Abstract
The oral health of older individuals can be negatively impacted by various systemic health factors, leading to rapid oral health deterioration.
This paper aims to present an overview of the published evidence on systemic health factors that contribute to rapid oral health deterioration in
older individuals, and to explore the implications of these factors for both general healthcare and oral healthcare provision. Older people are at
risk of experiencing adverse reactions to medications due to multimorbidity, polypharmacy, and changes in pharmacokinetics and pharmacodynamics.
Hyposalivation, a significant side effect of some medications, can be induced by both the type and number of medications used. Frailty, disability,
sarcopenia, care dependency, and limited access to professional oral healthcare can also compromise the oral health of older people. To prevent rapid
oral health deterioration, a comprehensive approach is required that involves effective communication between oral healthcare providers, other
healthcare providers, and informal caregivers. Oral healthcare providers have a responsibility to advocate for the importance of maintaining adequate
oral health and to raise awareness of the serious consequences of weakened oral health. By doing so, we can prevent weakened oral health from
becoming a geriatric syndrome.
Keywords:
oral health care; older people; multimorbidity; polypharmacy; frailty; sarcopenia; disability; care dependency 1. Introduction
The physical and psychological functions of many older adults are being negatively impacted by poor oral health. Difficulties with chewing, biting,
swallowing, tasting, speaking, communicating, smiling, appearance, aesthetics, and self-esteem are common [1]. Among the frailest and most care-dependent older adults, dental caries, periodontal disease, tooth loss, and xerostomia are particularly
prevalent [1,2,3,4]. Despite the fact that most chronic oral diseases are preventable and
treatable, a variety of factors make it difficult to maintain good oral health as people age. This paper aims to present an overview of the published
evidence on the systemic health factors that contribute to rapid oral health deterioration in older individuals, and to explore the implications of
these factors for both general healthcare and oral healthcare provision.
2. Ageing
Ageing is typically viewed as a gradual decline in the functioning of various bodily systems, stemming from the accumulation of damaged tissue and
substances caused by intrinsic or extrinsic mechanisms [5]. The process of biological ageing is a
multifaceted and intricate phenomenon, and although the exact molecular mechanisms behind its onset and progression remain unclear, ample evidence
suggests that oxidative stress may play a significant role [6]. Kinases, phosphatases, and
transcription factors are particularly sensitive to changes in cellular redox status, and chronic or severe disruptions in this homeostasis can result
in cell death or proliferation. Immune senescence, or the quantitative and qualitative changes in the immune system that accompany ageing, is another
hallmark of this process. While immune senescence does not necessarily entail a progressive decline in immune function, it often leads to cytokine
dysregulation, which can cause a chronic, low-grade inflammatory state. This inflammation may serve as a biological foundation for ageing and
contribute to the onset of age-related diseases, increasing the risk of multimorbidity and mortality [6,7,8,9].
3. Ageing and Telomere Length
Telomere length is considered a useful biomarker of cellular ageing, as it reflects the repeated sequences of nucleotides that protect the ends of
chromosomes [10]. With each replication of cells, telomeres shorten due to incomplete lagging strand
replication, leading to cellular senescence once they reach a critically short length [11]. Studies
have suggested that telomere length is sensitive to inflammation, as higher rates of telomere loss have been observed in a pro-inflammatory environment
with increased blood cell replication [12]. This has prompted some researchers to explore the
relationship between periodontal disease and telomere length [13,14,15,16]. In a NHANES
study involving 21,000 participants aged 35–75 years, a significant correlation was found between periodontal disease and telomere length,
particularly among women, overweight or obese individuals, and those with cardiometabolic comorbidities [17].
4. Diseases and Oral Health
Several studies have suggested a strong link between noncommunicable diseases and oral health, with demonstrated associations with oral diseases for
various conditions including cancer, diabetes, cardiovascular diseases, depression, neurodegenerative conditions, rheumatic diseases, inflammatory
bowel disease, gastric helicobacter pylori, obesity, and asthma [18]. The connection between oral
health and these diseases is largely attributed to inflammation, although there are two other pathways that may explain the association [19,20,21].
Firstly, some systemic diseases have direct links to negative impacts on oral health and oral health-related quality of life (OHRQoL), such as Crohn’s disease [22,23], Beçhet’s
disease [24,25,26], scleroderma [27,28], oral cancer [29,30,31,32], head and neck cancer [33], and Sjögren’s syndrome [34,35,36,37]. Secondly, some chronic diseases
may indirectly affect oral health, as they can lead to reduced motivation regarding oral hygiene and care. For example, psychiatric [38,39,40,41,42,43,44,45,46,47] and neurological diseases [48,49,50,51,52,53], as
well as Alzheimer’s disease [54,55],
rheumatic [56], oncological [57], and
cardiovascular diseases [58,59,60,61,62,63], can all have an impact.
Early diagnosis and treatment of oral conditions among older people with chronic diseases could prevent weak oral health and a decline in OHRQoL.
However, individuals with cognitive disorders and those receiving palliative care may lose their ability to communicate their oral health needs,
leading to under-reporting and underestimation of oral conditions [64]. This could result in
healthcare providers failing to fully appreciate the extent of the problem, leading to untreated oral conditions and prolonged discomfort among these
patients.
5. Multimorbidity and Polypharmacy
In 2013, a group of European researchers established a definition for multimorbidity, which refers to any combination of chronic disease with at
least one other disease (acute or chronic), bio-psychosocial factor (associated or not), or somatic risk factor. This definition recognizes that any
bio-psychosocial factor, somatic risk factor, social network, burden of diseases, healthcare consumption, and patient coping strategies may modify the
effects of the multimorbidity. Multimorbidity can lead to increased disability, decreased quality of life, or frailty. While the concept of
multimorbidity has been recognized and enhanced by European general practitioners [65], studies on
its prevalence have not yet been conducted.
In populations of older adults with multimorbidities, the use of multiple medications is common, a phenomenon referred to as polypharmacy.
Polypharmacy is associated with adverse outcomes, including medication–medication interactions, medication–disease interactions,
decreased renal and hepatic function, and reduced lean body mass, hearing, vision, cognition, and mobility [66]. A meta-analysis showed that 38% of community-dwelling adults aged 60 years and older use five or more medications daily [67]. Additionally, almost half of care home residents are exposed to potentially inappropriate medications [66]. A systematic review identified 138 definitions of polypharmacy, but a numerical definition alone is
insufficient to assess the safety and appropriateness of medication use. Therefore, a shift towards the term “appropriate polypharmacy”,
using a holistic approach that considers comorbidities present, is needed [68].
6. Frailty and Oral Health
The concept of frailty has become increasingly important in recent decades, but a consensus on its definition has not yet been reached. There are
two main approaches to defining frailty: one that focuses solely on physical functioning, and another that takes into account other domains, such as
memory and mood. For example, the Fried frailty phenotype considers unintentional weight loss, self-reported exhaustion, physical activity, hand grip
strength, and walking speed, while the multidimensional frailty index by Rockwood et al. also considers cognitive and psychological factors (Figure 1) [69,70]. The prevalence of frailty varies depending on the approach used, with higher rates found for multidimensional assessments
[71].

Figure 1.
Clinical Frailty Scale (Dalhousie University, Halifax, NS, Canada), used with permission [69,70].
A systematic review investigated the link between oral health and frailty, focusing on five longitudinal studies that used Fried’s frailty
phenotype. These studies found that the number of teeth, oral functions, accumulation of oral health problems, and dry mouth symptoms were
significantly associated with the incidence of frailty [72]. In community-dwelling older adults,
oral pain was associated with weight loss and weak handgrip, while chewing problems were associated with low physical activity and low gait speed.
Those who required dental prostheses were more likely to be prefrail or frail than others [73].
Further research is needed to determine whether oral health indicators can be used to assess frailty.
7. Sarcopenia and Oral Health
Sarcopenia is a condition that affects older individuals and causes a decline in muscle mass and strength. The prevalence of sarcopenia varies
widely, between 3.2% to 40%, with the highest incidence in people above the age of 80 and those living in institutions [69,70,71,72,73,74].
Several risk factors have been identified, including age, chronic diseases, and physical activity levels. Chronic obstructive pulmonary disease,
diabetes mellitus, and hypertension are among the chronic diseases that have been linked to sarcopenia [75]. Although it is a common issue in older adults, sarcopenia can be managed and even prevented with appropriate exercise and nutrition [74,76,77]. Interestingly, sarcopenia can also affect oral health in ways that are not well-known. As muscle mass declines, individuals may experience
weaker temporomandibular and orofacial muscles, resulting in difficulty chewing and swallowing [78].
Figure 2 presents an overview of the associations between weak oral health, malnutrition, and
sarcopenia [79].
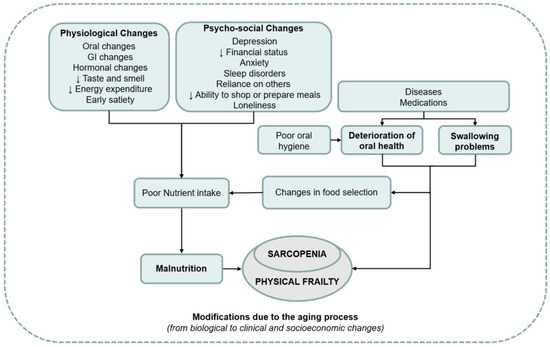
Figure 2.
Overview of the associations between weak oral health, malnutrition, and sarcopenia, used with permission [79].
8. Disability and Oral Health
Among older adults, there are bidirectional associations between oral health and disability, where both health outcomes can impact each other. Tooth
loss, for instance, may lead to disabilities such as limitations in activities of daily living, instrumental activities of daily living, and mobility [80,81,82,83,84]. Conversely, disability may be
associated with chronic illnesses, weak oral health, and reduced quality of life among older people [85]. The World Health Organization defines disability as an impairment that may be physical, cognitive, mental, sensory, emotional, developmental,
or a combination of these, and which may occur during a person’s lifetime or from birth. Disabilities can cause physical and cognitive
impairments, activity limitations, and participation restrictions [86]. To prevent disability among
older adults, self-efficacy must be improved, and physical activity must be promoted [87]. Physical
and cognitive functioning of older individuals can be assessed using several assessment instruments [88]. It is possible to analyze the order in which age-related declines occur by examining individuals and groups that are ageing physically and
cognitively at different rates [89,90].
9. Impact of Ageing and Age-Related Diseases on General Healthcare Provision
There is a new trend in healthcare provision for older people which focuses on preventing premature admission to care homes. This trend offers
various healthcare options, including the use of mobility aids, assistive technology devices, domiciliary healthcare, respite care, and telecare. By
using assistive technology, the rate of functional decline in frail older people can be slowed down, while domiciliary healthcare aims to maximize
independence, self-esteem, self-image, and quality of life [91,92]. Evidence suggests that domiciliary healthcare has positive outcomes, including improved quality of life, functional
status, and reduced costs [93]. Informal care provision through visiting nurses, hospice carers, and
physical therapists can also help older people live at home for a longer period. Respite care, which offers temporary relief to informal carers, has
shown some positive effects, but more research is needed to support this claim [94,95,96].
Telecare, which involves the use of personal and environmental sensors in older people’s homes, has been available for several decades. New
options include sensors for falls, epilepsy, enuresis, and security monitoring for temperature, carbon monoxide, and smoke detection. Although the
benefits of telecare are not yet fully understood due to limited research data, it presents an opportunity to identify what works best for each
individual and in which circumstances [97]. Despite the new healthcare options, informal carers,
such as spouses, children, relatives, and friends, still have to provide much of the domiciliary care to frail older people.
10. Impact of Multimorbidity and Polypharmacy on Oral Healthcare Provision
Multimorbidity can lead to a range of physical and psychosocial issues in older adults. The complexity of this condition means that symptoms may be
difficult to diagnose, and diseases may be masked or exacerbated by other health problems. In addition, treatment of one disease may be affected by the
presence of other diseases. This can result in a gradual decline in overall health. Oral healthcare providers who work with older adults should have a
thorough understanding of geriatrics and pharmacology, and collaborate closely with physicians and pharmacists to provide individualized care [98]. Older adults are particularly susceptible to adverse reactions to medication due to age-related
changes in pharmacokinetics and pharmacodynamics, as well as the prevalence of polypharmacy [99,100,101]. Many medications can cause a decrease in
saliva secretion rate, leading to dry mouth and a range of oral health problems [100]. Oral
healthcare providers should consider the impact of medication on oral health and be cautious in prescribing medication to patients with polypharmacy.
The modified Summated Xerostomia Inventory (Table 1) can be used to assess xerostomia. Practical
treatments are available to alleviate the symptoms of dry mouth and improve overall physical and psychosocial well-being [102,103].

Table 1.
Modified summated xerostomia inventory.
11. Impact of Frailty, Disability, and Care Dependency on Oral Healthcare Provision
It is crucial for both formal and informal caregivers of older adults to understand that those who are frail or disabled are at significant risk of
developing oral health problems. Caregivers should therefore take the responsibility of organizing a consultation with an oral healthcare provider. On
the other hand, sudden deterioration of oral health in older individuals can be an early indicator of frailty and should prompt oral healthcare
providers to arrange a consultation with a physician or geriatrician. Multiple epidemiological studies suggest that professional oral healthcare is
urgently needed to address the unmet needs of older adults. To improve oral healthcare provision, there should be integration of oral healthcare into
general healthcare, community programs that promote healthy behaviors, and access to preventive oral healthcare [104]. A crucial strategy is the development and implementation of an oral healthcare guideline to cater to older adults
living in the community. As older adults prefer to age in place, new options for oral healthcare provision such as domiciliary oral healthcare,
customised oral hygiene care aids, visiting dental hygienists and nurses, and oral hygiene telecare should be developed. Unfortunately, not all oral
healthcare offices are easily accessible for older adults who are frail, disabled, or care dependent. Therefore, it is the responsibility of oral
healthcare providers to make their premises easily and safely accessible for this group of individuals. Only when oral healthcare providers accept and
face this responsibility can dentistry be transformed into medical oral healthcare and dentists be upgraded to oral physicians.
12. Epilogue
The risk of rapid deterioration in oral health is heightened by ageing, age-related diseases, multimorbidity, polypharmacy, frailty, disability,
sarcopenia, and inappropriate oral hygiene care. Providing oral care to frail older people can be complex due to additional factors such as lifestyle
(nutrition and smoking habits) and the motivation of the patient or caregivers to achieve adequate oral hygiene. Depending on the degree of frailty,
the living environment, older people’s preferences regarding oral health (shared decision making), and life expectancy, an individualized oral
care plan must be made, in which the goals with regard to oral health are outlined. These goals should be formulated as concretely and measurably as
possible and be realistic and acceptable to all parties, whereas the principles of palliative care should often be used for frail older people.
Furthermore, physicians should also be aware of the potential impact of risk factors of rapid deterioration on the oral health of their patients.
Physicians should pay attention to their patients’ oral health when developing diagnosis and treatment plans. Furthermore, physicians should be
alert to the side effects of the medications they prescribe, as some medications can negatively impact oral health. They may consider prescribing
alternative medications with less harmful effects on oral health. For example, medication with a negative effect on saliva secretion rates as a
side-effect, can be replaced by other medication with a less xerogenic effect.
In general, it is important for physicians and dentists to view oral health as an integral part of a patient’s overall health. By working
together and providing proactive care, they can reduce the negative impact of these factors on oral health and improve patients’ quality of
life. Finally, it is crucial for all care providers to raise awareness about the importance of maintaining good oral health and the consequences of
neglecting it, in order to prevent weakened oral health from becoming a geriatric syndrome [4].
Author Contributions
Writing—original draft, G.-J.v.d.P.; Writing—review & editing, C.d.B. All authors have read and agreed to the published version of
the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- van der Putten, G.J.; De Visschere, L.; van der Maarel-Wierink, C.; Vanobbergen, J.; Schols, J. The importance of oral health in (frail) elderly people—A review. Eur. Geriatr. Med. 2013, 4, 339–344. [Google Scholar] [CrossRef]
- Kossioni, A.E.; Maggi, S.; Muller, F.; Petrovic, M. Oral health in older people: Time for action. Eur. Geriatr. Med. 2018, 9, 3–4. [Google Scholar] [CrossRef]
- Marengoni, A.; von Strauss, E.; Rizzuto, D.; Winblad, B.; Fratiglioni, L. The impact of chronic multimorbidity and disability on functional decline and survival in elderly persons. A community-based, longitudinal study. J. Intern. Med. 2009, 265, 288–295. [Google Scholar] [CrossRef] [PubMed]
- van der Putten, G.J.; de Baat, C.; De Visschere, L.; Schols, J. Poor oral health, a potential new geriatric syndrome. Gerodontology 2014, 31 (Suppl. 1), 17–24. [Google Scholar] [CrossRef] [PubMed]
- Strehler, B.L. Understanding aging. Methods Mol. Med. 2000, 38, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med. Dir. Assoc. 2013, 14, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A. The aging immune system: Primer and prospectus. Science 1996, 273, 70–74. [Google Scholar] [CrossRef]
- Sansoni, P.; Vescovini, R.; Fagnoni, F.; Biasini, C.; Zanni, F.; Zanlari, L.; Telera, A.; Lucchini, G.; Passeri, G.; Monti, D.; et al. The immune system in extreme longevity. Exp. Gerontol. 2008, 43, 61–65. [Google Scholar] [CrossRef]
- Sadighi Akha, A.A. Aging and the immune system: An overview. J. Immunol. Methods 2018, 463, 21–26. [Google Scholar] [CrossRef]
- Sanders, J.L.; Newman, A.B. Telomere length in epidemiology: A biomarker of aging, age-related disease, both, or neither? Epidemiol. Rev. 2013, 35, 112–131. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, Y.; Liu, D.; Songyang, Z.; Wan, M. Telomeres-structure, function, and regulation. Exp. Cell Res. 2013, 319, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rane, G.; Dai, X.; Shanmugam, M.K.; Arfuso, F.; Samy, R.P.; Lai, M.K.; Kappei, D.; Kumar, A.P.; Sethi, G. Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res. Rev. 2016, 25, 55–69. [Google Scholar] [CrossRef]
- Masi, S.; Gkranias, N.; Li, K.; Salpea, K.D.; Parkar, M.; Orlandi, M.; Suvan, J.E.; Eng, H.L.; Taddei, S.; Patel, K.; et al. Association between short leukocyte telomere length, endotoxemia, and severe periodontitis in people with diabetes: A cross-sectional survey. Diabetes Care 2014, 37, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Masi, S.; Salpea, K.D.; Li, K.; Parkar, M.; Nibali, L.; Donos, N.; Patel, K.; Taddei, S.; Deanfield, J.E.; D’Aiuto, F.; et al. Oxidative stress, chronic inflammation, and telomere length in patients with periodontitis. Free Radic. Biol. Med. 2011, 50, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.E.; Divaris, K.; Naorungroj, S.; Heiss, G.; Risques, R.A. Telomere length attrition and chronic periodontitis: An ARIC Study nested case-control study. J. Clin. Periodontol. 2015, 42, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Nishida, H.; Takeda, H.; Shin, K. Telomere length in leukocytes and cultured gingival fibroblasts from patients with aggressive periodontitis. J. Periodontol. 2004, 75, 84–90. [Google Scholar] [CrossRef]
- Song, W.; Yang, J.; Niu, Z. Association of periodontitis with leukocyte telomere length in US adults: A cross-sectional analysis of NHANES 1999 to 2002. J. Periodontol. 2021, 92, 833–843. [Google Scholar] [CrossRef]
- Botelho, J.; Mascarenhas, P.; Viana, J.; Proenca, L.; Orlandi, M.; Leira, Y.; Chambrone, L.; Mendes, J.J.; Machado, V. An umbrella review of the evidence linking oral health and systemic noncommunicable diseases. Nat. Commun. 2022, 13, 7614. [Google Scholar] [CrossRef]
- Gurenlian, J.R. Inflammation: The relationship between oral health and systemic disease. Dent. Assist. 2009, 78, 8–10, 12–14, 38–40; quiz 41–43. [Google Scholar] [PubMed]
- Peric, M.; Marhl, U.; Gennai, S.; Marruganti, C.; Graziani, F. Treatment of gingivitis is associated with reduction of systemic inflammation and improvement of oral health-related quality of life: A randomized clinical trial. J. Clin. Periodontol. 2022, 49, 899–910. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef] [PubMed]
- de Vries, S.A.G.; Tan, C.X.W.; Bouma, G.; Forouzanfar, T.; Brand, H.S.; de Boer, N.K. Salivary Function and Oral Health Problems in Crohn’s Disease Patients. Inflamm. Bowel Dis. 2018, 24, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Rikardsson, S.; Jonsson, J.; Hultin, M.; Gustafsson, A.; Johannsen, A. Perceived oral health in patients with Crohn’s disease. Oral Health Prev. Dent. 2009, 7, 277–282. [Google Scholar] [PubMed]
- Ali, S.; Nagieb, C.S.; Fayed, H.L. Effect of Behcet’s disease-associated oral ulcers on oral health related quality of life. Spec. Care Dent. 2022, 1–8. [Google Scholar] [CrossRef]
- Mumcu, G.; Ergun, T.; Inanc, N.; Fresko, I.; Atalay, T.; Hayran, O.; Direskeneli, H. Oral health is impaired in Behcet’s disease and is associated with disease severity. Rheumatology 2004, 43, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Senusi, A.; Higgins, S.; Fortune, F. The influence of oral health and psycho-social well-being on clinical outcomes in Behcet’s disease. Rheumatol. Int. 2018, 38, 1873–1883. [Google Scholar] [CrossRef]
- Albilia, J.B.; Lam, D.K.; Blanas, N.; Clokie, C.M.; Sandor, G.K. Small mouths … Big problems? A review of scleroderma and its oral health implications. J. Can. Dent. Assoc. 2007, 73, 831–836. [Google Scholar]
- Beaty, K.L.; Gurenlian, J.R.; Rogo, E.J. Oral Health Experiences of the Limited Scleroderma Patient. J. Dent. Hyg. 2021, 95, 59–69. [Google Scholar]
- Chung, M.; York, B.R.; Michaud, D.S. Oral Health and Cancer. Curr. Oral Health Rep. 2019, 6, 130–137. [Google Scholar] [CrossRef]
- Jobbins, J.; Bagg, J.; Finlay, I.G.; Addy, M.; Newcombe, R.G. Oral and dental disease in terminally ill cancer patients. BMJ 1992, 304, 1612. [Google Scholar] [CrossRef]
- Vermaire, J.A.; Partoredjo, A.S.K.; de Groot, R.J.; Brand, H.S.; Speksnijder, C.M. Mastication in health-related quality of life in patients treated for oral cancer: A systematic review. Eur. J. Cancer Care (Engl.) 2022, 31, e13744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bellocco, R.; Sandborgh-Englund, G.; Yu, J.; Sallberg Chen, M.; Ye, W. Poor Oral Health and Esophageal Cancer Risk: A Nationwide Cohort Study. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Morimata, J.; Otomaru, T.; Murase, M.; Haraguchi, M.; Sumita, Y.; Taniguchi, H. Investigation of factor affecting health-related quality of life in head and neck cancer patients. Gerodontology 2013, 30, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Azuma, N.; Katada, Y.; Yoshikawa, T.; Yokoyama, Y.; Nishioka, A.; Sekiguchi, M.; Kitano, M.; Kitano, S.; Sano, H.; Matsui, K. Evaluation of changes in oral health-related quality of life over time in patients with Sjogren’s syndrome. Mod. Rheumatol. 2021, 31, 669–677. [Google Scholar] [CrossRef]
- Cartee, D.L.; Maker, S.; Dalonges, D.; Manski, M.C. Sjogren’s Syndrome: Oral Manifestations and Treatment, a Dental Perspective. J. Dent. Hyg. 2015, 89, 365–371. [Google Scholar]
- Enger, T.B.; Palm, O.; Garen, T.; Sandvik, L.; Jensen, J.L. Oral distress in primary Sjogren’s syndrome: Implications for health-related quality of life. Eur. J. Oral Sci. 2011, 119, 474–480. [Google Scholar] [CrossRef]
- Vujovic, S.; Desnica, J.; Stevanovic, M.; Mijailovic, S.; Vojinovic, R.; Selakovic, D.; Jovicic, N.; Rosic, G.; Milovanovic, D. Oral Health and Oral Health-Related Quality of Life in Patients with Primary Sjogren’s Syndrome. Medicina 2023, 59, 473. [Google Scholar] [CrossRef] [PubMed]
- Al-Mobeeriek, A. Oral health status among psychiatric patients in Riyadh, Saudi Arabia. West Indian Med. J. 2012, 61, 549–554. [Google Scholar] [CrossRef]
- Bertaud-Gounot, V.; Kovess-Masfety, V.; Perrus, C.; Trohel, G.; Richard, F. Oral health status and treatment needs among psychiatric inpatients in Rennes, France: A cross-sectional study. BMC Psychiatry 2013, 13, 227. [Google Scholar] [CrossRef]
- Buunk-Werkhoven, Y.A.; Dijkstra, A.; Schaub, R.M.; van der Schans, C.P.; Spreen, M. Oral health related quality of life among imprisoned Dutch forensic psychiatric patients. J. Forensic Nurs. 2010, 6, 137–143. [Google Scholar] [CrossRef]
- Goud, V.; Kannaiyan, K.; Rao, B.V.; Abidullah, M.; Dharani, V.; Nayak, M. Oral Health Status and Treatment Needs of Psychiatric Outpatients Aged 18–64 Years in District Civil Hospital, Raichur, Karnataka: A Cross-Sectional Study. J. Pharm. Bioallied Sci. 2021, 13, S598–S601. [Google Scholar] [CrossRef] [PubMed]
- Kisely, S.; Najman, J.M. A study of the association between psychiatric symptoms and oral health outcomes in a population-based birth cohort at 30-year-old follow-up. J. Psychosom. Res. 2022, 157, 110784. [Google Scholar] [CrossRef] [PubMed]
- Kossioni, A.E.; Kossionis, G.E.; Polychronopoulou, A. Oral health status of elderly hospitalised psychiatric patients. Gerodontology 2012, 29, 272–283. [Google Scholar] [CrossRef]
- Lewis, D. Summary of: Prevalence of oral diseases and oral-health-related quality of life in people with severe mental illness undertaking community-based psychiatric care. Br. Dent. J. 2012, 213, 462–463. [Google Scholar] [CrossRef]
- Ngo, D.Y.J.; Thomson, W.M.; Subramaniam, M.; Abdin, E.; Ang, K.Y. The oral health of long-term psychiatric inpatients in Singapore. Psychiatry Res. 2018, 266, 206–211. [Google Scholar] [CrossRef]
- Shah, V.R.; Jain, P.; Patel, N. Oral health of psychiatric patients: A cross-sectional comparision study. Dent. Res. J. 2012, 9, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, R.; Nordstrom, G. Oral health status of psychiatric patients. J. Clin. Nurs. 2000, 9, 632–638. [Google Scholar] [CrossRef]
- Auffret, M.; Meuric, V.; Boyer, E.; Bonnaure-Mallet, M.; Verin, M. Oral Health Disorders in Parkinson’s Disease: More than Meets the Eye. J. Parkinson’s Dis. 2021, 11, 1507–1535. [Google Scholar] [CrossRef]
- Nakayama, Y.; Washio, M.; Mori, M. Oral health conditions in patients with Parkinson’s disease. J. Epidemiol. 2004, 14, 143–150. [Google Scholar] [CrossRef]
- Persson, M.; Osterberg, T.; Granerus, A.K.; Karlsson, S. Influence of Parkinson’s disease on oral health. Acta Odontol. Scand. 1992, 50, 37–42. [Google Scholar] [CrossRef]
- Ribeiro, G.R.; Campos, C.H.; Garcia, R.C. Oral Health in Elders with Parkinson’s Disease. Braz. Dent. J. 2016, 27, 340–344. [Google Scholar] [CrossRef]
- Saft, C.; Andrich, J.E.; Muller, T.; Becker, J.; Jackowski, J. Oral and dental health in Huntington’s disease—An observational study. BMC Neurol. 2013, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Manchery, N.; Henry, J.D.; Nangle, M.R. A systematic review of oral health in people with multiple sclerosis. Community Dent. Oral Epidemiol. 2020, 48, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Hamza, S.A.; Asif, S.; Bokhari, S.A.H. Oral health of individuals with dementia and Alzheimer’s disease: A review. J. Indian Soc. Periodontol. 2021, 25, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Tiisanoja, A.; Syrjala, A.M.; Tertsonen, M.; Komulainen, K.; Pesonen, P.; Knuuttila, M.; Hartikainen, S.; Ylostalo, P. Oral diseases and inflammatory burden and Alzheimer’s disease among subjects aged 75 years or older. Spec. Care Dent. 2019, 39, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Ahola, K.; Saarinen, A.; Kuuliala, A.; Leirisalo-Repo, M.; Murtomaa, H.; Meurman, J.H. Impact of rheumatic diseases on oral health and quality of life. Oral Dis. 2015, 21, 342–348. [Google Scholar] [CrossRef]
- Carvalho, C.G.; Medeiros-Filho, J.B.; Ferreira, M.C. Guide for health professionals addressing oral care for individuals in oncological treatment based on scientific evidence. Support. Care Cancer 2018, 26, 2651–2661. [Google Scholar] [CrossRef]
- Gianos, E.; Jackson, E.A.; Tejpal, A.; Aspry, K.; O’Keefe, J.; Aggarwal, M.; Jain, A.; Itchhaporia, D.; Williams, K.; Batts, T.; et al. Oral health and atherosclerotic cardiovascular disease: A review. Am. J. Prev. Cardiol. 2021, 7, 100179. [Google Scholar] [CrossRef]
- Holmlund, A.; Lampa, E.; Lind, L. Oral health and cardiovascular disease risk in a cohort of periodontitis patients. Atherosclerosis 2017, 262, 101–106. [Google Scholar] [CrossRef]
- Joshy, G.; Arora, M.; Korda, R.J.; Chalmers, J.; Banks, E. Is poor oral health a risk marker for incident cardiovascular disease hospitalisation and all-cause mortality? Findings from 172 630 participants from the prospective 45 and Up Study. BMJ Open 2016, 6, e012386. [Google Scholar] [CrossRef]
- Kotronia, E.; Brown, H.; Papacosta, A.O.; Lennon, L.T.; Weyant, R.J.; Whincup, P.H.; Wannamethee, S.G.; Ramsay, S.E. Oral health and all-cause, cardiovascular disease, and respiratory mortality in older people in the UK and USA. Sci. Rep. 2021, 11, 16452. [Google Scholar] [CrossRef]
- Meurman, J.H.; Bascones-Martinez, A. Oral Infections and Systemic Health—More than Just Links to Cardiovascular Diseases. Oral Health Prev. Dent. 2021, 19, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Najafipour, H.; Malek Mohammadi, T.; Rahim, F.; Haghdoost, A.A.; Shadkam, M.; Afshari, M. Association of oral health and cardiovascular disease risk factors “results from a community based study on 5900 adult subjects”. ISRN Cardiol. 2013, 2013, 782126. [Google Scholar] [CrossRef] [PubMed]
- Venkatasalu, M.R.; Murang, Z.R.; Ramasamy, D.T.R.; Dhaliwal, J.S. Oral health problems among palliative and terminally ill patients: An integrated systematic review. BMC Oral Health 2020, 20, 79. [Google Scholar] [CrossRef]
- Le Reste, J.Y.; Nabbe, P.; Manceau, B.; Lygidakis, C.; Doerr, C.; Lingner, H.; Czachowski, S.; Munoz, M.; Argyriadou, S.; Claveria, A.; et al. The European General Practice Research Network presents a comprehensive definition of multimorbidity in family medicine and long term care, following a systematic review of relevant literature. J. Am. Med. Dir. Assoc. 2013, 14, 319–325. [Google Scholar] [CrossRef]
- Le Reste, J.Y.; Nabbe, P.; Lazic, D.; Assenova, R.; Lingner, H.; Czachowski, S.; Argyriadou, S.; Sowinska, A.; Lygidakis, C.; Doerr, C.; et al. How do general practitioners recognize the definition of multimorbidity? A European qualitative study. Eur. J. Gen. Pract. 2016, 22, 159–168. [Google Scholar] [CrossRef]
- Loya, A.M.; Gonzalez-Stuart, A.; Rivera, J.O. Prevalence of polypharmacy, polyherbacy, nutritional supplement use and potential product interactions among older adults living on the United States-Mexico border: A descriptive, questionnaire-based study. Drugs Aging 2009, 26, 423–436. [Google Scholar] [CrossRef]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef]
- Rockwood, K.; Mitnitski, A. Frailty in relation to the accumulation of deficits. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Oude Voshaar, R.C. Prevalence of frailty in community-dwelling older persons: A systematic review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef]
- Kamdem, B.; Seematter-Bagnoud, L.; Botrugno, F.; Santos-Eggimann, B. Relationship between oral health and Fried’s frailty criteria in community-dwelling older persons. BMC Geriatr. 2017, 17, 174. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, F.B.; Lebrao, M.L.; Santos, J.L.; Duarte, Y.A. Relationship between oral health and frailty in community-dwelling elderly individuals in Brazil. J. Am. Geriatr. Soc. 2013, 61, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.F.; Scariot, E.L.; da Rosa, L.H.T. The effect of different exercise programs on sarcopenia criteria in older people: A systematic review of systematic reviews with meta-analysis. Arch. Gerontol. Geriatr. 2023, 105, 104868. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhu, A.; Chu, S.; Zhou, Q.; Zhou, Y.; Qu, X.; Tang, Q.; Zhang, Y. Correlation between nutrition, oral health, and different sarcopenia groups among elderly outpatients of community hospitals: A cross-sectional study of 1505 participants in China. BMC Geriatr. 2022, 22, 332. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, R.; Song, G.; Teng, J.; Shen, S.; Fu, X.; Yan, Y.; Liu, C. The Effect of Resistance Training on the Rehabilitation of Elderly Patients with Sarcopenia: A Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 15491. [Google Scholar] [CrossRef]
- Yoo, J.I.; Ha, Y.C.; Cha, Y. Nutrition and Exercise Treatment of Sarcopenia in Hip Fracture Patients: Systematic Review. J. Bone Metab. 2022, 29, 63–73. [Google Scholar] [CrossRef]
- Hatta, K.; Ikebe, K. Association between oral health and sarcopenia: A literature review. J. Prosthodont. Res. 2021, 65, 131–136. [Google Scholar] [CrossRef]
- Azzolino, D.; Passarelli, P.C.; De Angelis, P.; Piccirillo, G.B.; D’Addona, A.; Cesari, M. Poor Oral Health as a Determinant of Malnutrition and Sarcopenia. Nutrients 2019, 11, 2898. [Google Scholar] [CrossRef]
- Holm-Pedersen, P.; Schultz-Larsen, K.; Christiansen, N.; Avlund, K. Tooth loss and subsequent disability and mortality in old age. J. Am. Geriatr. Soc. 2008, 56, 429–435. [Google Scholar] [CrossRef]
- Komiyama, T.; Ohi, T.; Miyoshi, Y.; Murakami, T.; Tsuboi, A.; Tomata, Y.; Tsuji, I.; Watanabe, M.; Hattori, Y. Association Between Tooth Loss, Receipt of Dental Care, and Functional Disability in an Elderly Japanese Population: The Tsurugaya Project. J. Am. Geriatr. Soc. 2016, 64, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, T.; Ohi, T.; Tomata, Y.; Tanji, F.; Tsuji, I.; Watanabe, M.; Hattori, Y. Dental Status is Associated With Incident Functional Disability in Community-Dwelling Older Japanese: A Prospective Cohort Study Using Propensity Score Matching. J. Epidemiol. 2020, 30, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, Y.; Listl, S.; Jurges, H.; Watt, R.G.; Aida, J.; Tsakos, G. Causal Effect of Tooth Loss on Functional Capacity in Older Adults in England: A Natural Experiment. J. Am. Geriatr. Soc. 2021, 69, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Yang, J.; Huang, C.; Sun, H.; Wu, Y. Eating and communication difficulties as mediators of the relationship between tooth loss and functional disability in middle-aged and older adults. J. Dent. 2020, 96, 103331. [Google Scholar] [CrossRef]
- Kotronia, E.; Brown, H.; Papacosta, O.; Lennon, L.T.; Weyant, R.J.; Whincup, P.H.; Wannamethee, S.G.; Ramsay, S.E. Oral health problems and risk of incident disability in two studies of older adults in the United Kingdom and the United States. J. Am. Geriatr. Soc. 2022, 70, 2080–2092. [Google Scholar] [CrossRef]
- World Health Organization (WHO). World Report on Disability; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Motl, R.W.; McAuley, E. Physical activity, disability, and quality of life in older adults. Phys. Med. Rehabil. Clin. N. Am. 2010, 21, 299–308. [Google Scholar] [CrossRef]
- Clouston, S.A.; Brewster, P.; Kuh, D.; Richards, M.; Cooper, R.; Hardy, R.; Rubin, M.S.; Hofer, S.M. The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol. Rev. 2013, 35, 33–50. [Google Scholar] [CrossRef]
- Bossers, W.J.; van der Woude, L.H.; Boersma, F.; Scherder, E.J.; van Heuvelen, M.J. Recommended measures for the assessment of cognitive and physical performance in older patients with dementia: A systematic review. Dement. Geriatr. Cogn. Dis. Extra 2012, 2, 589–609. [Google Scholar] [CrossRef]
- Gill, T.M. Assessment of function and disability in longitudinal studies. J. Am. Geriatr. Soc. 2010, 58 (Suppl. 2), S308–S312. [Google Scholar] [CrossRef]
- Mann, W.C.; Ottenbacher, K.J.; Fraas, L.; Tomita, M.; Granger, C.V. Effectiveness of assistive technology and environmental interventions in maintaining independence and reducing home care costs for the frail elderly. A randomized controlled trial. Arch. Fam. Med. 1999, 8, 210–217. [Google Scholar] [CrossRef]
- Parsons, J.; Rouse, P.; Robinson, E.M.; Sheridan, N.; Connolly, M.J. Goal setting as a feature of homecare services for older people: Does it make a difference? Age Ageing 2012, 41, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Ryburn, B.; Wells, Y.; Foreman, P. Enabling independence: Restorative approaches to home care provision for frail older adults. Health Soc. Care Community 2009, 17, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Evans, D. Exploring the concept of respite. J. Adv. Nurs. 2013, 69, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.; McNamara, R.; Abrams, K.; Cannings-John, R.; Hood, K.; Longo, M.; Myles, S.; O’Mahony, S.; Roe, B.; Williams, K. Systematic review of respite care in the frail elderly. Health Technol. Assess. 2009, 13, 1–246. [Google Scholar] [CrossRef]
- Hogan, L.; Boron, J.B.; Masters, J.; MacArthur, K.; Manley, N. Characteristics of dementia family caregivers who use paid professional in-home respite care. Home Health Care Serv. Q. 2022, 41, 310–329. [Google Scholar] [CrossRef]
- Parker, S.G.; Hawley, M.S. Telecare for an ageing population? Age Ageing 2013, 42, 424–425. [Google Scholar] [CrossRef]
- Dolan, T.A. Professional education to meet the oral health needs of older adults and persons with disabilities. Spec. Care Dentist. 2013, 33, 190–197. [Google Scholar] [CrossRef]
- Corsonello, A.; Pedone, C.; Incalzi, R.A. Age-related pharmacokinetic and pharmacodynamic changes and related risk of adverse drug reactions. Curr. Med. Chem. 2010, 17, 571–584. [Google Scholar] [CrossRef]
- Liu, B.; Dion, M.R.; Jurasic, M.M.; Gibson, G.; Jones, J.A. Xerostomia and salivary hypofunction in vulnerable elders: Prevalence and etiology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 52–60. [Google Scholar] [CrossRef]
- Ship, J.A.; Pillemer, S.R.; Baum, B.J. Xerostomia and the geriatric patient. J. Am. Geriatr. Soc. 2002, 50, 535–543. [Google Scholar] [CrossRef]
- van der Putten, G.J.; Brand, H.S.; Schols, J.M.; de Baat, C. The diagnostic suitability of a xerostomia questionnaire and the association between xerostomia, hyposalivation and medication use in a group of nursing home residents. Clin. Oral Investig. 2011, 15, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Thomson, W.M.; van der Putten, G.J.; de Baat, C.; Ikebe, K.; Matsuda, K.; Enoki, K.; Hopcraft, M.S.; Ling, G.Y. Shortening the xerostomia inventory. Oral Surg Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.O.; Jones, J.A.; Brunson, D.; Griffin, P.M.; Bailey, W.D. Burden of oral disease among older adults and implications for public health priorities. Am. J. Public Health 2012, 102, 411–418. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).