Scholar] [CrossRef]
- Weinzierl, A.; Schmauss, D.; Brucato, D.; Harder, Y. Implant-Based Breast Reconstruction after Mastectomy, from the Subpectoral to the Prepectoral Approach: An Evidence-Based Change of Mind? J. Clin. Med. 2022, 11, 3079. [Google Scholar] [CrossRef]
- Harless, C.; Jacobson, S.R. Current strategies with 2-staged prosthetic breast reconstruction. Gland Surg. 2015, 4, 204–211. [Google Scholar] [CrossRef]
- Corban, J.; Shash, H.; Safran, T.; Sheppard-Jones, N.; Fouda–Neel, O. A systematic review of complications associated with direct implants vs. tissue expanders following Wise pattern skin-sparing mastectomy. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 1191–1199. [Google Scholar] [CrossRef]
- Rietjens, M.; Villa, G.; Toesca, A.; Rizzo, S.; Raimondi, S.; Rossetto, F.; Sangalli, C.; De Lorenzi, F.; Manconi, A.; Matthes, A.G.Z.; et al. Appropriate Use of Magnetic Resonance Imaging and Ultrasound to Detect Early Silicone Gel Breast Implant Rupture in Postmastectomy Reconstruction. Plast. Reconstr. Surg. 2014, 134, 13e–20e. [Google Scholar] [CrossRef]
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology 2019, 292, 520–536. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, J.-A.; Kim, S.Y. Incidental Extramammary Findings on Preoperative Breast MRI in Breast Cancer Patients: A Pictorial Essay. J. Korean Soc. Radiol. 2023, 84, 372. [Google Scholar] [CrossRef]
- Neri, E.; Bali, M.A.; Ba-Ssalamah, A.; Boraschi, P.; Brancatelli, G.; Alves, F.C.; Grazioli, L.; Helmberger, T.; Lee, J.M.; Manfredi, R.; et al. ESGAR consensus statement on liver MR imaging and clinical use of liver-specific contrast agents. Eur. Radiol. 2016, 26, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Ahn, J.-H.; Kim, M.K.; Sym, S.J.; Gong, G.; Ahn, S.D.; Kim, S.-B.; Kim, W.K. Brain metastases in breast cancer: Prognostic factors and management. Breast Cancer Res. Treat. 2008, 111, 523–530. [Google Scholar] [CrossRef]
- Panagiotidis, E.; Pant, V.; Vinjamuri, S. Review of the role of MRI and 18F-sodium fluoride PET/computed tomography in the characterisation of spinal bone metastases in a cohort of patients with breast cancer. Nucl. Med. Commun. 2023, 44, 219–225. [Google Scholar] [CrossRef]
- Chen, D.H.; Tyebally, S.; Malloupas, M.; Roylance, R.; Spurrell, E.; Raja, F.; Ghosh, A.K. Cardiovascular Disease Amongst Women Treated for Breast Cancer: Traditional Cytotoxic Chemotherapy, Targeted Therapy, and Radiation Therapy. Curr. Cardiol. Rep. 2021, 23, 16. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiaffino, S.; Cozzi, A.; Pompei, B.; Scarano, A.L.; Catanese, C.; Catic, A.; Rossi, L.; Del Grande, F.; Harder, Y. MRI-Conditional Breast Tissue Expander: First In-Human Multi-Case Assessment of MRI-Related Complications and Image Quality. J. Clin. Med. 2023, 12, 4410. https://doi.org/10.3390/jcm12134410
Schiaffino S, Cozzi A, Pompei B, Scarano AL, Catanese C, Catic A, Rossi L, Del Grande F, Harder Y. MRI-Conditional Breast Tissue Expander: First In-Human Multi-Case Assessment of MRI-Related Complications and Image Quality. Journal of Clinical Medicine. 2023; 12(13):4410. https://doi.org/10.3390/jcm12134410
Chicago/Turabian StyleSchiaffino, Simone, Andrea Cozzi, Barbara Pompei, Angela Lia Scarano, Carola Catanese, Armin Catic, Lorenzo Rossi, Filippo Del Grande, and Yves Harder. 2023. "MRI-Conditional Breast Tissue Expander: First In-Human Multi-Case Assessment of MRI-Related Complications and Image Quality" Journal of Clinical Medicine 12, no. 13: 4410. https://doi.org/10.3390/jcm12134410
APA StyleSchiaffino, S., Cozzi, A., Pompei, B., Scarano, A. L., Catanese, C., Catic, A., Rossi, L., Del Grande, F., & Harder, Y. (2023). MRI-Conditional Breast Tissue Expander: First In-Human Multi-Case Assessment of MRI-Related Complications and Image Quality. Journal of Clinical Medicine, 12(13), 4410. https://doi.org/10.3390/jcm12134410






