Multimodality Imaging Evaluation to Detect Subtle Right Ventricular Involvement in Patients with Acute Myocarditis and Preserved Left Ventricular Ejection Fraction
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Transthoracic Echocardiography
2.3. CMR Acquisition Protocol
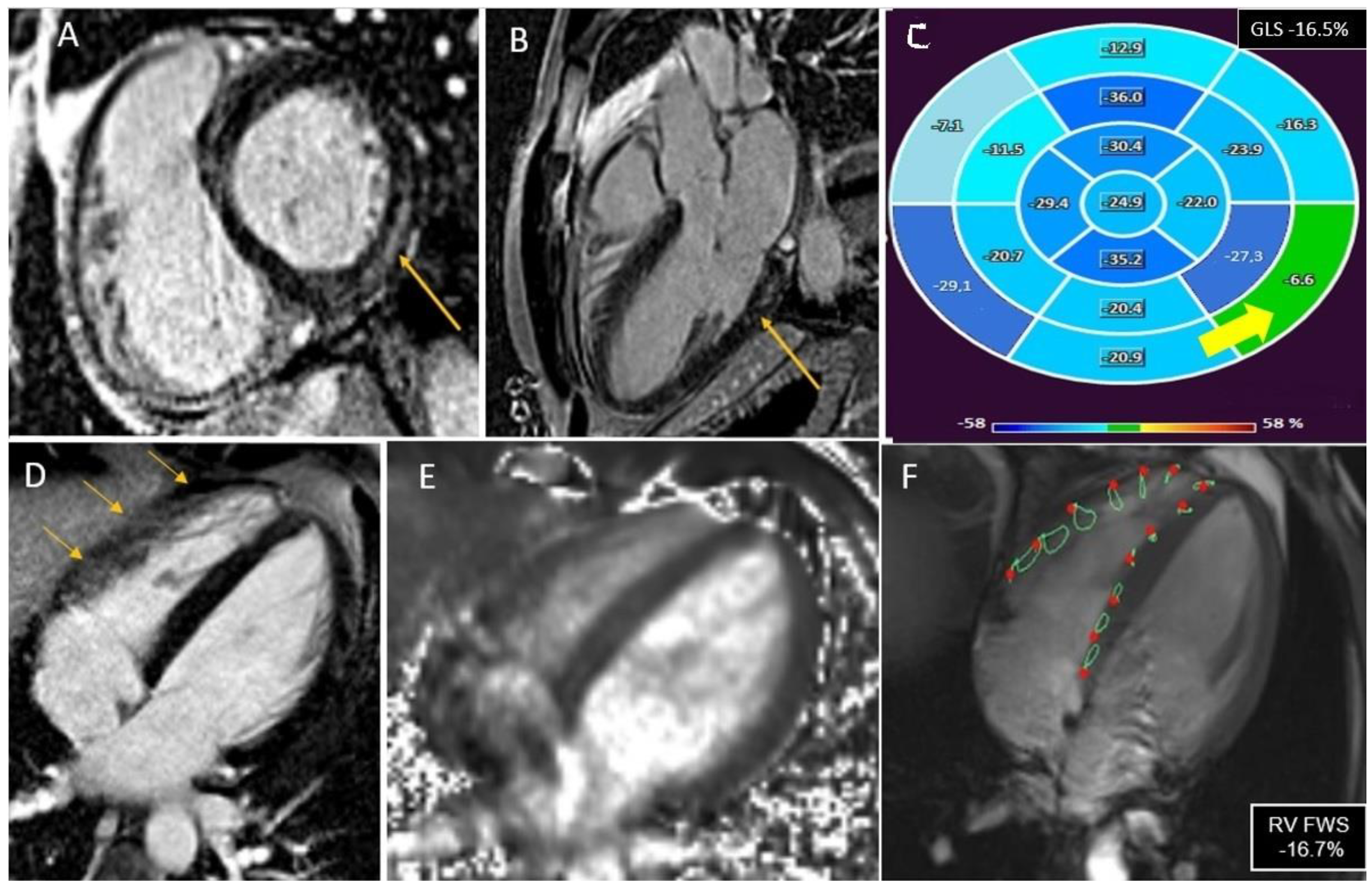
2.4. CMR Analysis
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristic of Population
3.2. 2D Transthoracic Echocardiography
3.3. Cardiovascular Magnetic Resonance Parameters
3.4. Right Ventricle Involvement
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MY | Acute myocarditis |
| CMR | Cardiovascular magnetic resonance |
| CMR-FT | Feature tracking CMR |
| EF | Ejection fraction |
| FAC | Fractional area change |
| GLS | Global longitudinal strain |
| LGE | Late gadolinium enhancement |
| LV | Left ventricle |
| LV-GLS | Left ventricle global longitudinal strain |
| RLS | Regional longitudinal strain |
| RV | Right ventricle |
| RV FWS | Right ventricle free wall longitudinal strain |
| TAPSE | Tricuspid annular plane systolic excursion |
| TTE | Transthoracic echocardiography |
References
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Helio, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Bernhard, B.; Schnyder, A.; Garachemani, D.; Fischer, K.; Tanner, G.; Safarkhanlo, Y.; Stark, A.W.; Schütze, J.; Pavlicek-Bahlo, M.; Greulich, S.; et al. Prognostic Value of Right Ventricular Function in Patients with Suspected Myocarditis Undergoing Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2023, 16, 28–41. [Google Scholar] [CrossRef]
- Stiermaier, T.; Fohrenbach, F.; Klingel, K.; Kandolf, R.; Boudriot, E.; Sandri, M.; Linke, A.; Rommel, K.P.; Desch, S.; Schuler, G.; et al. Biventricular endomyocardial biopsy in patients with suspected myocarditis: Feasibility, complication rate and additional diagnostic value. Int. J. Cardiol. 2017, 230, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Perfetti, M.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Pepe, A.; Todiere, G.; Lanzillo, C.; Scatteia, A.; et al. Cardiac MR with Late Gadolinium Enhancement in Acute Myocarditis with Pre-served Systolic Function: ITAMY Study. J. Am. Coll. Cardiol. 2017, 70, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Duan, X.; Feng, G.; Sirajuddin, A.; Yin, G.; Zhuang, B.; He, J.; Xu, J.; Yang, W.; Wu, W.; et al. Mul-tiparametric Cardiovascular Magnetic Resonance in Acute Myocarditis: Comparison of 2009 and 2018 Lake Louise Criteria with Endomyocardial Biopsy Confirmation. Front. Cardiovasc. Med. 2021, 8, 739892. [Google Scholar] [CrossRef] [PubMed]
- Luetkens, J.A.; Homsi, R.; Sprinkart, A.M.; Doerner, J.; Dabir, D.; Kuetting, D.L.; Block, W.; Andrie, R.; Stehning, C.; Fimmers, R.; et al. Incremental value of quantitative CMR including parametric mapping for the diagnosis of acute myocarditis. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Negri, F.; De Luca, A.; Todiere, G.; Bianco, F.; Barison, A.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; et al. Role of right ventricular involvement in acute myocarditis, assessed by cardiac magnetic resonance. Int. J. Cardiol. 2018, 271, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Gherbesi, E.; Bergamaschi, L.; Cusmano, I.; Tien, T.T.; Paolisso, P.; Foa, A.; Pizzi, C.; Barosi, A. The usefulness of speckle tracking echocardiography in identifying subclinical myocardial dysfunction in young adults recovered from mild COVID-19. Echocardiography 2022, 39, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, Z.; Xu, L.; Liu, J.; Li, Y.; Zhang, N.; Liu, D.; Wen, Z. Diagnostic and Prognostic Value of Cardiac Magnetic Resonance Strain in Suspected Myocarditis with Preserved LV-EF: A Comparison between Patients with Negative and Positive Late Gadolinium Enhancement Findings. J. Magn. Reson. Imaging 2022, 55, 1109–1119. [Google Scholar] [CrossRef]
- Amundsen, B.H.; Helle-Valle, T.; Edvardsen, T.; Torp, H.; Crosby, J.; Lyseggen, E.; Stoylen, A.; Ihlen, H.; Lima, J.A.; Smiseth, O.A.; et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: Validation against sonomicrometry and tagged magnetic resonance imaging. J. Am. Coll. Cardiol. 2006, 47, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Gavazzoni, M.; Badano, L.P.; Vizzardi, E.; Raddino, R.; Genovese, D.; Taramasso, M.; Sciatti, E.; Palermo, C.; Metra, M.; Muraru, D. Prognostic value of right ventricular free wall longitudinal strain in a large cohort of outpatients with left-side heart disease. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Obrist, S.J.; Erne, S.A.; Stark, A.W.; Marggraf, M.; Kaneko, K.; Guensch, D.P.; Huber, A.T.; Greulich, S.; Aghayev, A.; et al. Feature Tracking Myocardial Strain Incrementally Improves Prognostication in Myocarditis Beyond Traditional CMR Imaging Features. JACC Cardiovasc. Imaging 2020, 13, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Linder, O.L.; Erne, S.A.; Stark, A.W.; Obrist, S.J.; Bernhard, B.; Guensch, D.P.; Huber, A.T.; Kwong, R.Y.; Grani, C. Reproducibility and its confounders of CMR feature tracking myocardial strain analysis in patients with suspected myocarditis. Eur. Radiol. 2021, 32, 3436–3446. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S.; et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int. J. Cardiovasc. Imaging 2002, 18, 539–542. [Google Scholar] [PubMed]
- Grani, C.; Eichhorn, C.; Biere, L.; Murthy, V.L.; Agarwal, V.; Kaneko, K.; Cuddy, S.; Aghayev, A.; Steigner, M.; Blankstein, R.; et al. Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients with Suspected Myocarditis. J. Am. Coll. Cardiol. 2017, 70, 1964–1976. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Kindermann, I.; Kindermann, M.; Mahfoud, F.; Ukena, C.; Athanasiadis, A.; Hill, S.; Mahrholdt, H.; Voehringer, M.; Schieber, M.; et al. Comparative evaluation of left and right ventricular endomyocardial biopsy: Differences in complication rate and diagnostic performance. Circulation 2010, 122, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, C.; Pieroni, M.; Maseri, A.; Frustaci, A. Histologic findings in patients with clinical and instrumental diagnosis of sporadic arrhythmogenic right ventricular dysplasia. J. Am. Coll. Cardiol. 2004, 43, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
| Myocarditis | Controls | p Value | ||
|---|---|---|---|---|
| n = 54 | n = 20 | |||
| Clinical Characteristics | ||||
| Age (years) | Mean ± SD | 36.6 ± 17.3 | 55.6 ± 8.8 | <0.001 |
| Male | n (%) | 49 (92.2) | 12 (60) | <0.001 |
| Body Surface Area (m2) | Mean ± SD | 1.90 ± 0.16 | 1.85 ± 0.12 | 0.110 |
| Dyslipidemia | n (%) | 9 (17.3) | 7 (35) | 0.106 |
| Arterial Hypertension | n (%) | 4 (7.7) | 4 (20) | 0.137 |
| Diabetes | n (%) | 2 (3.8) | 3 (15) | 0.09 |
| Current smoker | n (%) | 18 (34.6) | 10 (50) | 0.246 |
| Familiarity | n (%) | 7 (13.5) | 4 (20%) | 0.490 |
| History of CAD | n (%) | 2 (3.8) | 2 (10) | 0.307 |
| Echocardiography results | ||||
| EDVi mL/m2 | Median [IQR] | 60.7 [40.2–79.1] | 59.2 [36.1–76.2] | 0.645 |
| LV EF 2D % | Median [IQR] | 58.6 [53.2–60.1] | 59.3 [54.1–64.2] | 0.071 |
| LV GLS, % | Median [IQR] | −16.4 [−14.2–−20.1] | −22.4 [−18.2–−25.1] | 0.017 |
| LV Mass Index g/m2 | Median [IQR] | 79.1 [61.0–85.4] | 69.8 [53.0–79.4] | 0.063 |
| E/A | Mean ± SD | 1.24 ± 0.75 | 1.03 ± 0.32 | 0.327 |
| E/e’ | Mean ± SD | 6.23 ± 2.96 | 7.55 ± 2.2 | 0.076 |
| LAVi mL/m2 | Mean ± SD | 23.8 ± 7.0 | 22.5 ± 4.9 | 0.460 |
| TAPSE mm | Mean ± SD | 22.7 ± 2.2 | 23.9 ± 2.6 | 0.068 |
| FAC % | Mean ± SD | 41.6 ± 3.8 | 42.8 ± 3.2 | 0.212 |
| CMR results | ||||
| LV EF% | Median [IQR] | 57.6 [55.2–61.3] | 59.3 [54.6–65.3] | 0.073 |
| LV GLS % | Median [IQR] | −19.0 [−15.2–−27.3] | −21.0 [−18.4–−29.7] | 0.029 |
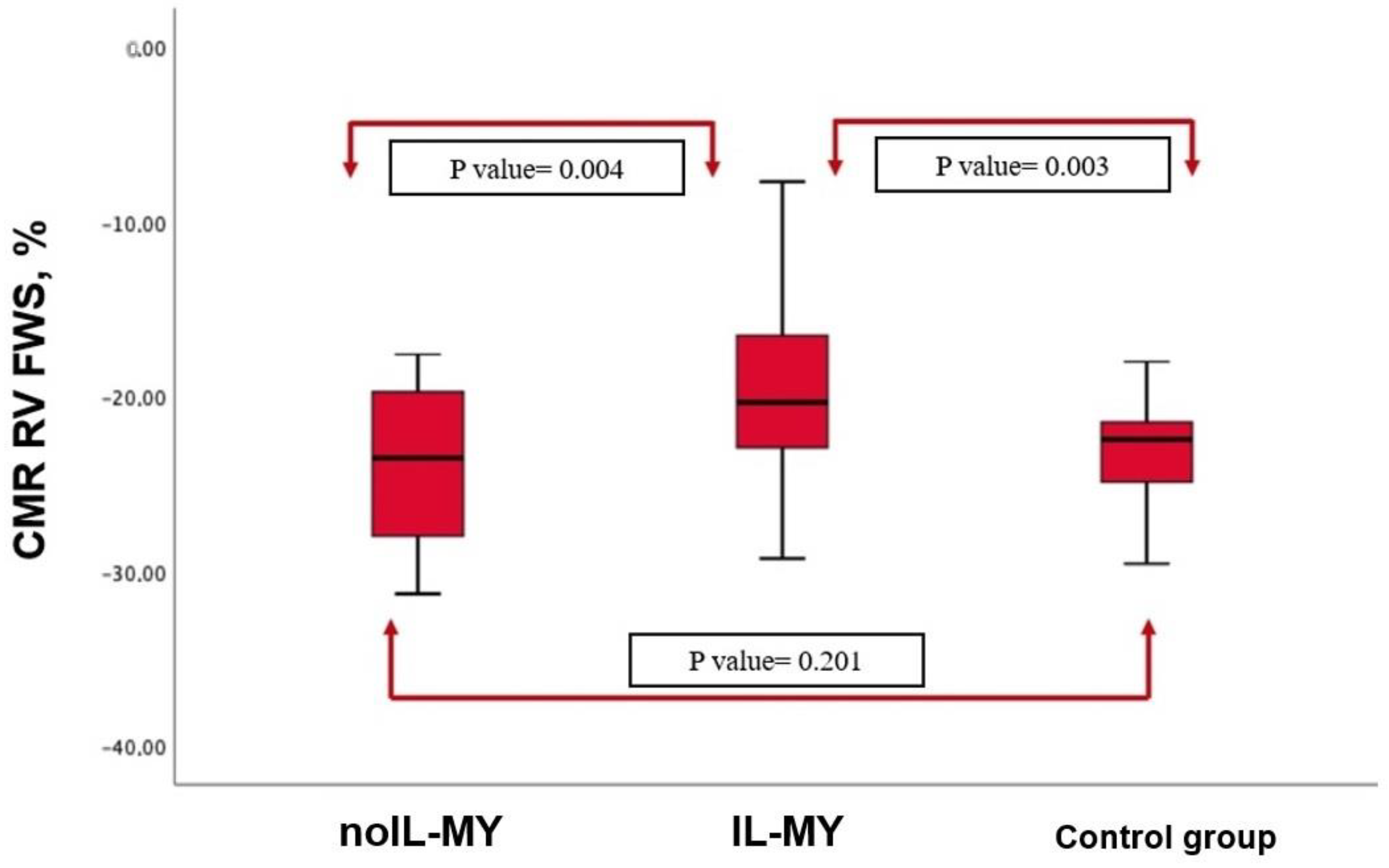
| RV FWS% | Median [IQR] | −21.2 [−17.4–−29.3] | −23.2 [−18.2–−27.3] | 0.201 |
| RV EDVi mL/m2 | Median [IQR] | 80.1 [69.2–92.1] | 88.1 [69.5–98.1] | 0.076 |
| RV ESVi mL/m2 | Median [IQR] | 27.5 [24.2–37.2] | 28.0 [21.2–36.1] | 0.231 |
| RV EF% | Median [IQR] | 49.6 [47.2–59.3] | 48.7 [46.6–57.3] | 0.456 |
| All Myocarditis | IL-MY | noIL-MY | p Value * | ||
|---|---|---|---|---|---|
| n = 54 | n = 34 | n = 20 | |||
| Echocardiography results | |||||
| EDVi mL/m2 | mean ± SD | 60.75 ± 15.2 | 60.47 ± 17.96 | 66.39 ± 20.84 | 0.290 |
| EF 2D % | mean ± SD | 56.23 ± 7.22 | 55.32 ± 7.93 | 57.94 ± 5.44 | 0.216 |
| LV Mass Index g/m2 | mean ± SD | 79 ± 20 | 80.82 ± 23.39 | 75.56 ± 11.35 | 0.373 |
| E/A | mean ± SD | 1.24 ± 0.75 | 1.24 ± 0.91 | 1.25 ± 0.34 | 0.976 |
| E/e’ | mean ± SD | 6.23 ± 2.96 | 6.38 ± 3.01 | 5.94 ± 2.92 | 0.617 |
| LAVi mL/m2 | mean ± SD | 23.8 ± 7.05 | 23.6 ± 8.0 | 24.2 ± 4.8 | 0.772 |
| TAPSE mm | mean ± SD | 22.7 ± 2.2 | 22.5 ± 1.9 | 23.0 ± 2.8 | 0.460 |
| FAC % | mean ± SD | 41.6 ± 3.8 | 41.2 ± 3.9 | 42.3 ± 3.8 | 0.301 |
| CMR results | |||||
| LV EF% | median (IQR) | 58.6 [53.2–60.1] | 59.4 [54.2–63.1] | 57.3 [55.1–64.2] | 0.256 |
| LV GLS % | median (IQR) | −19.0 [−15.2–−27.3] | −17.4 [−14.2–−27.3] | −18.8 [−15.7–−26.8] | 0.365 |
| LV segments with edema | median (IQR) | 2 [1–6] | 3 [2–7] | 2 [1–6] | 0.015 |
| LV segments with LGE | median (IQR) | 3 [1–5] | 3 [2–6] | 2 [1–6] | 0.022 |
| RV EF% | median (IQR) | 67.7 [51.3–81.5] | 47.1 [45.2–53.4] | 48.6 [46.3–58.2] | 0.256 |
| RV EDV mL/m2 | median (IQR) | 80.1 [69.2–92.1] | 85.1 [76.0–95.4] | 79.0 [69.0–91.1] | 0.06 |
| RV ESV mL/m2 | median (IQR) | 27.5 [24.2–37.2] | 28.2 [23.4–39.1] | 31.3 [24.5–39.2] | 0.560 |
| RV FWS % | median (IQR) | −21.2 [−17.4–−29.3] | −18.17 [−11.2–−22.8] | −24.2 [−19.4–−28.7] | 0.004 |
| RV FWS < −23% | n (%) | 32 (61%) | 25 (73%) | 7 (38%) | 0.003 |
| Pericardial effusion | n (%) | 7 (13.5) | 5 (14.7) | 2 (11.1) | 0.718 |
| Pericardial LGE | n (%) | 14 (27) | 11 (32) | 3 (17) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonanni, M.; Angelini, G.; Leo, L.A.; Schlossbauer, S.A.; Bergamaschi, L.; Landi, A.; Sangiorgi, G.M.; Forleo, C.; Pasotti, E.; Pedrazzini, G.; et al. Multimodality Imaging Evaluation to Detect Subtle Right Ventricular Involvement in Patients with Acute Myocarditis and Preserved Left Ventricular Ejection Fraction. J. Clin. Med. 2023, 12, 4308. https://doi.org/10.3390/jcm12134308
Bonanni M, Angelini G, Leo LA, Schlossbauer SA, Bergamaschi L, Landi A, Sangiorgi GM, Forleo C, Pasotti E, Pedrazzini G, et al. Multimodality Imaging Evaluation to Detect Subtle Right Ventricular Involvement in Patients with Acute Myocarditis and Preserved Left Ventricular Ejection Fraction. Journal of Clinical Medicine. 2023; 12(13):4308. https://doi.org/10.3390/jcm12134308
Chicago/Turabian StyleBonanni, Michela, Gianmarco Angelini, Laura Anna Leo, Susanne Anna Schlossbauer, Luca Bergamaschi, Antonio Landi, Giuseppe Massimo Sangiorgi, Cinzia Forleo, Elena Pasotti, Giovanni Pedrazzini, and et al. 2023. "Multimodality Imaging Evaluation to Detect Subtle Right Ventricular Involvement in Patients with Acute Myocarditis and Preserved Left Ventricular Ejection Fraction" Journal of Clinical Medicine 12, no. 13: 4308. https://doi.org/10.3390/jcm12134308
APA StyleBonanni, M., Angelini, G., Leo, L. A., Schlossbauer, S. A., Bergamaschi, L., Landi, A., Sangiorgi, G. M., Forleo, C., Pasotti, E., Pedrazzini, G., Valgimigli, M., Faletra, F. F., Guglielmo, M., & Pavon, A. G. (2023). Multimodality Imaging Evaluation to Detect Subtle Right Ventricular Involvement in Patients with Acute Myocarditis and Preserved Left Ventricular Ejection Fraction. Journal of Clinical Medicine, 12(13), 4308. https://doi.org/10.3390/jcm12134308












