Circulating Syndecan-1 Levels Are Associated with Chronological Coagulofibrinolytic Responses and the Development of Disseminated Intravascular Coagulation (DIC) after Trauma: A Retrospective Observational Study
Abstract
1. Introduction
2. Method
2.1. Study Design
2.2. Patient Selection and Criteria
2.3. Blood Sampling and Measurement
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
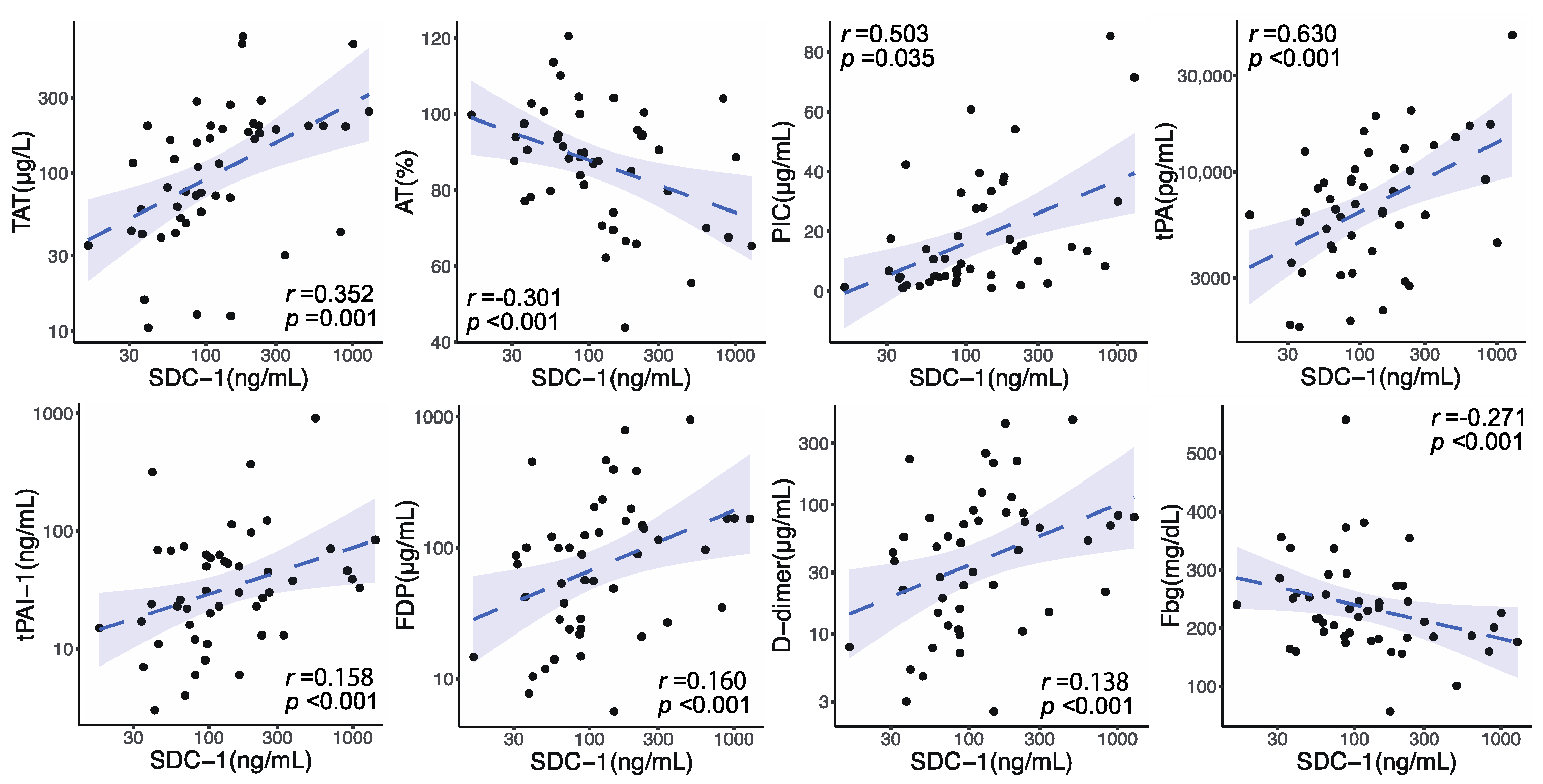
3.2. Circulating SDC-1 Changes after Trauma and Relationship between Circulating SDC-1 Levels and Coagulofibrinolytic Responses on Admission
3.3. Association between Circulating SDC-1 Elevation and Chronological Changes in Coagulofibrinolytic Markers
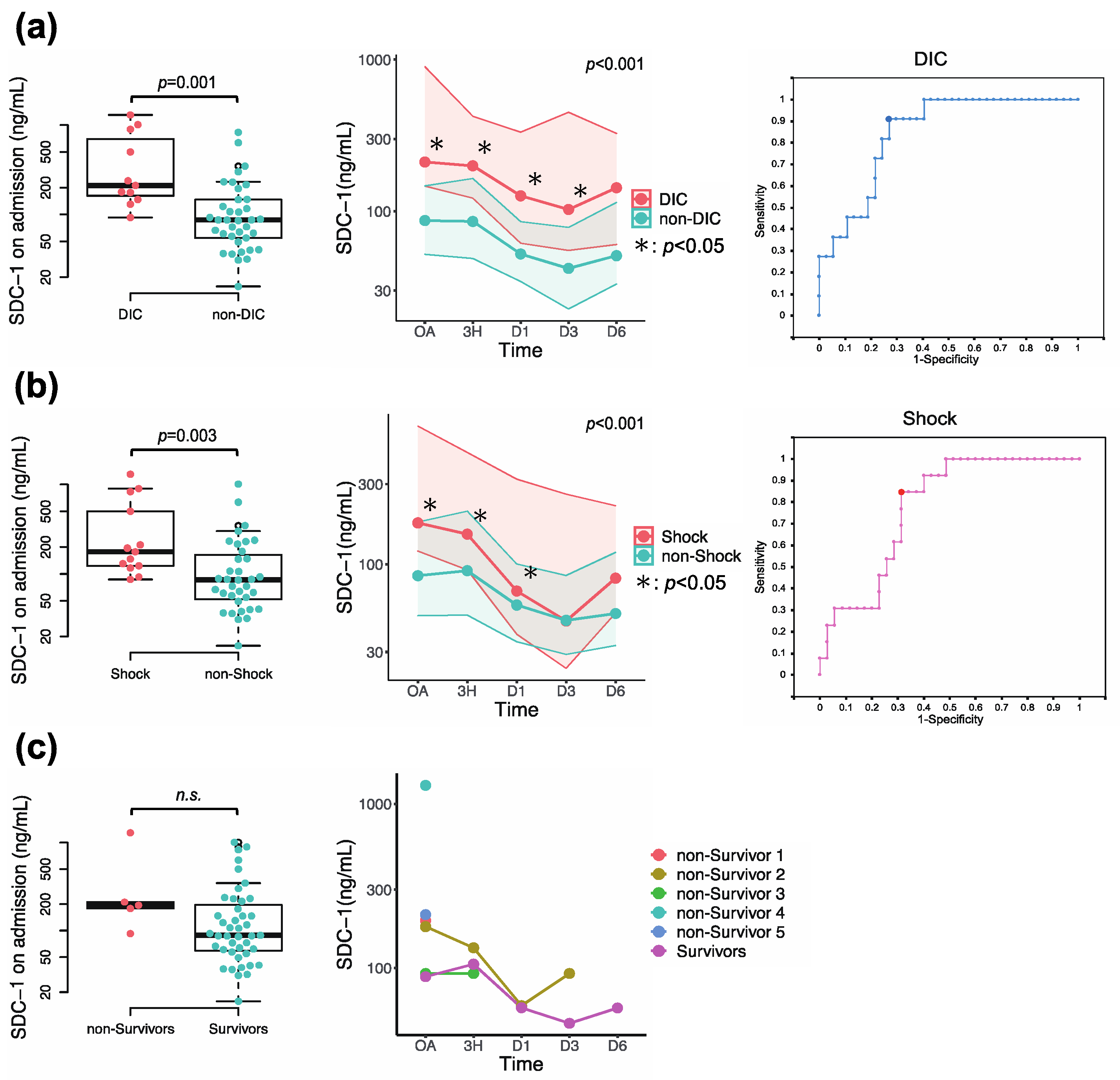
3.4. Association of Circulating SDC-1 with Development of DIC, Prevalence of Shock, and In-Hospital Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Norton, R.; Kobusingye, O. Injuries. N. Engl. J. Med. 2013, 368, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Brohi, K.; Singh, J.; Heron, M.; Coats, T. Acute traumatic coagulopathy. J. Trauma 2003, 54, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, J.B.A.M.; Lynn, M.; McKenney, M.G.; Cohn, S.M.; Murtha, M.R. Early Coagulopathy Predicts Mortality in Trauma. J. Trauma Inj. Infect. Crit. Care 2003, 55, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, J.B.; Tilley, B.C.; Baraniuk, S.; Fox, E.E.; Wade, C.E.; Podbielski, J.M.; del Junco, J.L.; Brasel, K.J.; Bulger, E.M.; PROPPR Study Group; et al. Transfusion of plasma, platelets, and red cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma The PROPPR randomized clinical trial. JAMA 2015, 313, 471–482. [Google Scholar] [CrossRef]
- Gando, S.; Otomo, Y. Local hemostasis, immunothrombosis, and systemic disseminated intravascular coagulation in trauma and traumatic shock. Crit. Care 2015, 19, 1–11. [Google Scholar] [CrossRef]
- Gando, S. Disseminated Intravascular Coagulation in Trauma Patients. Semin. Thromb. Hemost. 2001, 27, 585–592. [Google Scholar] [CrossRef]
- Moore, H.B.; Moore, E.E.; Neal, M.D.; Sheppard, F.R.; Kornblith, L.Z.; Draxler, D.F.; Sauaia, A. Fibrinolysis Shutdown in Trauma: Historical Review and Clinical Implications. Anesth. Analg. 2019, 129, 762–773. [Google Scholar] [CrossRef]
- Uchimido, R.; Schmidt, E.P.; Shapiro, N.I. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit. Care 2019, 23, 1–12. [Google Scholar] [CrossRef]
- Bertrand, J.; Bollmann, M. Soluble syndecans: Biomarkers for diseases and therapeutic options. Br. J. Pharmacol. 2019, 176, 67–81. [Google Scholar] [CrossRef]
- Teng, Y.H.-F.; Aquino, R.S.; Park, P.W. Molecular functions of syndecan-1 in disease. Matrix Biol. 2012, 31, 3–16. [Google Scholar] [CrossRef]
- Okada, H.; Takemura, G.; Suzuki, K.; Oda, K.; Takada, C.; Hotta, Y.; Miyazaki, N.; Tsujimoto, A.; Muraki, I.; Ando, Y.; et al. Three-dimensional ultrastructure of capillary endothelial glycocalyx under normal and experimental endotoxemic conditions. Crit. Care 2017, 21, 261. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.G.; Ostrowski, S.R.; Cardenas, J.C.; Baer, L.A.; Tomasek, J.S.; Henriksen, H.H.; Wade, C.E. Syndecan-1: A quantitative marker for the endotheliopathy of trauma. J. Am. Coll. Surg. 2017, 225, 419–427. [Google Scholar] [CrossRef]
- Rodriguez, E.G.; Cardenas, J.C.; Lopez, E.; Cotton, B.A.; Tomasek, J.S.; Ostrowski, S.R.; Wade, C.E. Early Identification of the Patient with Endotheliopathy of Trauma by Arrival Serum Albumin. Shock 2018, 50, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.I.; Stensballe, J.; Rasmussen, L.S.; Ostrowski, S.R. A High Admission Syndecan-1 Level, A Marker of Endothelial Glycocalyx Degradation, Is Associated with Inflammation, Protein C Depletion, Fibrinolysis, and Increased Mortality in Trauma Patients. Ann. Surg. 2011, 254, 194–200. [Google Scholar] [CrossRef]
- Ostrowski, S.R.; Sørensen, A.M.; Windeløv, N.A.; Perner, A.; Welling, K.L.; Wanscher, M.; Johansson, P.I. High levels of soluble VEGF receptor 1 early after trauma are associated with shock, sympathoadrenal activation, glycocalyx degradation and inflammation in severely injured patients: A prospective study. Scand. J. Trauma Resusc. Emerg. Med. 2012, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.I.; Henriksen, H.H.; Stensballe, J.; Gybel-Brask, M.; Cardenas, J.C.; Baer, L.A.; Ostrowski, S.R. Traumatic endotheliopathy: A prospective observational study of 424 severely injured patients. Ann. Surg. 2017, 265, 597–603. [Google Scholar] [CrossRef]
- Suzuki, K.; Okada, H.; Sumi, K.; Tomita, H.; Kobayashi, R.; Ishihara, T.; Mizuno, Y.; Yamaji, F.; Kamidani, R.; Miura, T.; et al. Syndecan-1 as a severity biomarker for patients with trauma. Front. Med. 2022, 9, 985955. [Google Scholar] [CrossRef]
- Qi, F.; Zhou, H.; Gu, P.; Tang, Z.-H.; Zhu, B.-F.; Chen, J.-R.; Zhang, J.-S.; Li, F. Endothelial glycocalyx degradation is associated with early organ impairment in polytrauma patients. BMC Emerg. Med. 2021, 21, 52. [Google Scholar] [CrossRef]
- Gando, S.; Shiraishi, A.; Wada, T.; Yamakawa, K.; Fujishima, S.; Saitoh, D.; Kushimoto, S.; Ogura, H.; Abe, T.; Otomo, Y.; et al. A multicenter prospective validation study on disseminated intravascular coagulation in trauma-induced coagulopathy. J. Thromb. Haemost. 2020, 18, 2232–2244. [Google Scholar] [CrossRef]
- Hayakawa, M. Pathophysiology of trauma-induced coagulopathy: Disseminated intravascular coagulation with the fibrinolytic phenotype. J. Intensiv. Care 2017, 5, 14. [Google Scholar] [CrossRef]
- Taylor, F.B., Jr.; Toh, C.H.; Hoots, W.K.; Wada, H.; Levi, M. Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb. Haemost. 2001, 86, 1327–1330. [Google Scholar]
- Patel, P.; Walborn, A.; Rondina, M.; Fareed, J.; Hoppensteadt, D. Markers of Inflammation and Infection in Sepsis and Disseminated Intravascular Coagulation. Clin. Appl. Thromb. 2019, 25, 1076029619843338. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levy, J.H. Derangement of the endothelial glycocalyx in sepsis. J. Thromb. Haemost. 2019, 17, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, K.; Ito, T.; Madokoro, Y.; Kamikokuryo, C.; Niiyama, S.; Yamada, S.; Maruyama, I.; Kakihana, Y. Circulating Syndecan-1 as a Predictor of Persistent Thrombocytopenia and Lethal Outcome: A Population Study of Patients with Suspected Sepsis Requiring Intensive Care. Front. Cardiovasc. Med. 2021, 8, 730553. [Google Scholar] [CrossRef]
- Ostrowski, S.R.; Haase, N.; Müller, R.B.; Møller, M.H.; Pott, F.C.; Perner, A.; Johansson, P.I. Association between biomarkers of endothelial injury and hypocoagulability in patients with severe sepsis: A prospective study. Crit. Care 2015, 19, 191. [Google Scholar] [CrossRef] [PubMed]
- Anand, D.; Ray, S.; Srivastava, L.M.; Bhargava, S. Evolution of serum hyaluronan and syndecan levels in prognosis of sepsis patients. Clin. Biochem. 2016, 49, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Puskarich, M.A.; Cornelius, D.; Tharp, J.; Nandi, U.; Jones, A.E. Plasma syndecan-1 levels identify a cohort of patients with severe sepsis at high risk for intubation after large-volume intravenous fluid resuscitation. J. Crit. Care 2016, 36, 125–129. [Google Scholar] [CrossRef]
- Hahn, R.G.; Patel, V.; Dull, R.O. Human glycocalyx shedding: Systematic review and critical appraisal. Acta Anaesthesiol. Scand. 2021, 65, 590–606. [Google Scholar] [CrossRef]
- Filho, I.P.T.; Torres, L.N.; Salgado, C.; Dubick, M.A. Plasma syndecan-1 and heparan sulfate correlate with microvascular glycocalyx degradation in hemorrhaged rats after different resuscitation fluids. Am. J. Physiol. Circ. Physiol. 2016, 310, H1468–H1478. [Google Scholar] [CrossRef]
- Hayashida, K.; Parks, W.C.; Park, P.W. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood 2009, 114, 3033–3043. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, J.; Zhang, H.; Wang, X.; Liu, D. Elevated endothelial dysfunction-related biomarker levels indicate the severity and predict sepsis incidence. Sci. Rep. 2022, 12, 21935. [Google Scholar] [CrossRef]
- Connolly-Andersen, A.-M.; Thunberg, T.; Ahlm, C. Endothelial Activation and Repair During Hantavirus Infection: Association with Disease Outcome. Open Forum Infect. Dis. 2014, 1, ofu027. [Google Scholar] [CrossRef] [PubMed]
- Kajita, Y.; Terashima, T.; Mori, H.; Islam, M.; Irahara, T.; Tsuda, M.; Kano, H.; Takeyama, N. A longitudinal change of syndecan-1 predicts risk of acute respiratory distress syndrome and cumulative fluid balance in patients with septic shock: A preliminary study. J. Intensiv. Care 2021, 9, 27. [Google Scholar] [CrossRef]
- Hahn, R.G.; Zdolsek, M.; Zdolsek, J. Plasma concentrations of syndecan-1 are dependent on kidney function. Acta Anaesthesiol. Scand. 2021, 65, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.E.; Moore, H.B.; Kornblith, L.Z.; Neal, M.D.; Hoffman, M.; Mutch, N.J.; Sauaia, A. Trauma-induced coagulopathy. Nat. Rev. Dis. Prim. 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.B.; Gando, S.; Iba, T.; Kim, P.Y.; Yeh, C.H.; Brohi, K.; Hunt, B.J.; Levy, J.H.; Draxler, D.F.; Stanworth, S.; et al. Defining trauma-induced coagulopathy with respect to future implications for patient management: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2020, 18, 740–747. [Google Scholar] [CrossRef]
- Schenck, H.; Netti, E.; Teernstra, O.; De Ridder, I.; Dings, J.; Niemelä, M.; Temel, Y.; Hoogland, G.; Haeren, R. The Role of the Glycocalyx in the Pathophysiology of Subarachnoid Hemorrhage-Induced Delayed Cerebral Ischemia. Front. Cell Dev. Biol. 2021, 9, 731641. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Matsumoto, H.; Ogura, H.; Hirose, T.; Shimizu, K.; Yamamoto, K.; Maruyama, I.; Shimazu, T. Circulating syndecan-1 predicts the development of disseminated intravascular coagulation in patients with sepsis. J. Crit. Care 2018, 43, 48–53. [Google Scholar] [CrossRef]
- Johansson, P.I.; Sørensen, A.M.; Perner, A.; Welling, K.L.; Wanscher, M.; Larsen, C.F.; Ostrowski, S.R. Disseminated intravascular coagulation or acute coagulopathy of trauma shock early after trauma? An observational study. Crit. Care 2011, 15, R272. [Google Scholar] [CrossRef]
- Chappell, D.; Bruegger, D.; Potzel, J.; Jacob, M.; Brettner, F.; Vogeser, M.; Conzen, P.; Becker, B.F.; Rehm, M. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit. Care 2014, 18, 538. [Google Scholar] [CrossRef]
- Bruegger, D.; Schwartz, L.; Chappell, D.; Jacob, M.; Rehm, M.; Vogeser, M.; Christ, F.; Reichart, B.; Becker, B.F. Release of atrial natriuretic peptide precedes shedding of the endothelial glycocalyx equally in patients undergoing on- and off-pump coronary artery bypass surgery. Basic Res. Cardiol. 2011, 106, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Straat, M.; Müller, M.C.; Meijers, J.C.; Arbous, M.S.; de Man, A.M.S.; Beurskens, C.J.; Vroom, M.B.; Juffermans, N.P. Effect of transfusion of fresh frozen plasma on parameters of endothelial condition and inflammatory status in non-bleeding critically ill patients: A prospective substudy of a randomized trial. Crit. Care 2015, 19, 163. [Google Scholar] [CrossRef] [PubMed]
- Schöchl, H.; Schlimp, C.J. Trauma bleeding management: The concept of goal-directed primary care. Anesth. Analg. 2014, 119, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Duranteau, J.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Maegele, M.; Nardi, G.; Riddez, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care 2019, 23, 98. [Google Scholar] [CrossRef]


| ALL (n = 48) | SDC-1 on day 0 | ||||
|---|---|---|---|---|---|
| >99.6 ng/mL (n = 24) | ≤99.6 ng/mL (n = 24) | p Value | |||
| Patient characteristics | |||||
| Age | years | 61.5 (42.0–72.6) | 67.0 (42.3–75.3) | 55.0 (41.3–71.0) | 0.672 |
| Sex; male/female | n, (%) | 37 (77.1)/11 (22.9) | 16 (66.7)/8 (33.3) | 21 (87.5)/3 (12.5) | 0.227 |
| Injury Severity Score (ISS) | 19 (13–29) | 27 (15–34) | 17 (9–24) | 0.007 | |
| shock | n, (%) | 13 (27.1) | 11 (45.8) | 2 (8.3) | 0.003 |
| ISTH-overt DIC (+) | n, (%) | 11 (22.9) | 10 (41.7) | 1 (4.2) | 0.002 |
| Timing of DIC diagnosis n, (%) | |||||
| OA | 2 (4.2) | 2 (8.3) | 0 (0.0) | 0.149 | |
| OA–3H | 7 (14.6) | 6 (25.0) | 1 (4.2) | 0.041 | |
| 3H–24H | 2 (4.2) | 2 (8.3) | 0 (0.0) | 0.149 | |
| 24H– | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | |
| Duration from injury to blood sampling | |||||
| minutes | 60 (43–110) | 62 (36–118) | 58 (46–82) | 1.000 | |
| Laboratory data [normal range] | |||||
| HGB [11.3–15.2] | g/dL | 13.1 (11.6–14.0) | 13.0 (10.8–13.7) | 13.3 (11.9–15.0) | 0.095 |
| HCT [34.3–45.2] | % | 38.2 (34.2–41.3) | 38.2 (31.7–40.1) | 38.7 (35.3–43.0) | 0.111 |
| PLT [13.1–36.9] | ×104/μL | 21.8 (17.9–26.8) | 22.3 (18.2–26.8) | 21.4 (17.7–26.7) | 0.703 |
| PT-INR [0.85–1.15] | 0.97 (0.92–1.08) | 1.03 (0.95–1.21) | 0.93 (0.92–1.00) | 0.018 | |
| APTT [21.5–43.1] | sec | 23.9 (21.7–28.1) | 26.4 (21.9–30.5) | 23.0 (21.7–25.0) | 0.039 |
| Fbg [200–400] | mg/dL | 223 (184–270) | 206 (178–246) | 246 (197–294) | 0.036 |
| FDP [<5.0] | μg/mL | 89.4 (27.3–164.3) | 154.3 (64.2–226.1) | 39.9 (16.6–96.9) | <0.001 |
| D-dimer [<1.0] | μg/mL | 46.1 (14.7–82.5) | 78.0 (33.9–122.2) | 20.5 (8.4–49.9) | <0.001 |
| TAT [<3.0] | μg/L | 115.3 (49.3–200.0) | 194.2 (127.0–235.4) | 60.1 (41.4–114.1) | <0.001 |
| PIC [0.0–0.8] | μg/mL | 10.4 (4.8–27.9) | 16.5 (8.8–37.8) | 5.2 (3.3–10.8) | 0.001 |
| tPA [1270–8840] | pg/mL | 6468 (4208–10,411) | 9696 (5638–15,685) | 5863 (3287–8075) | 0.006 |
| tPAI-1 [<50] | ng/mL | 31 (15–63) | 46 (28–81) | 21 (11–57) | 0.010 |
| AT [80.0–120.0] | % | 88.7 (77.4–97.1) | 82.4 (66.8–93.3) | 92.5 (87.9–100.5) | 0.003 |
| PC [82.0–112.0] | % | 82.6 (71.3–108.4) | 75.0 (63.4–97.2) | 87.8 (74.5–111.6) | 0.039 |
| α2PI [80.0–130.0] | % | 83.6 (67.6–100.3) | 74.3 (58.8–88.1) | 96.9 (80.3–106.8) | 0.001 |
| PLG [80.0–130.0] | % | 91.2 (75.7–103.2) | 82.8 (68.9–96.5) | 98.5 (86.5–106.7) | 0.028 |
| IL-6 [<4.0] | pg/mL | 122 (33–277) | 177 (87–395) | 63 (22–262) | 0.097 |
| Lactate [3.3–14.9] | mg/dL | 22.0 (14.0–42.5) | 38.0 (20.0–52.8) | 14.0 (9.5–22.5) | <0.001 |
| Alb [3.9–4.9] | g/dL | 3.9 (3.6–4.2) | 3.7 (3.3–4.0) | 4.1 (3.7–4.2) | 0.044 |
| BUN [7–21] | mg/dL | 17 (15–21) | 18 (14–23) | 17 (15–21) | 0.535 |
| Cre [0.65–1.07] | mg/dL | 0.88 (0.75–1.07) | 0.98 (0.78–1.12) | 0.87 (0.73–0.98) | 0.166 |
| SDC-1 | ng/mL | 99.6 (61.1–214.3) | 213.3 (146.6–462.3) | 61.4 (38.5–86.5) | <0.001 |
| Transfusion | |||||
| PRBC | Units | 1 (0–10) | 5 (0–14) | 0 (0–6) | 0.033 |
| FFP | Units | 0 (0–8) | 7 (0–16) | 0 (0–0) | 0.005 |
| Platelets | Units | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.020 |
| Intervention | |||||
| Craniotomy | n, (%) | 2 (4.2) | 2 (8.3) | 0 (0.0) | 0.149 |
| Thoracotomy | n, (%) | 2 (4.2) | 1 (4.2) | 1 (4.2) | 1.000 |
| Laparotomy | n, (%) | 3 (7.1) | 2 (8.3) | 1 (4.2) | 0.551 |
| IVR | n, (%) | 10 (23.8) | 8 (33.3) | 2 (8.3) | 0.033 |
| ORIF | n, (%) | 20 (47.6) | 8 (33.3) | 12 (50.0) | 0.242 |
| Outcome | |||||
| In-hospital mortality | n, (%) | 5 (10.4) | 4 (16.7) | 1 (4.2) | 0.156 |
| Cut-Off Value | Sensitivity (%) | Specificity (%) | AUC | Standard Error | p Value | 95%CI | ||
|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||||
| DIC | 130.38 ng/mL | 90.9 | 73.0 | 0.845 | 0.057 | 0.001 | 0.734 | 0.957 |
| Shock | 116.48 ng/mL | 84.6 | 68.6 | 0.774 | 0.067 | 0.004 | 0.643 | 0.905 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, H.; Annen, S.; Mukai, N.; Ohshita, M.; Murata, S.; Harima, Y.; Ogawa, S.; Okita, M.; Nakabayashi, Y.; Kikuchi, S.; et al. Circulating Syndecan-1 Levels Are Associated with Chronological Coagulofibrinolytic Responses and the Development of Disseminated Intravascular Coagulation (DIC) after Trauma: A Retrospective Observational Study. J. Clin. Med. 2023, 12, 4386. https://doi.org/10.3390/jcm12134386
Matsumoto H, Annen S, Mukai N, Ohshita M, Murata S, Harima Y, Ogawa S, Okita M, Nakabayashi Y, Kikuchi S, et al. Circulating Syndecan-1 Levels Are Associated with Chronological Coagulofibrinolytic Responses and the Development of Disseminated Intravascular Coagulation (DIC) after Trauma: A Retrospective Observational Study. Journal of Clinical Medicine. 2023; 12(13):4386. https://doi.org/10.3390/jcm12134386
Chicago/Turabian StyleMatsumoto, Hironori, Suguru Annen, Naoki Mukai, Muneaki Ohshita, Satoru Murata, Yutaka Harima, Shirou Ogawa, Mitsuo Okita, Yuki Nakabayashi, Satoshi Kikuchi, and et al. 2023. "Circulating Syndecan-1 Levels Are Associated with Chronological Coagulofibrinolytic Responses and the Development of Disseminated Intravascular Coagulation (DIC) after Trauma: A Retrospective Observational Study" Journal of Clinical Medicine 12, no. 13: 4386. https://doi.org/10.3390/jcm12134386
APA StyleMatsumoto, H., Annen, S., Mukai, N., Ohshita, M., Murata, S., Harima, Y., Ogawa, S., Okita, M., Nakabayashi, Y., Kikuchi, S., Takeba, J., & Sato, N. (2023). Circulating Syndecan-1 Levels Are Associated with Chronological Coagulofibrinolytic Responses and the Development of Disseminated Intravascular Coagulation (DIC) after Trauma: A Retrospective Observational Study. Journal of Clinical Medicine, 12(13), 4386. https://doi.org/10.3390/jcm12134386






