Abstract
Sodium imbalance is a hallmark of chronic kidney disease (CKD). Excess tissue sodium in CKD is associated with hypertension, inflammation, and cardiorenal disease. Sodium magnetic resonance imaging (23Na MRI) has been increasingly utilized in CKD clinical trials especially in the past few years. These studies have demonstrated the association of excess sodium tissue accumulation with declining renal function across whole CKD spectrum (early- to end-stage), biomarkers of systemic inflammation, and cardiovascular dysfunction. In this article, we review recent advances of 23Na MRI in CKD and discuss its future role with a focus on the skin, the heart, and the kidney itself.
1. Introduction
Declining kidney function is a characteristic feature of chronic kidney disease (CKD) that leads to fluid and electrolyte imbalance, particularly sodium, in the body. High dietary salt is implicated in various diseases including hypertension [1] and cardiovascular disease [2,3]. Hypertensive individuals are at a 75% higher risk of developing CKD compared to those with normal blood pressure [4]. Furthermore, individuals with CKD face a heightened risk of cardiovascular disease and mortality [5,6]. Given the susceptibility of CKD patients to sodium-related physiological changes, understanding how sodium is managed in CKD can offer insights into the mechanisms of the disease and aid in optimizing and personalizing therapeutic interventions.
Techniques such as radioactive 22NaCl [7], ashing [8,9], and inductively coupled plasma-optical emission spectrometry [10] have been previously reported for measuring tissue sodium content. However, these techniques are not suitable for the clinical setting due to their destructive nature. Sodium magnetic resonance imaging (23Na MRI) allows repeated noninvasive in vivo quantification of soft tissue total sodium content (TSC) [11]. 23Na MRI is an increasingly utilized imaging technology that can provide a direct biomarker to assess emerging sodium removal therapeutics in CKD. Despite its first human use almost four decades ago [12], 23Na MRI had not been widely used in clinical research up until the past couple of decades. This has been enabled by advances in sodium imaging technology [13,14,15] that have made it possible to produce quality sodium images within acceptable signal collection times. 23Na MRI validity in determining TSC was demonstrated by comparing 23Na MRI signal to the directly measured sodium content in the muscle and skin of animals and humans using gold standard chemical analysis [16]. The first use of 23Na MRI in CKD research was reported around a decade ago [17]. Subsequently, its utility in CKD research has been increasingly appreciated in recent years. A comprehensive review of 23Na MRI in CKD has previously been provided by Martin et al. [18], augmented since by additional studies [19,20,21,22,23,24,25]. A list of studies involved in the use of 23Na MRI in kidney disease is summarized in Table 1. In this article, we review the past and the most recent potential clinical applications of 23Na MRI in CKD and discuss its future potentials (Figure 1) in managing CKD with a focus on skin, the heart, and the kidney itself.

Table 1.
23Na MRI studies in CKD, HD, PD, AKI, and kidney transplant.
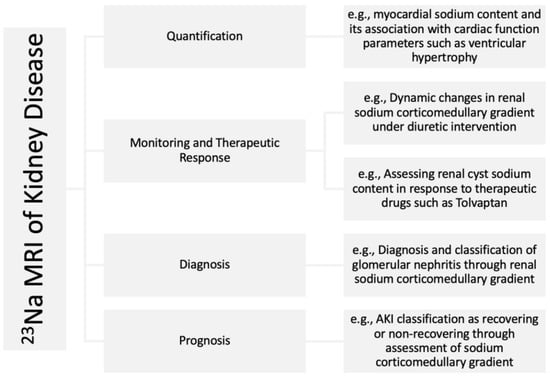
Figure 1.
Examples of potential applications of 23Na MRI in kidney disease.
2. 23Na MRI Evaluation of the Skin Sodium Content
Our understanding of sodium storage in the body has evolved over the past 20 years. In classical physiological teaching, ingested sodium when combined with inadequate excretion leads to expansion of the extracellular water compartment, congestion, and increasing blood pressure [35]. This model is now being augmented with additional appreciation of retained sodium being stored without associated water, within skin, muscle, and bone [16,17,36].
Skin is the largest organ in the body that provides a major extracellular reservoir for sodium storage, by providing proteoglycan chains available for sodium to bind without water [37,38]. It has previously been demonstrated that elevated sodium concentration in the skin microenvironment provides a barrier to infection [39]. A high skin sodium concentration also helps to modulate immune cell function [40,41] through enhancing proinflammatory and antimicrobial macrophage function and T-cell activation [39,42,43,44,45]. Animal studies show that interstitial sodium clearance in the skin is handled by mononuclear phagocyte system-derived vascular endothelial growth factor C-(VEGF-C-) mediated lymphangiogenesis [46,47], reducing VEGF-C bioavailability results in sodium accumulation in the skin and salt-sensitive hypertension [46,47,48].
Skin sodium content naturally increases with age. This was demonstrated by Kopp et al. [16] in healthy adults (men and women) for the first time using 23Na MRI, and these observations were further strengthened by subsequent studies [17,32,33]. More recently, Salerno et al. [25] have extended the range of individuals studied to include children and adolescents (healthy and with CKD). The authors reported significantly lower skin sodium content in healthy children and adolescents (age ≤ 17) compared with healthy adults. Aging is described as a pro-inflammatory process [49] that may be associated with tissue sodium accumulation due to reduced clearance of sodium by VEGF-C-mediated lymphangiogenesis. The skin sodium accumulation is aggravated in the CKD population, independent of age, worsening as kidney function declines. The first 23Na MRI study of the lower leg in hemodialysis (HD) showed older HD patients (≥60 years old) have significantly higher skin sodium content and lower serum VEGF-C compared with age-matched healthy controls [17]. Furthermore, recent 23Na MRI investigations of the lower leg in CKD (stages 3–5) [32], HD, and peritoneal dialysis (PD) patients [32,33] have revealed skin sodium concentration increases progressively across the whole CKD spectrum, with HD and PD having the highest associated sodium concentration compared to healthy controls. Moreover, Qirjazi et al. [32] reported a negative correlation between skin sodium concentration and estimated glomerular filtration rate (eGFR) in a merged control-CKD group, linking the excess skin sodium accumulation to worsening renal function. The excess skin sodium storage is also shown to be modifiable and associated with VEFG-C level. Quantitative 23Na MRI of the lower leg conducted by Dahlmann et al. [17] demonstrated that dialysis reduces skin sodium in HD patients. They also reported that HD patients with low VEFG-C have significantly higher skin sodium content compared with HD patients with high VEFG-C post HD. Recently, Lemoine et al. [34] demonstrated that the removal of stored sodium in the skin could further be controlled through modulating the dialysate sodium concentration. It is interesting to note that Dahlmann et al. [20] demonstrated that renal transplantation led to lower skin and muscle sodium accumulation in 31 stage-5 CKD patients receiving living-doner kidneys. They also reported improved renal function, normalization of blood pressure, and an increase in VEGF-C concentration post-transplantation.
Low-grade chronic systemic inflammation is a hallmark of CKD [50]. Markers of systemic inflammation such as interleukin 6 (IL-6), high-sensitivity C-reactive protein (hsCRP), and serum albumin have been associated with skin sodium content using 23Na MRI. Mitsides et al. [31] demonstrated elevated skin sodium, IL-6, and VEGF-C in stage 5 CKD patients. Similarly, Sahinoz et al. [33] reported higher IL-6 and hsCRP levels correlated with increased skin sodium content in dialysis patients. Furthermore, a negative correlation was found between serum albumin and skin sodium content in HD patients by Salerno et al. [22]. Sodium-glucose cotransporter 2 (SGLT2) inhibitors increase urinary sodium excretion by preventing sodium reabsorption in the renal proximal tubule [51]. A recent review of clinical trials has reported that SGLT2 inhibitors lower the risk of kidney disease progression and hospitalization for heart failure both in CKD and heart failure patients [52]. These results highlight the importance of the link between excess skin sodium accumulation and chronic systemic inflammation and the significance of sodium removal on improving clinical outcomes in CKD patients. Other 23Na MRI studies have demonstrated the efficacy of sodium removal therapeutic interventions. This was first reported over a single HD session, using 23Na MRI of the HD patients’ lower leg [17]. Recently, Lemoine et al. [34] investigated the effects of modulating dialysate sodium concentration on skin sodium clearance using 23Na MRI in the longer term (pre-dialysis state). The authors demonstrated that dialysate with a sodium concentration of 137 mmol/L (over a 12-month period) was associated with a lower amount of skin sodium compared to patients randomized to receive dialysis against a sodium concentration of 140 mmol/L. Additionally, in a case report of an 80-year-old male, Penny et al. [19] demonstrated a reduction in skin sodium content after transitioning from high-flux HD to expanded HD using a medium cut-off dialysis membrane (potentially with greater permissiveness to sodium removal compared to conventional dialysis membrane characteristics). They also reported improved depression, anxiety, and uremic pruritus scores following the switch to the medium cut-off dialysis membrane. The use of 23Na MRI in assessing sodium removal therapeutic interventions is increasing. Novel therapeutic approaches to sodium removal are emerging that are also amenable to the assessment of tissue sodium content using 23Na MRI. For example, a current clinical trial (ClinicalTrials.gov registered NCT04603014, accessed on 16 June 2023) is underway that utilizes 23Na MRI to assess interdialytic zero sodium containing PD fluid to augment sodium removal and ameliorate congestion in HD patients struggling with volume removal reliant on HD alone.
CKD is an umbrella term comprising a large group of heterogeneous pathophysiological conditions that affect salt balance in the body differently. As such, tubular disorders such as Bartter’s, Gitelman’s, and Fanconi syndromes are associated with kidney salt wasting. This is in contrast to proteinuric glomerular diseases, such as minimal-change disease and focal segmental glomerulosclerosis, which are associated with sodium retention [53,54]. The association between tissue sodium accumulation and CKD etiology is not very well explored. Recently, Salerno et al. [25] conducted a first-time 23Na MRI of the lower leg of pediatric CKD (age ≤ 17). Once the group was stratified based on etiology, the authors found high whole-leg sodium Z-scores in four patients with glomerular disease and one kidney transplant recipient due to atypical hemolytic-uremic syndrome and low whole-leg sodium Z-scores in two patients with tubular disorders.
Insulin resistance (IR) is highly prevalent in CKD and becomes almost universal towards end-stage renal failure [55,56]. IR in CKD has implications for increased cardiovascular risk factors including oxidative stress [57], chronic inflammation [57], and endothelial dysfunction [58]. The muscle is considered the primary site of IR [55]. The severity of IR has been associated with enhanced muscle catabolism in maintenance hemodialysis (MHD) patients [59,60]. Elevated tissue sodium is reported in MHD patients [17] and is shown to be even higher in HD patients with type 2 diabetes [30]. Deger et al. [29] investigated the association of lower leg tissue sodium accumulation measured by 23Na MRI with IR in eleven MHD patients. They reported an inverse relationship between muscle sodium content and glucose and leucine disposal rates suggesting muscle sodium content might be a determinant of IR in MHD patients. This finding highlights the fact that standard HD prescription does not successfully address the normalization of tissue sodium. Further therapeutic intervention studies are needed to investigate the effect of tissue sodium normalization on IR in CKD and particularly MHD patients.
Dietary salt intake studies reveal the contribution of high salt consumption to hypertension [1] and the increased risk of cardiovascular disease [2,3]. Sodium toxicity in CKD has been known about since 1949, when restricted daily dietary sodium intake (150 mg/day) was used to manage hypertension through the ‘Rice Diet’ experiment [61]. At the same time, hypertension, by itself, could cause CKD [4]. As shown by a meta-analysis of pooled data, the risk of developing CKD (GFR < 60 mL/min/1.73 m2) in hypertensive patients is 75% higher than in normotensive individuals [4]. Furthermore, excess dietary salt intake is shown to be associated with higher mortality rates in HD patients [62]. These findings are in parallel with 23Na MRI studies. Schneider et al. [27] reported a strong correlation between skin sodium content and left ventricular mass in CKD patients (a well-recognized risk factor for mortality). A recent study by Lemoine et al. [21] revealed that HD patients accumulate a similar amount of sodium in their skin as compared with heart failure patients, highlighting that excess sodium in HD may exacerbate cardiovascular risks in heart failure patients. Moreover, Salerno et al. [22] demonstrated for the first time the association between excess skin sodium content in dialysis patients and observed mortality.
3. Emerging 23Na MRI and CKD-Related Cardiomyopathy
The pathophysiology of hemodialysis-associated cardiomyopathy (HD-CMP) is complex. Abnormal cardiac and vascular structure along with function play a key part in excessive cardiovascular events and premature mortality experienced by patients with end-stage renal disease (ESRD) requiring HD [63,64]. Drug and device-based therapies that reduce cardiovascular morbidity in the general population have been ineffective in those receiving HD [65,66,67,68,69]. The pattern of heart disease associated with kidney failure is characterized by cardiac hypertrophy and fibrosis [70]. These two mechanisms are closely linked, as cardiac hypertrophy is associated with interstitial matrix expansion and increased expression of proteoglycans such as syndecan-4 and small leucine-rich proteoglycans [71,72] (capable of binding sequestered Na+, analogous to interstitial dermal storage). Sodium then potentially exacerbates this process by recruiting and activating fibroblasts yet further via tonicity-responsive enhanced binder protein activation [73,74]. Proton MRI detects signs of diffuse myocardial fibrosis that are present even from the early stages of chronic kidney disease [75]. This cardiac pathology becomes even more evident in patients receiving HD, where fibrotic features (imaged with cardiac MRI) are associated with increased left ventricular mass and serum biomarkers of cardiac fibrosis (e.g., galectin-3) [76].
Stored sodium is not biologically inert and is associated with a variety of pathophysiological consequences, including inflammation [32,33], aberrant tissue remodeling [77], hypertension [36], and fibrosis [78]. These effects are independent from the well-appreciated effects of pressure and volume overload [77,78]. Sodium MRI clinical data showing increased salt storage is observed in cardiovascular high-risk patients, e.g., CKD patients and, in particular, those requiring maintenance dialysis [17,32]. Schneider et al. reported that increased skin sodium concentration in patients with CKD is associated with left ventricular hypertrophy (LVH) independent from blood pressure and total body overhydration [27]. As suggested by Bottomley [79], increased imaged myocardial sodium signal may be the result of increased intracellular and/or extracellular sodium. Lemoine et al. [21] have recently demonstrated that heart failure exacerbates sodium storage when combined with CKD. They have shown that patients with cardiorenal syndrome have significantly higher tissue sodium concentrations than those with similar levels of renal function, but with no evidence of heart failure [21]. Parallel with these findings, a study by Friedrich et al. [23] revealed higher skin and muscle sodium content and serum IL-6 in HD patients with CVD, but with no significant difference in excess extracellular water from HD patients without CVD. Furthermore, in a more recent study, 23Na MRI has shown that skin tissue sodium concentration is associated with a marked increase in (predominantly cardiovascular) mortality in dialysis patients [22].
Direct evidence of toxic myocardial sodium deposition is still somewhat scant. In a small animal model looking at imaged and directly measured myocardial sodium concentration, preclinical evidence has shown that LVH and fibrosis is associated with increased intracellular myocardial sodium [80]. More recently, Christa et al. showed that increased myocardial sodium content in humans with primary hyperaldosteronism may be associated with pathways that lead to LVH [81]. The authors suggested that increased myocardial sodium signal may be the result of water-free interstitial cardiac sodium accumulation, similarly to mechanisms observed in the skin.
Direct 23Na MRI evaluation of myocardial sodium content could provide a previously unconsidered therapeutic target for the management of the principal cause of morbidity and mortality in patients with CKD. Understanding the role of myocardial sodium accumulation may also allow for the optimal selection of patients that might benefit from the range of emerging treatments targeting enhanced sodium removal and allow clinicians to follow the response to treatment in advance of longer term and potentially irreversible effects of tissue injury becoming apparent.
4. Emerging Renal Functional 23Na MRI
There is an urgent need to identify and develop additional techniques to diagnose kidney disease, provide patient-specific prognostic data, and follow response to treatment. There has been very little in the way of novel renal diagnostics developed within the last 20 years. Methods remain relatively crude, usually providing information averaged over the whole renal mass. Blood and urine-based methods provide limited information (especially in the non-proteinuric patient) to reliably predict outcomes on an individual basis.
Kidney function is usually assessed by the estimation or measurement of the glomerular filtration rate (GFR), focusing exclusively on the filtration process. However, kidney disease often has impacts on other components of the kidney, within the medulla, such as tubules and peritubular circulation. Many studies have shown the highly predictive value of interstitial fibrosis and tubular atrophy in CKD progression (irrespective of etiology) in both native kidney and allograft tissue-based studies [82] and the references therewithin. Corticomedullary gradient (CMG) exploration would provide a relevant assessment of tubular dysfunction independently of glomerular function and could be of significant prognostic value [83,84]. Increased medullary sodium concentration (combined with urea in the inner medulla) sets up an oncotic gradient that increases the reabsorption of water into the parenchyma, to be redistributed to the circulation [85]. Therefore, there is a physiologically significant difference in sodium concentration between the cortex and medulla. This CMG has previously been studied by invasive micro puncture experiments in rodent kidneys thus far [85,86,87]. Because of the lack of a non-invasive tool to explore this gradient in humans, all our fundamental knowledge is based on these animal studies. An imaging-based approach would provide such a non-invasive tool.
Previous animal 23Na MRI studies have demonstrated the feasibility to quantify sodium CMG in steady state, post renal obstruction [88], and diuretic infusion [89,90], providing tubular functional information. Dynamic measurement of sodium CMG has also been performed recently in pigs after diuretic challenge [90]. Limited studies have attempted to measure the sodium CMG in humans in steady state, post water deprivation [91], and water load [92], with variable technical success. Recently, the first application of 23Na MRI in CKD was reported by Akbari et al. [24]. The authors demonstrated a correlation (r2 = 0.22; p < 0.001) between sodium corticomedullary ratio and urinary osmolarity in both healthy volunteers and CKD patients with cardiorenal syndrome.
23Na MRI could potentially have relevant application in the following populations:
Chronic kidney disease: Recent epidemiological studies have shown significant associations with the incidence or progression of diseases including CKD and low osmolarity [83,84,93]. However, urine osmolarity is representative of overall renal mass function. The use of 23Na MRI could provide direct visualization and quantification of abnormalities or disruptions in sodium CMG in different regions of the kidney that may not be captured by urine osmolarity alone. Furthermore, 23Na MRI could be applied in assessing kidney function in ESRD where the collection of a urine sample would be challenging due to little to no remaining residual renal function (RRF). RRF is strongly associated with survival and quality of life in dialysis patients, and methods of evaluating RRF are still being refined [94].
Glomerulonephritis (GN): GN causes inflammation in the glomeruli and other parts of the kidney, and its diagnosis and classification are based on renal biopsy [95]. GN remains the second most common cause of end-stage kidney failure worldwide [95]. The renal sodium CMG may be altered since elevated tissue sodium is associated with inflammation [31,32,44]. Sodium MRI has the potential to be used as a non-invasive diagnostic and classification tool for GN, potentially rectifying the need for renal biopsy, an invasive procedure that carries risks of complications and discomfort for the patient.
Acute kidney injury (AKI): AKI leads to a sudden drop in kidney function and could be due to glomerular or tubular dysfunction [96]. Prognosis in AKI is currently difficult to predict but is very important because of the high mortality rate associated with more severe and slowly resolving AKI. In critically ill patients, the mortality was reported at 53% in the Acute Renal Failure Trial Network (ATN) study [97] and 44.7% in the Randomized Evaluation of Normal versus Augmented Level of Renal Replacement Trial (RENAL) [98]. A 23Na MRI of the lower leg in AKI patients revealed that skin and muscle sodium content did not change after 5–6 days of hemodialysis [28]. This may suggest that the kidney itself may be a better site for 23Na MRI to assess AKI in the early days of the injury. Furthermore, preclinical studies have demonstrated the utility of 23Na MRI in monitoring kidney recovery post AKI [99,100,101]. Maril et al. reported a 21% reduction in the sodium CMG of rats post acute tubular necrosis [99]. In a rat study, a positive correlation between total 23Na kidney content and fibrotic markers was reported following renal ischemia-reperfusion injury (IRI) [100]. Rasmussen et al. reported a reduction in sodium CMG in pigs post IRI [101]. It could be hypothesized that the recovery of medullary salination is a vital earlier component of renal recovery and evaluation of sodium CMG using 23Na MRI could classify AKI as either non-recovering or recovering.
Transplanted patients: The feasibility and usability of 23Na MRI of a human renal allograft was demonstrated in a single case report alone [102]. Renal transplant recipients often exhibit renal function deterioration and have a higher vasopressin concentration than healthy controls [103]. In a 23Na MRI study, Moon et al. reported significantly lower sodium CMG in six kidney transplant patients compared to six healthy individuals [26]. It is currently very challenging to elucidate causes of acute and chronic graft dysfunction without resorting to renal biopsy and to predict recovery in the setting of graft primary non-function. Sodium CMG measured using 23Na MRI may help in monitoring and predicting recovery non-invasively.
Autosomal dominant polycystic kidney disease (ADPKD): ADPKD is the most common inherited progressive renal disease caused by the mutation of two major genes, PKD1 and PKD2, and the rare genes, GANAB and DNAJB11 [104,105]. ADPKD leads to the formation and multiplication of fluid-filled renal cysts causing renal function to decline [104,105]. The management of ADPKD is limited to the treatment of symptoms and complications [106]. TEMPO and REPRISE studies have demonstrated Tolvaptan’s (TVP) ability to slow the decline in GFR and modulate changes in the total kidney volume [107,108]. A high degree of variability in TVP diuresis response has been reported in several studies [109,110,111,112]. It could be hypothesized that 23Na MRI might provide a therapeutic monitoring tool, through the measurement of sodium CMG, to determine the TVP dose personalized to each individual patient.
Diuretic resistance in CKD with heart failure (HF) syndrome: CKD and HF represent concurrent disease epidemics [113,114]. Both conditions have increasing incidence and prevalence in older age groups as well as persons with hypertension, diabetes mellitus, or other cardiovascular and kidney disease risk factors [115]. The presence of one condition appears to accelerate the progression of the other; having both conditions increases the risk of hospitalization, rehospitalization, the need for intensive care or kidney replacement therapy, and death [116,117,118,119,120]. The prevalence of moderate to severe kidney impairment is approximately 30–60% in all patients with HF [121]. The Acute Decompensated Heart Failure National Registry (ADHERE) database reported data on over 100,000 patients with HF requiring hospitalization, and only 9% had a normal kidney function [122]. The cornerstone of managing fluid retention in these patients is loop diuretics (used in >90% of patients) [123], with furosemide being the most common. The pathophysiology of diuretic resistance is multi-factorial and involves sympathetic nervous system activation, renin–angiotensin–aldosterone system (RAAS) activation, nephron remodeling, pre-existing renal function alterations, disrupted pharmacokinetics/pharmacodynamics of diuretics, and intravascular fluid depletion [124]. Diuretics rely on tubular delivery to have their effect, and therefore, pre-existing or worsening renal dysfunction further limits their effectiveness. A threshold effect for sodium excretion exists, requiring a minimum tubular drug concentration before effect [125]. This makes it impossible to extrapolate the response to a previous lower dose to predict the effects of a larger dose. Doses sufficient to control fluid retention and symptoms of congestion may be as low as 20 mg of furosemide, or as high as 1000+ mg per day. Dose escalation is often too slow or incomplete (resulting in catastrophic fluid retention and transfer from ambulatory care to hospital) or precipitous (resulting in excessive fluid removal and acute kidney injury), and clinical practice currently relies on ‘trial and error’. Approaches based upon monitoring urinary sodium and volume have been attempted [126,127,128]. Although several studies have illustrated the prognostic value of urinary sodium following a first administration of a loop diuretic, prognostic value during consecutive days is unproven [129] and the efficacy of subsequent doses is falling off due to the braking phenomenon and tubular remodeling [130]. The response of CMG measured by 23Na MRI to fluid restriction and diuretic challenge might help to guide diuretic dose prescription and subsequently improve efficacy and patient safety for the management of patients with HF and renal dysfunction. This contention is currently under clinical study (ClinicalTrials.gov registered NCT04170855, accessed on 16 June 2023).
5. Conclusions
In this article, we have reviewed 23Na MRI studies in CKD that demonstrate the association of sodium with hypertension, systemic inflammation, and cardiovascular disease. This association may point to sodium as a new therapeutic target. Fortunately, new therapies targeting sodium removal are becoming available for patients with HF and CKD, alone or in combination. In patients with at least some degree of RRF, pharmacological therapies such as SGLT2 inhibitors promote natriuresis (and deplete tissue-bound sodium pools) [131]. Sodium intake can be reduced via dietary restriction; however, this is often difficult to achieve in patients who are already severely restricted and struggling to maintain adequate nutrition [132]. Oral binders/inhibitors of absorption are under development to limit systemic exposure to ingested sodium [133]. Targeting sodium directly, rather than sodium and water together, is now becoming a therapeutic reality. Direct sodium removal (DSR) is a therapeutic application of a novel implanted instillation and drainage system, combined with 0% sodium containing PD fluid. The potential impact has already been demonstrated in large animal models and an initial study in humans with severe heart failure [134].
23Na MRI has been increasingly utilized in CKD clinical research especially in the past few years. Its ability to repeatedly and non-invasively quantify soft tissue TSC in the skin has been very well demonstrated, and its use in assessing myocardial and renal sodium content in the setting of CKD is emerging. The ability of 23Na MRI to provide a direct assessment of soft tissue TSC makes it a valuable research and clinical tool in diagnostic, prognostic, and monitoring therapeutics in kidney diseases. However, it is important to note that 23Na MRI is still a relatively new technique in the clinical setting. The findings discussed in this review are mostly conducted in small populations. Further large-scale clinical trials are required to validate these findings.
Author Contributions
Conceptualization, A.A. and C.W.M.; writing—original draft preparation, A.A.; writing—review and editing, C.W.M.; supervision, C.W.M.; project administration, C.W.M. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
C.W.M. receives research funding and associated honoraria from Baxter Healthcare and Sequana Healthcare.
References
- He, F.J.; Li, J.; Macgregor, G.A. Effect of Longer Term Modest Salt Reduction on Blood Pressure: Cochrane Systematic Review and Meta-Analysis of Randomised Trials. BMJ 2013, 346, f1325. [Google Scholar] [CrossRef]
- He, F.J.; MacGregor, G.A. Salt Reduction Lowers Cardiovascular Risk: Meta-Analysis of Outcome Trials. Lancet 2011, 378, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Strazzullo, P.; D’Elia, L.; Kandala, N.-B.; Cappuccio, F.P. Salt Intake, Stroke, and Cardiovascular Disease: Meta-Analysis of Prospective Studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Borrelli, S.; Pacilio, M.; Minutolo, R.; Chiodini, P.; de Nicola, L.; Conte, G. Hypertension and Prehypertension and Prediction of Development of Decreased Estimated GFR in the General Population: A Meta-Analysis of Cohort Studies. Am. J. Kidney Dis. 2016, 67, 89–97. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Streeten, D.H.; Rapoport, A.; Conn, J.W. Existence of a slowly exchangeable pool of body sodium in normal subjects and its diminution in patients with primary aldosteronism. J. Clin. Endocrinol. Metab. 1963, 23, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Titze, J.; Lang, R.; Ilies, C.; Schwind, K.H.; Kirsch, K.A.; Dietsch, P.; Luft, F.C.; Hilgers, K.F. Osmotically Inactive Skin Na+ Storage in Rats. Am. J. Physiol.-Ren. Physiol. 2003, 285, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Nikpey, E.; Karlsen, T.V.; Rakova, N.; Titze, J.M.; Tenstad, O.; Wiig, H. High-Salt Diet Causes Osmotic Gradients and Hyperosmolality in Skin without Affecting Interstitial Fluid and Lymph. Hypertension 2017, 69, 660–668. [Google Scholar] [CrossRef]
- Fischereder, M.; Michalke, B.; Schmöckel, E.; Habicht, A.; Kunisch, R.; Pavelic, I.; Szabados, B.; Schönermarck, U.; Nelson, P.J.; Stangl, M. Sodium Storage in Human Tissues Is Mediated by Glycosaminoglycan Expression. Am. J. Physiol. Renal Physiol. 2017, 313, F319–F325. [Google Scholar] [CrossRef]
- Madelin, G.; Regatte, R.R. Biomedical Applications of Sodium MRI In Vivo. J. Magn. Reson. Imaging 2013, 38, 511–529. [Google Scholar] [CrossRef]
- Hilal, S.K.; Maudsley, A.A.; Ra, J.B.; Simon, H.E.; Roschmann, P.; Wittekoek, S.; Cho, Z.H.; Mun, S.K. In Vivo NMR Imaging of Sodium-23 in the Human Head. J. Comput. Assist. Tomogr. 1985, 9, 1–7. [Google Scholar] [CrossRef]
- Boada, F.E.; Gillen, J.S.; Shen, G.X.; Chang, S.Y.; Thulborn, K.R. Fast Three Dimensional Sodium Imaging. Magn. Reson. Med. 1997, 37, 706–715. [Google Scholar] [CrossRef]
- Gurney, P.T.; Hargreaves, B.A.; Nishimura, D.G. Design and Analysis of a Practical 3D Cones Trajectory. Magn. Reson. Med. 2006, 55, 575–582. [Google Scholar] [CrossRef]
- Nagel, A.M.; Laun, F.B.; Weber, M.-A.; Matthies, C.; Semmler, W.; Schad, L.R. Sodium MRI Using a Density-Adapted 3D Radial Acquisition Technique. Magn. Reson. Med. 2009, 62, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Kopp, C.; Linz, P.; Wachsmuth, L.; Dahlmann, A.; Horbach, T.; Schöfl, C.; Renz, W.; Santoro, D.; Niendorf, T.; Müller, D.N.; et al. 23Na Magnetic Resonance Imaging of Tissue Sodium. Hypertension 2012, 59, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Dahlmann, A.; Dörfelt, K.; Eicher, F.; Linz, P.; Kopp, C.; Mössinger, I.; Horn, S.; Büschges-Seraphin, B.; Wabel, P.; Hammon, M.; et al. Magnetic Resonance-Determined Sodium Removal from Tissue Stores in Hemodialysis Patients. Kidney Int. 2015, 87, 434–441. [Google Scholar] [CrossRef]
- Martin, K.; Tan, S.-J.; Toussaint, N.D. Magnetic Resonance Imaging Determination of Tissue Sodium in Patients with Chronic Kidney Disease. Nephrology 2022, 27, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Penny, J.D.; Salerno, F.R.; Akbari, A.; McIntyre, C.W. Pruritus: Is There a Grain of Salty Truth? Hemodial. Int. 2021, 25, E10–E14. [Google Scholar] [CrossRef] [PubMed]
- Dahlmann, A.; Linz, P.; Zucker, I.; Haag, V.; Jantsch, J.; Dienemann, T.; Nagel, A.M.; Neubert, P.; Rosenhauer, D.; Rauh, M.; et al. Reduction of Tissue Na+ Accumulation after Renal Transplantation. Kidney Int. Rep. 2021, 6, 2338–2347. [Google Scholar] [CrossRef]
- Lemoine, S.; Salerno, F.R.; Akbari, A.; McKelvie, R.S.; McIntyre, C.W. Tissue Sodium Storage in Patients with Heart Failure: A New Therapeutic Target? Circ. Cardiovasc. Imaging 2021, 14, e012910. [Google Scholar] [CrossRef] [PubMed]
- Salerno, F.R.; Akbari, A.; Lemoine, S.; Filler, G.; Scholl, T.J.; McIntyre, C.W. Outcomes and Predictors of Skin Sodium Concentration in Dialysis Patients. Clin. Kidney J. 2022, 27708, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, A.-C.; Linz, P.; Nagel, A.M.; Rosenhauer, D.; Horn, S.; Schiffer, M.; Uder, M.; Kopp, C.; Dahlmann, A. Hemodialysis Patients with Cardiovascular Disease Reveal Increased Tissue Na+ Deposition. Kidney Blood Press. Res. 2022, 47, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Lemoine, S.; Salerno, F.; Marcus, T.L.; Duffy, T.; Scholl, T.J.; Filler, G.; House, A.A.; McIntyre, C.W. Functional Sodium MRI Helps to Measure Corticomedullary Sodium Content in Normal and Diseased Human Kidneys. Radiology 2022, 303, 384–389. [Google Scholar] [CrossRef]
- Salerno, F.R.; Akbari, A.; Lemoine, S.; Scholl, T.J.; McIntyre, C.W.; Filler, G. Effects of Pediatric Chronic Kidney Disease and Its Etiology on Tissue Sodium Concentration: A Pilot Study. Pediatr. Nephrol. 2023, 38, 499–507. [Google Scholar] [CrossRef]
- Moon, C.H.; Furlan, A.; Kim, J.H.; Zhao, T.; Shapiro, R.; Bae, K.T. Quantitative Sodium MR Imaging of Native versus Transplanted Kidneys Using a Dual-Tuned Proton/Sodium (1H/23Na) Coil: Initial Experience. Eur. Radiol. 2014, 24, 1320–1326. [Google Scholar] [CrossRef]
- Schneider, M.P.; Raff, U.; Kopp, C.; Scheppach, J.B.; Toncar, S.; Wanner, C.; Schlieper, G.; Saritas, T.; Floege, J.; Schmid, M.; et al. Skin Sodium Concentration Correlates with Left Ventricular Hypertrophy in CKD. J. Am. Soc. Nephrol. 2017, 28, 1867–1876. [Google Scholar] [CrossRef]
- Hammon, M.; Grossmann, S.; Linz, P.; Seuss, H.; Hammon, R.; Rosenhauer, D.; Janka, R.; Cavallaro, A.; Luft, F.C.; Titze, J.; et al. 3 Tesla 23Na Magnetic Resonance Imaging During Acute Kidney Injury. Acad. Radiol. 2017, 24, 1086–1093. [Google Scholar] [CrossRef]
- Deger, S.M.; Wang, P.; Fissell, R.; Ellis, C.D.; Booker, C.; Sha, F.; Morse, J.L.; Stewart, T.G.; Gore, J.C.; Siew, E.D.; et al. Tissue Sodium Accumulation and Peripheral Insulin Sensitivity in Maintenance Hemodialysis Patients. J. Cachexia Sarcopenia Muscle 2017, 8, 500–507. [Google Scholar] [CrossRef]
- Kopp, C.; Linz, P.; Maier, C.; Wabel, P.; Hammon, M.; Nagel, A.M.; Rosenhauer, D.; Horn, S.; Uder, M.; Luft, F.C.; et al. Elevated Tissue Sodium Deposition in Patients with Type 2 Diabetes on Hemodialysis Detected by 23Na Magnetic Resonance Imaging. Kidney Int. 2018, 93, 1191–1197. [Google Scholar] [CrossRef]
- Mitsides, N.; Alsehli, F.M.S.; Mc Hough, D.; Shalamanova, L.; Wilkinson, F.; Alderdice, J.; Mitra, R.; Swiecicka, A.; Brenchley, P.; Parker, G.J.M.; et al. Salt and Water Retention Is Associated with Microinflammation and Endothelial Injury in Chronic Kidney Disease. Nephron 2019, 143, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Qirjazi, E.; Salerno, F.R.; Akbari, A.; Hur, L.; Penny, J.; Scholl, T.; McIntyre, C.W. Tissue Sodium Concentrations in Chronic Kidney Disease and Dialysis Patients by Lower Leg Sodium-23 Magnetic Resonance Imaging. Nephrol. Dial. Transplant. 2021, 36, 1234–1243. [Google Scholar] [CrossRef]
- Sahinoz, M.; Tintara, S.; Deger, S.M.; Alsouqi, A.; Crescenzi, R.L.; Mambungu, C.; Vincz, A.; Mason, O.J.; Prigmore, H.L.; Guide, A.; et al. Tissue Sodium Stores in Peritoneal Dialysis and Hemodialysis Patients Determined by Sodium-23 Magnetic Resonance Imaging. Nephrol. Dial. Transplant. 2021, 36, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, S.; Salerno, F.R.; Akbari, A.; McIntyre, C.W. Influence of Dialysate Sodium Prescription on Skin and Muscle Sodium Concentration. Am. J. Kidney Dis. 2021, 78, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Guyton, A.C. Blood Pressure Control--Special Role of the Kidneys and Body Fluids. Science 1991, 252, 1813–1816. [Google Scholar] [CrossRef]
- Kopp, C.; Linz, P.; Dahlmann, A.; Hammon, M.; Jantsch, J.; Müller, D.N.; Schmieder, R.E.; Cavallaro, A.; Eckardt, K.-U.; Uder, M.; et al. 23Na Magnetic Resonance Imaging-Determined Tissue Sodium in Healthy Subjects and Hypertensive Patients. Hypertension 2013, 61, 635–640. [Google Scholar] [CrossRef]
- Titze, J.; Shakibaei, M.; Schafflhuber, M.; Schulze-Tanzil, G.; Porst, M.; Schwind, K.H.; Dietsch, P.; Hilgers, K.F. Glycosaminoglycan Polymerization May Enable Osmotically Inactive Na+ Storage in the Skin. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H203–H208. [Google Scholar] [CrossRef]
- Wiig, H.; Luft, F.C.; Titze, J.M. The Interstitium Conducts Extrarenal Storage of Sodium and Represents a Third Compartment Essential for Extracellular Volume and Blood Pressure Homeostasis. Acta Physiol. 2018, 222, e13006. [Google Scholar] [CrossRef] [PubMed]
- Jantsch, J.; Schatz, V.; Friedrich, D.; Schröder, A.; Kopp, C.; Siegert, I.; Maronna, A.; Wendelborn, D.; Linz, P.; Binger, K.J.; et al. Cutaneous Na+ Storage Strengthens the Antimicrobial Barrier Function of the Skin and Boosts Macrophage-Driven Host Defense. Cell Metab. 2015, 21, 493–501. [Google Scholar] [CrossRef]
- Schatz, V.; Neubert, P.; Schröder, A.; Binger, K.; Gebhard, M.; Müller, D.N.; Luft, F.C.; Titze, J.; Jantsch, J. Elementary Immunology: Na+ as a Regulator of Immunity. Pediatr. Nephrol. 2017, 32, 201–210. [Google Scholar] [CrossRef]
- Wilck, N.; Balogh, A.; Markó, L.; Bartolomaeus, H.; Müller, D.N. The Role of Sodium in Modulating Immune Cell Function. Nat. Rev. Nephrol. 2019, 15, 546–558. [Google Scholar] [CrossRef]
- Binger, K.J.; Gebhardt, M.; Heinig, M.; Rintisch, C.; Schroeder, A.; Neuhofer, W.; Hilgers, K.; Manzel, A.; Schwartz, C.; Kleinewietfeld, M.; et al. High Salt Reduces the Activation of IL-4- and IL-13-Stimulated Macrophages. J. Clin. Investig. 2015, 125, 4223–4238. [Google Scholar] [CrossRef] [PubMed]
- Kleinewietfeld, M.; Manzel, A.; Titze, J.; Kvakan, H.; Yosef, N.; Linker, R.A.; Muller, D.N.; Hafler, D.A. Sodium Chloride Drives Autoimmune Disease by the Induction of Pathogenic TH17 Cells. Nature 2013, 496, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yosef, N.; Thalhamer, T.; Zhu, C.; Xiao, S.; Kishi, Y.; Regev, A.; Kuchroo, V.K. Induction of Pathogenic TH17 Cells by Inducible Salt-Sensing Kinase SGK1. Nature 2013, 496, 513–517. [Google Scholar] [CrossRef]
- Zhang, W.-C.; Zheng, X.-J.; Du, L.-J.; Sun, J.-Y.; Shen, Z.-X.; Shi, C.; Sun, S.; Zhang, Z.; Chen, X.-Q.; Qin, M.; et al. High Salt Primes a Specific Activation State of Macrophages, M(Na). Cell Res. 2015, 25, 893–910. [Google Scholar] [CrossRef] [PubMed]
- Machnik, A.; Dahlmann, A.; Kopp, C.; Goss, J.; Wagner, H.; van Rooijen, N.; Eckardt, K.-U.; Müller, D.N.; Park, J.-K.; Luft, F.C.; et al. Mononuclear Phagocyte System Depletion Blocks Interstitial Tonicity-Responsive Enhancer Binding Protein/Vascular Endothelial Growth Factor C Expression and Induces Salt-Sensitive Hypertension in Rats. Hypertension 2010, 55, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Machnik, A.; Neuhofer, W.; Jantsch, J.; Dahlmann, A.; Tammela, T.; Machura, K.; Park, J.-K.; Beck, F.-X.; Müller, D.N.; Derer, W.; et al. Macrophages Regulate Salt-Dependent Volume and Blood Pressure by a Vascular Endothelial Growth Factor-C-Dependent Buffering Mechanism. Nat. Med. 2009, 15, 545–552. [Google Scholar] [CrossRef]
- Wiig, H.; Schröder, A.; Neuhofer, W.; Jantsch, J.; Kopp, C.; Karlsen, T.V.; Boschmann, M.; Goss, J.; Bry, M.; Rakova, N.; et al. Immune Cells Control Skin Lymphatic Electrolyte Homeostasis and Blood Pressure. J. Clin. Investig. 2013, 123, 2803–2815. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Aging, Cellular Senescence, and Cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- Petreski, T.; Piko, N.; Ekart, R.; Hojs, R.; Bevc, S. Review on Inflammation Markers in Chronic Kidney Disease. Biomedicines 2021, 9, 182. [Google Scholar] [CrossRef]
- Tang, J.; Ye, L.; Yan, Q.; Zhang, X.; Wang, L. Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Water and Sodium Metabolism. Front. Pharmacol. 2022, 13, 800490. [Google Scholar] [CrossRef]
- Van der Aart-van der Beek, A.B.; de Boer, R.A.; Heerspink, H.J.L. Kidney and Heart Failure Outcomes Associated with SGLT2 Inhibitor Use. Nat. Rev. Nephrol. 2022, 18, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Svenningsen, P.; Bistrup, C.; Friis, U.G.; Bertog, M.; Haerteis, S.; Krueger, B.; Stubbe, J.; Jensen, O.N.; Thiesson, H.C.; Uhrenholt, T.R.; et al. Plasmin in Nephrotic Urine Activates the Epithelial Sodium Channel. J. Am. Soc. Nephrol. 2009, 20, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Artunc, F.; Wörn, M.; Schork, A.; Bohnert, B.N. Proteasuria-The Impact of Active Urinary Proteases on Sodium Retention in Nephrotic Syndrome. Acta Physiol. 2019, 225, e13249. [Google Scholar] [CrossRef]
- Spoto, B.; Pisano, A.; Zoccali, C. Insulin Resistance in Chronic Kidney Disease: A Systematic Review. Am. J. Physiol. Renal Physiol. 2016, 311, F1087–F1108. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Kato, K.; Ohkido, I.; Yokoo, T. Role and Treatment of Insulin Resistance in Patients with Chronic Kidney Disease: A Review. Nutrients 2021, 13, 4349. [Google Scholar] [CrossRef]
- Liao, M.-T.; Sung, C.-C.; Hung, K.-C.; Wu, C.-C.; Lo, L.; Lu, K.-C. Insulin Resistance in Patients with Chronic Kidney Disease. J. Biomed. Biotechnol. 2012, 2012, 691369. [Google Scholar] [CrossRef] [PubMed]
- De Boer, I.H.; Mehrotra, R. Insulin Resistance in Chronic Kidney Disease: A Step Closer to Effective Evaluation and Treatment. Kidney Int. 2014, 86, 243–245. [Google Scholar] [CrossRef]
- Siew, E.D.; Pupim, L.B.; Majchrzak, K.M.; Shintani, A.; Flakoll, P.J.; Ikizler, T.A. Insulin Resistance Is Associated with Skeletal Muscle Protein Breakdown in Non-Diabetic Chronic Hemodialysis Patients. Kidney Int. 2007, 71, 146–152. [Google Scholar] [CrossRef]
- Siew, E.D.; Ikizler, T.A. Insulin Resistance and Protein Energy Metabolism in Patients with Advanced Chronic Kidney Disease. Semin. Dial. 2010, 23, 378–382. [Google Scholar] [CrossRef]
- Kempner, W. Treatment of Heart and Kidney Disease and of Hypertensive and Arteriosclerotic Vascular Disease with the Rice Diet. Ann. Intern. Med. 1949, 31, 821–856. [Google Scholar] [CrossRef] [PubMed]
- Mc Causland, F.R.; Waikar, S.S.; Brunelli, S.M. Increased Dietary Sodium Is Independently Associated with Greater Mortality among Prevalent Hemodialysis Patients. Kidney Int. 2012, 82, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, A.J.; Russell, G.B.; Satko, S.G. Sudden and Cardiac Death Rates in Hemodialysis Patients. Kidney Int. 1999, 55, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N. Clinical Epidemiology of Cardiac Disease in Dialysis Patients: Left Ventricular Hypertrophy, Ischemic Heart Disease, and Cardiac Failure. Semin. Dial. 2003, 16, 111–117. [Google Scholar] [CrossRef]
- Weir, M.A.; Herzog, C.A. Beta Blockers in Patients with End-Stage Renal Disease-Evidence-Based Recommendations. Semin. Dial. 2018, 31, 219–225. [Google Scholar] [CrossRef]
- Cuculich, P.S.; Sánchez, J.M.; Kerzner, R.; Greenberg, S.L.; Sengupta, J.; Chen, J.; Faddis, M.N.; Gleva, M.J.; Smith, T.W.; Lindsay, B.D. Poor Prognosis for Patients with Chronic Kidney Disease despite ICD Therapy for the Primary Prevention of Sudden Death. Pacing Clin. Electrophysiol. 2007, 30, 207–213. [Google Scholar] [CrossRef]
- Amin, M.S.; Fox, A.D.; Kalahasty, G.; Shepard, R.K.; Wood, M.A.; Ellenbogen, K.A. Benefit of Primary Prevention Implantable Cardioverter-Defibrillators in the Setting of Chronic Kidney Disease: A Decision Model Analysis. J. Cardiovasc. Electrophysiol. 2008, 19, 1275–1280. [Google Scholar] [CrossRef]
- Fu, L.; Zhou, Q.; Zhu, W.; Lin, H.; Ding, Y.; Shen, Y.; Hu, J.; Hong, K. Do Implantable Cardioverter Defibrillators Reduce Mortality in Patients with Chronic Kidney Disease at All Stages? Int. Heart J. 2017, 58, 371–377. [Google Scholar] [CrossRef]
- Dasgupta, A.; Montalvo, J.; Medendorp, S.; Lloyd-Jones, D.M.; Ghossein, C.; Goldberger, J.; Passman, R. Increased Complication Rates of Cardiac Rhythm Management Devices in ESRD Patients. Am. J. Kidney Dis. 2007, 49, 656–663. [Google Scholar] [CrossRef]
- Hayer, M.K.; Radhakrishnan, A.; Price, A.M.; Liu, B.; Baig, S.; Weston, C.J.; Biasiolli, L.; Ferro, C.J.; Townend, J.N.; Steeds, R.P.; et al. Defining Myocardial Abnormalities Across the Stages of Chronic Kidney Disease: A Cardiac Magnetic Resonance Imaging Study. JACC Cardiovasc. Imaging 2020, 13, 2357–2367. [Google Scholar] [CrossRef]
- Finsen, A.V.; Lunde, I.G.; Sjaastad, I.; Østli, E.K.; Lyngra, M.; Jarstadmarken, H.O.; Hasic, A.; Nygård, S.; Wilcox-Adelman, S.A.; Goetinck, P.F.; et al. Syndecan-4 Is Essential for Development of Concentric Myocardial Hypertrophy via Stretch-Induced Activation of the Calcineurin-NFAT Pathway. PLoS ONE 2011, 6, e28302. [Google Scholar] [CrossRef] [PubMed]
- Waehre, A.; Vistnes, M.; Sjaastad, I.; Nygård, S.; Husberg, C.; Lunde, I.G.; Aukrust, P.; Yndestad, A.; Vinge, L.E.; Behmen, D.; et al. Chemokines Regulate Small Leucine-Rich Proteoglycans in the Extracellular Matrix of the Pressure-Overloaded Right Ventricle. J. Appl. Physiol. 2012, 112, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, H.; Woo, S.K.; Dahl, S.C.; Handler, J.S.; Kwon, H.M. Tonicity-Responsive Enhancer Binding Protein, a Rel-like Protein That Stimulates Transcription in Response to Hypertonicity. Proc. Natl. Acad. Sci. USA 1999, 96, 2538–2542. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.H.; Woo, S.K.; Han, K.H.; Kim, Y.H.; Handler, J.S.; Kim, J.; Kwon, H.M. Hydration Status Affects Nuclear Distribution of Transcription Factor Tonicity Responsive Enhancer Binding Protein in Rat Kidney. J. Am. Soc. Nephrol. 2001, 12, 2221–2230. [Google Scholar] [CrossRef]
- Edwards, N.C.; Moody, W.E.; Yuan, M.; Hayer, M.K.; Ferro, C.J.; Townend, J.N.; Steeds, R.P. Diffuse Interstitial Fibrosis and Myocardial Dysfunction in Early Chronic Kidney Disease. Am. J. Cardiol. 2015, 115, 1311–1317. [Google Scholar] [CrossRef]
- Rutherford, E.; Talle, M.A.; Mangion, K.; Bell, E.; Rauhalammi, S.M.; Roditi, G.; McComb, C.; Radjenovic, A.; Welsh, P.; Woodward, R.; et al. Defining Myocardial Tissue Abnormalities in End-Stage Renal Failure with Cardiac Magnetic Resonance Imaging Using Native T1 Mapping. Kidney Int. 2016, 90, 845–852. [Google Scholar] [CrossRef]
- Frohlich, E.D.; Chien, Y.; Sesoko, S.; Pegram, B.L. Relationship between Dietary Sodium Intake, Hemodynamics, and Cardiac Mass in SHR and WKY Rats. Am. J. Physiol. 1993, 264, R30–R34. [Google Scholar] [CrossRef]
- Yu, H.C.; Burrell, L.M.; Black, M.J.; Wu, L.L.; Dilley, R.J.; Cooper, M.E.; Johnston, C.I. Salt Induces Myocardial and Renal Fibrosis in Normotensive and Hypertensive Rats. Circulation 1998, 98, 2621–2628. [Google Scholar] [CrossRef]
- Bottomley, P.A. Sodium MRI in Human Heart: A Review. NMR Biomed. 2016, 29, 187–196. [Google Scholar] [CrossRef]
- Popov, S.; Venetsanou, K.; Chedrese, P.J.; Pinto, V.; Takemori, H.; Franco-Cereceda, A.; Eriksson, P.; Mochizuki, N.; Soares-da-Silva, P.; Bertorello, A.M. Increases in Intracellular Sodium Activate Transcription and Gene Expression via the Salt-Inducible Kinase 1 Network in an Atrial Myocyte Cell Line. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H57–H65. [Google Scholar] [CrossRef]
- Christa, M.; Weng, A.M.; Geier, B.; Wörmann, C.; Scheffler, A.; Lehmann, L.; Oberberger, J.; Kraus, B.J.; Hahner, S.; Störk, S.; et al. Increased Myocardial Sodium Signal Intensity in Conn’s Syndrome Detected by 23Na Magnetic Resonance Imaging. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Schelling, J.R. Tubular Atrophy in the Pathogenesis of Chronic Kidney Disease Progression. Pediatr. Nephrol. 2016, 31, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Tabibzadeh, N.; Wagner, S.; Metzger, M.; Flamant, M.; Houillier, P.; Boffa, J.-J.; Vrtovsnik, F.; Thervet, E.; Stengel, B.; Haymann, J.-P.; et al. Fasting Urinary Osmolality, CKD Progression, and Mortality: A Prospective Observational Study. Am. J. Kidney Dis. 2019, 73, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Hebert, L.A.; Greene, T.; Levey, A.; Falkenhain, M.E.; Klahr, S. High Urine Volume and Low Urine Osmolality Are Risk Factors for Faster Progression of Renal Disease. Am. J. Kidney Dis. 2003, 41, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Jamison, R.L. The Renal Concentrating Mechanism: Micropuncture Studies of the Renal Medulla. Fed. Proc. 1983, 42, 2392–2397. [Google Scholar] [PubMed]
- Buerkert, J.; Martin, D.; Prasad, J.; Trigg, D. Role of Deep Nephrons and the Terminal Collecting Duct in a Mannitol-Induced Diuresis. Am. J. Physiol. 1981, 240, F411–F422. [Google Scholar] [CrossRef]
- Gennari, F.J.; Johns, C.; Caflisch, C.R.; Cortell, S. Dissociation of Saline-Induced Natriuresis from Urea Washout in the Rat. Am. J. Physiol. 1981, 241, F250–F256. [Google Scholar] [CrossRef]
- Maril, N.; Margalit, R.; Mispelter, J.; Degani, H. Functional Sodium Magnetic Resonance Imaging of the Intact Rat Kidney. Kidney Int. 2004, 65, 927–935. [Google Scholar] [CrossRef]
- Maril, N.; Margalit, R.; Mispelter, J.; Degani, H. Sodium Magnetic Resonance Imaging of Diuresis: Spatial and Kinetic Response. Magn. Reson. Med. 2005, 53, 545–552. [Google Scholar] [CrossRef]
- Grist, J.T.; Riemer, F.; Hansen, E.S.S.; Tougaard, R.S.; McLean, M.A.; Kaggie, J.; Bøgh, N.; Graves, M.J.; Gallagher, F.A.; Laustsen, C. Visualization of Sodium Dynamics in the Kidney by Magnetic Resonance Imaging in a Multi-Site Study. Kidney Int. 2020, 98, 1174–1178. [Google Scholar] [CrossRef]
- Maril, N.; Rosen, Y.; Reynolds, G.H.; Ivanishev, A.; Ngo, L.; Lenkinski, R.E. Sodium MRI of the Human Kidney at 3 Tesla. Magn. Reson. Med. 2006, 56, 1229–1234. [Google Scholar] [CrossRef]
- Haneder, S.; Konstandin, S.; Morelli, J.N.; Nagel, A.M.; Zoellner, F.G.; Schad, L.R.; Schoenberg, S.O.; Michaely, H.J. Quantitative and Qualitative (23)Na MR Imaging of the Human Kidneys at 3 T: Before and after a Water Load. Radiology 2011, 260, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Chang, T.I.; Lee, J.; Kim, Y.H.; Oh, K.-H.; Lee, S.W.; Kim, S.W.; Park, J.T.; Yoo, T.-H.; Kang, S.-W.; et al. Urine Osmolality and Renal Outcome in Patients with Chronic Kidney Disease: Results from the KNOW-CKD. Kidney Blood Press. Res. 2019, 44, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Shafi, T.; Levey, A.S. Measurement and Estimation of Residual Kidney Function in Patients on Dialysis. Adv. Chronic Kidney Dis. 2018, 25, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Chadban, S.J.; Atkins, R.C. Glomerulonephritis. Lancet 2008, 365, 1797–1806. [Google Scholar] [CrossRef]
- Bellomo, R.; Kellum, J.A.; Ronco, C. Acute Kidney Injury. Lancet 2012, 380, 756–766. [Google Scholar] [CrossRef]
- VA/NIH Acute Renal Failure Trial Network; Palevsky, P.M.; Zhang, J.H.; O’Connor, T.Z.; Chertow, G.M.; Crowley, S.T.; Choudhury, D.; Finkel, K.; Kellum, J.A.; Paganini, E.; et al. Intensity of Renal Support in Critically Ill Patients with Acute Kidney Injury. N. Engl. J. Med. 2008, 359, 7–20. [Google Scholar] [CrossRef]
- RENAL Replacement Therapy Study Investigators; Bellomo, R.; Cass, A.; Cole, L.; Finfer, S.; Gallagher, M.; Lo, S.; McArthur, C.; McGuinness, S.; Myburgh, J.; et al. Intensity of Continuous Renal-Replacement Therapy in Critically Ill Patients. N. Engl. J. Med. 2009, 361, 1627–1638. [Google Scholar] [CrossRef]
- Maril, N.; Margalit, R.; Rosen, S.; Heyman, S.N.; Degani, H. Detection of Evolving Acute Tubular Necrosis with Renal 23Na MRI: Studies in Rats. Kidney Int. 2006, 69, 765–768. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Mariager, C.Ø.; Rasmussen, D.G.K.; Mølmer, M.; Genovese, F.; Karsdal, M.A.; Laustsen, C.; Nørregaard, R. Noninvasive Assessment of Fibrosis Following Ischemia/Reperfusion Injury in Rodents Utilizing Na Magnetic Resonance Imaging. Pharmaceutics 2020, 12, 775. [Google Scholar] [CrossRef]
- Rasmussen, C.W.; Bøgh, N.; Bech, S.K.; Thorsen, T.H.; Hansen, E.S.S.; Bertelsen, L.B.; Laustsen, C. Fibrosis Imaging with Multiparametric Proton and Sodium MRI in Pig Injury Models. NMR Biomed. 2023, 36, e4838. [Google Scholar] [CrossRef]
- Rosen, Y.; Lenkinski, R.E. Sodium MRI of a Human Transplanted Kidney. Acad. Radiol. 2009, 16, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, E.B.; Danielsen, H.; Nielsen, A.H.; Knudsen, F.; Jensen, T.; Kornerup, H.J.; Madsen, M. Relationship between Urinary Concentrating Ability, Arginine Vasopressin in Plasma and Blood Pressure after Renal Transplantation. Scand. J. Clin. Lab. Investig. 1985, 45, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C.; Guay-Woodford, L.M.; Harris, P.C.; Horie, S.; Peters, D.J.M.; Torres, V.E. Polycystic Kidney Disease. Nat. Rev. Dis. Prim. 2018, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Sommerer, C.; Zeier, M. Clinical Manifestation and Management of ADPKD in Western Countries. Kidney Dis. 2016, 2, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Chebib, F.T.; Perrone, R.D.; Chapman, A.B.; Dahl, N.K.; Harris, P.C.; Mrug, M.; Mustafa, R.A.; Rastogi, A.; Watnick, T.; Yu, A.S.L.; et al. A Practical Guide for Treatment of Rapidly Progressive ADPKD with Tolvaptan. J. Am. Soc. Nephrol. 2018, 29, 2458–2470. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Grantham, J.J.; Higashihara, E.; Perrone, R.D.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S.; et al. Tolvaptan in Patients with Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2012, 367, 2407–2418. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Perrone, R.D.; Koch, G.; Ouyang, J.; McQuade, R.D.; Blais, J.D.; Czerwiec, F.S.; et al. Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2017, 377, 1930–1942. [Google Scholar] [CrossRef]
- Irazabal, M.V.; Torres, V.E.; Hogan, M.C.; Glockner, J.; King, B.F.; Ofstie, T.G.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S. Short-Term Effects of Tolvaptan on Renal Function and Volume in Patients with Autosomal Dominant Polycystic Kidney Disease. Kidney Int. 2011, 80, 295–301. [Google Scholar] [CrossRef]
- Boertien, W.E.; Meijer, E.; de Jong, P.E.; ter Horst, G.J.; Renken, R.J.; van der Jagt, E.J.; Kappert, P.; Ouyang, J.; Engels, G.E.; van Oeveren, W.; et al. Short-Term Effects of Tolvaptan in Individuals with Autosomal Dominant Polycystic Kidney Disease at Various Levels of Kidney Function. Am. J. Kidney Dis. 2015, 65, 833–841. [Google Scholar] [CrossRef]
- Kramers, B.J.; van Gastel, M.D.A.; Boertien, W.E.; Meijer, E.; Gansevoort, R.T. Determinants of Urine Volume in ADPKD Patients Using the Vasopressin V2 Receptor Antagonist Tolvaptan. Am. J. Kidney Dis. 2019, 73, 354–362. [Google Scholar] [CrossRef]
- Borrego Utiel, F.J.; Merino García, E. Glomerular Filtration Rate Is the Main Predictor of Urine Volume in Autosomal Dominant Polycystic Kidney Disease Patients Treated with Tolvaptan When Daily Osmolar Excretion Is Expressed as Urinary Osmolality/Creatinine Ratio. Clin. Kidney J. 2021, 14, 1031–1033. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Philbin, E.F.; Spertus, J.A.; Kaatz, S.; Sandberg, K.R.; Weaver, W.D.; Resource Utilization among Congestive Heart Failure (REACH) Study. Confirmation of a Heart Failure Epidemic: Findings from the Resource Utilization among Congestive Heart Failure (REACH) Study. J. Am. Coll. Cardiol. 2002, 39, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Bakris, G.L.; Owen, W.F.; Klassen, P.S.; Califf, R.M. Slowing the Progression of Diabetic Nephropathy and Its Cardiovascular Consequences. Am. Heart J. 2004, 148, 243–251. [Google Scholar] [CrossRef]
- McCullough, P.A.; Kellum, J.A.; Haase, M.; Müller, C.; Damman, K.; Murray, P.T.; Cruz, D.; House, A.A.; Schmidt-Ott, K.M.; Vescovo, G.; et al. Pathophysiology of the Cardiorenal Syndromes: Executive Summary from the Eleventh Consensus Conference of the Acute Dialysis Quality Initiative (ADQI). Contrib. Nephrol. 2013, 182, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Haase, M.; Müller, C.; Damman, K.; Murray, P.T.; Kellum, J.A.; Ronco, C.; McCullough, P.A. Pathogenesis of Cardiorenal Syndrome Type 1 in Acute Decompensated Heart Failure: Workgroup Statements from the Eleventh Consensus Conference of the Acute Dialysis Quality Initiative (ADQI). Contrib. Nephrol. 2013, 182, 99–116. [Google Scholar] [CrossRef]
- Cruz, D.N.; Schmidt-Ott, K.M.; Vescovo, G.; House, A.A.; Kellum, J.A.; Ronco, C.; McCullough, P.A. Pathophysiology of Cardiorenal Syndrome Type 2 in Stable Chronic Heart Failure: Workgroup Statements from the Eleventh Consensus Conference of the Acute Dialysis Quality Initiative (ADQI). Contrib. Nephrol. 2013, 182, 117–136. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Hoste, E.A.; Braam, B.; Briguori, C.; Kellum, J.A.; McCullough, P.A.; Ronco, C. Cardiorenal Syndrome Type 3: Pathophysiologic and Epidemiologic Considerations. Contrib. Nephrol. 2013, 182, 137–157. [Google Scholar] [CrossRef]
- Tumlin, J.A.; Costanzo, M.R.; Chawla, L.S.; Herzog, C.A.; Kellum, J.A.; McCullough, P.A.; Ronco, C. Cardiorenal Syndrome Type 4: Insights on Clinical Presentation and Pathophysiology from the Eleventh Consensus Conference of the Acute Dialysis Quality Initiative (ADQI). Contrib. Nephrol. 2013, 182, 158–173. [Google Scholar] [CrossRef]
- Clark, A.L.; Kalra, P.R.; Petrie, M.C.; Mark, P.B.; Tomlinson, L.A.; Tomson, C.R. Change in Renal Function Associated with Drug Treatment in Heart Failure: National Guidance. Heart 2019, 105, 904–910. [Google Scholar] [CrossRef]
- Heywood, J.T.; Fonarow, G.C.; Costanzo, M.R.; Mathur, V.S.; Wigneswaran, J.R.; Wynne, J.; ADHERE Scientific Advisory Committee and Investigators. High Prevalence of Renal Dysfunction and Its Impact on Outcome in 118,465 Patients Hospitalized with Acute Decompensated Heart Failure: A Report from the ADHERE Database. J. Card. Fail. 2007, 13, 422–430. [Google Scholar] [CrossRef]
- Chioncel, O.; Mebazaa, A.; Harjola, V.-P.; Coats, A.J.; Piepoli, M.F.; Crespo-Leiro, M.G.; Laroche, C.; Seferovic, P.M.; Anker, S.D.; Ferrari, R.; et al. Clinical Phenotypes and Outcome of Patients Hospitalized for Acute Heart Failure: The ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1242–1254. [Google Scholar] [CrossRef]
- Hoorn, E.J.; Ellison, D.H. Diuretic Resistance. Am. J. Kidney Dis. 2017, 69, 136–142. [Google Scholar] [CrossRef]
- Faris, R.F.; Flather, M.; Purcell, H.; Poole-Wilson, P.A.; Coats, A.J.S. Diuretics for Heart Failure. Cochrane Database Syst. Rev. 2012, 2, CD003838. [Google Scholar] [CrossRef]
- Singh, D.; Shrestha, K.; Testani, J.M.; Verbrugge, F.H.; Dupont, M.; Mullens, W.; Tang, W.H.W. Insufficient Natriuretic Response to Continuous Intravenous Furosemide Is Associated with Poor Long-Term Outcomes in Acute Decompensated Heart Failure. J. Card. Fail. 2014, 20, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Testani, J.M.; Hanberg, J.S.; Cheng, S.; Rao, V.; Onyebeke, C.; Laur, O.; Kula, A.; Chen, M.; Wilson, F.P.; Darlington, A.; et al. Rapid and Highly Accurate Prediction of Poor Loop Diuretic Natriuretic Response in Patients with Heart Failure. Circ. Heart Fail. 2016, 9, e002370. [Google Scholar] [CrossRef]
- Bart, B.A.; Goldsmith, S.R.; Lee, K.L.; Givertz, M.M.; O’Connor, C.M.; Bull, D.A.; Redfield, M.M.; Deswal, A.; Rouleau, J.L.; LeWinter, M.M.; et al. Ultrafiltration in Decompensated Heart Failure with Cardiorenal Syndrome. N. Engl. J. Med. 2012, 367, 2296–2304. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Nijst, P.; Dupont, M.; Penders, J.; Tang, W.H.W.; Mullens, W. Urinary Composition during Decongestive Treatment in Heart Failure with Reduced Ejection Fraction. Circ. Heart Fail. 2014, 7, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Wile, D. Diuretics: A Review. Ann. Clin. Biochem. 2012, 49, 419–431. [Google Scholar] [CrossRef]
- Karg, M.V.; Bosch, A.; Kannenkeril, D.; Striepe, K.; Ott, C.; Schneider, M.P.; Boemke-Zelch, F.; Linz, P.; Nagel, A.M.; Titze, J.; et al. SGLT-2-Inhibition with Dapagliflozin Reduces Tissue Sodium Content: A Randomised Controlled Trial. Cardiovasc. Diabetol. 2018, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Ivey-Miranda, J.B.; Almeida-Gutierrez, E.; Herrera-Saucedo, R.; Posada-Martinez, E.L.; Chavez-Mendoza, A.; Mendoza-Zavala, G.H.; Cigarroa-Lopez, J.A.; Magaña-Serrano, J.A.; Rivera-Leaños, R.; Treviño-Mejia, A.; et al. Sodium Restriction in Patients with Chronic Heart Failure and Reduced Ejection Fraction: A Randomized Controlled Trial. Cardiol. J. 2021, 30, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Linz, B.; Saljic, A.; Hohl, M.; Gawałko, M.; Jespersen, T.; Sanders, P.; Böhm, M.; Linz, D. Inhibition of Sodium-Proton-Exchanger Subtype 3-Mediated Sodium Absorption in the Gut: A New Antihypertensive Concept. Int. J. Cardiol. Heart Vasc. 2020, 29, 100591. [Google Scholar] [CrossRef]
- Rao, V.S.; Turner, J.M.; Griffin, M.; Mahoney, D.; Asher, J.; Jeon, S.; Yoo, P.S.; Boutagy, N.; Feher, A.; Sinusas, A.; et al. First-in-Human Experience with Peritoneal Direct Sodium Removal Using a Zero-Sodium Solution: A New Candidate Therapy for Volume Overload. Circulation 2020, 141, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).