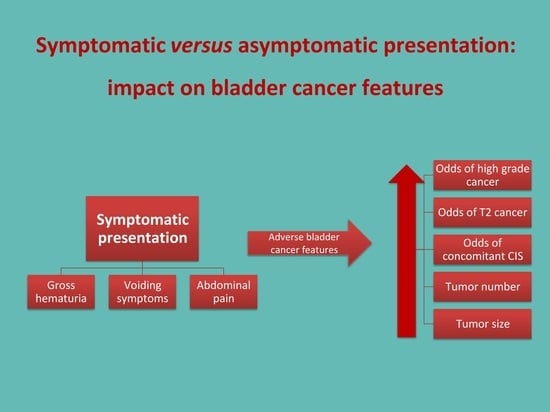
The Impact of the Initial Clinical Presentation of Bladder Cancer on Histopathological and Morphological Tumor Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection and Definition of Variables
2.3. Outcomes
- Demographic characteristics of patients;
- The mode of initial clinical presentation (symptomatic vs. asymptomatic);
- Histopathological characteristics;
- Morphological characteristics.
2.4. Statistical Analysis
3. Results
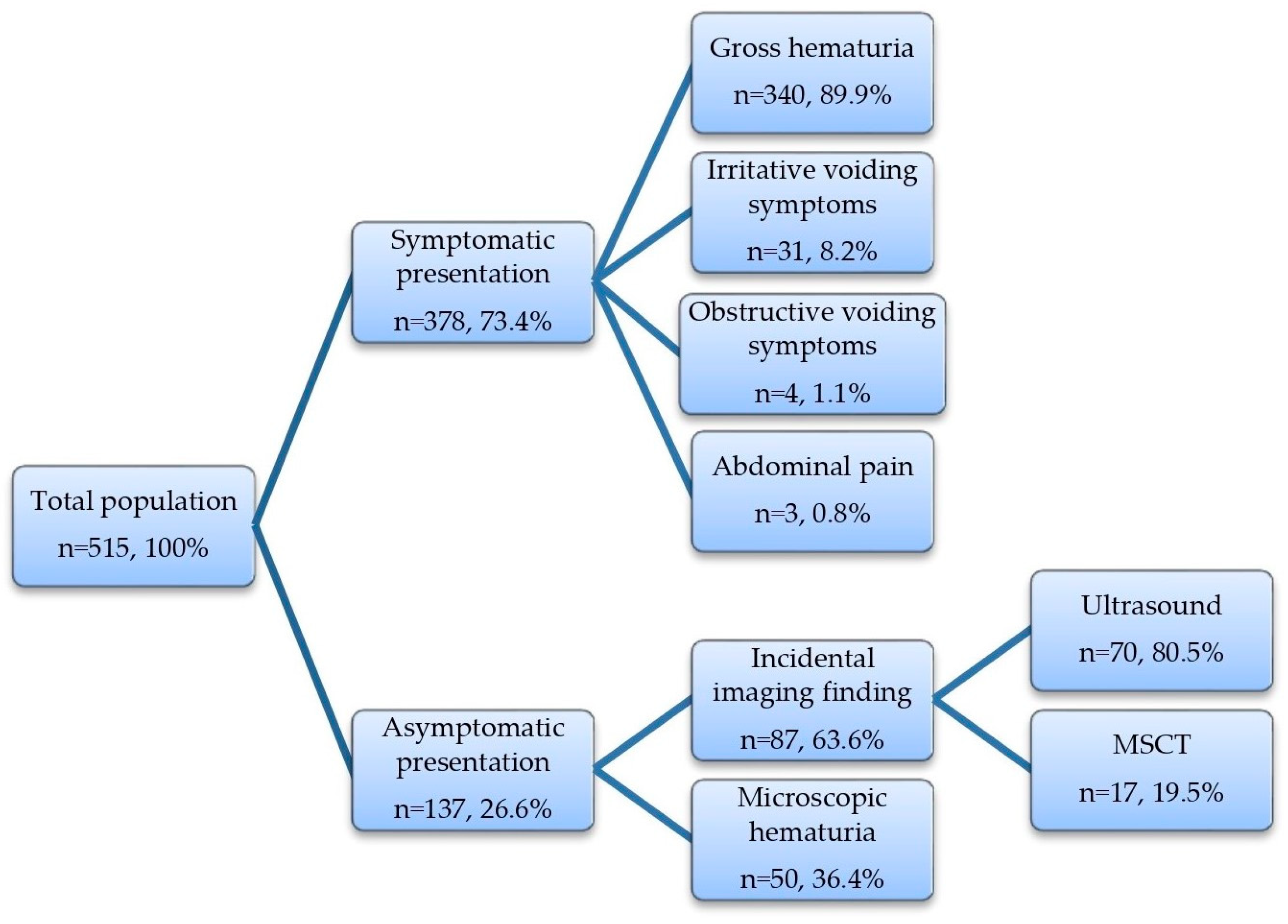
3.1. Baseline Patient Demographics and the Mode of Initial Clinical Presentation
3.2. Symptomatic vs. Asymptomatic Presentation
- Detection of high-grade bladder cancer vs. PUNLMP/low-grade bladder cancer (p < 0.001);
- Presence of concomitant CIS vs. no CIS (p = 0.012);
- Detection of T2 stage bladder cancer vs. Ta/T1 stage bladder cancer (p < 0.001);
- Detection of a higher number of tumors (p = 0.005);
- Larger tumor size (p < 0.001).
3.3. Gross vs. Microscopic Hematuria
- Detection of high-grade bladder cancer vs. PUNLMP/low-grade bladder cancer (p = 0.020);
- Detection of T2 stage bladder cancer vs. Ta/T1 stage bladder cancer (p = 0.015);
- Larger tumor size (p < 0.001).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.; Nishiyama, H. Epidemiology of Urothelial Carcinoma. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2017, 24, 730–734. [Google Scholar] [CrossRef]
- Mariani, A.J.; Mariani, M.C.; Macchioni, C.; Stams, U.K.; Hariharan, A.; Moriera, A. The Significance of Adult Hematuria: 1000 Hematuria Evaluations Including a Risk-Benefit and Cost-Effectiveness Analysis. J. Urol. 1989, 141, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Arianayagam, R.; Arianayagam, M.; Rashid, P. Bladder Cancer--Current Management. Aust. Fam. Physician 2011, 40, 209–213. [Google Scholar] [PubMed]
- Kirkali, Z.; Chan, T.; Manoharan, M.; Algaba, F.; Busch, C.; Cheng, L.; Kiemeney, L.; Kriegmair, M.; Montironi, R.; Murphy, W.M.; et al. Bladder Cancer: Epidemiology, Staging and Grading, and Diagnosis. Urology 2005, 66 (Suppl. S1), 4–34. [Google Scholar] [CrossRef]
- Tohi, Y.; Miyauchi, Y.; Yamasaki, M.; Fujiwara, K.; Harada, S.; Matsuda, I.; Ito, A.; Matsuoka, Y.; Kato, T.; Taoka, R.; et al. Incidental Bladder Cancer Found on Cystoscopy during Prostate Biopsy: Prevalence, Pathological Findings, and Oncological Outcome. Urol. Int. 2022, 106, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Rayn, K.N.; Hale, G.R.; Bloom, J.B.; Gold, S.A.; Carvalho, F.L.F.; Mehralivand, S.; Czarniecki, M.; Wood, B.J.; Merino, M.J.; Choyke, P.; et al. Incidental Bladder Cancers Found on Multiparametric MRI of the Prostate Gland: A Single Center Experience. Diagn. Interv. Radiol. Ank. Turk. 2018, 24, 316–320. [Google Scholar] [CrossRef]
- Ramirez, D.; Gupta, A.; Canter, D.; Harrow, B.; Dobbs, R.W.; Kucherov, V.; Mueller, E.; Streeper, N.; Uhlman, M.A.; Svatek, R.S.; et al. Microscopic Haematuria at Time of Diagnosis Is Associated with Lower Disease Stage in Patients with Newly Diagnosed Bladder Cancer. BJU Int. 2016, 117, 783–786. [Google Scholar] [CrossRef]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Siow, W.Y.; Yip, S.K.; Ng, L.G.; Tan, P.H.; Cheng, W.S.; Foo, K.T. Renal Cell Carcinoma: Incidental Detection and Pathological Staging. J. R. Coll. Surg. Edinb. 2000, 45, 291–295. [Google Scholar]
- Barocas, D.A.; Boorjian, S.A.; Alvarez, R.D.; Downs, T.M.; Gross, C.P.; Hamilton, B.D.; Kobashi, K.C.; Lipman, R.R.; Lotan, Y.; Ng, C.K.; et al. Microhematuria: AUA/SUFU Guideline. J. Urol. 2020, 204, 778–786. [Google Scholar] [CrossRef]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: Cham, Switzerland, 2017; ISBN 978-3-319-40617-6. [Google Scholar]
- Gaya, J.M.; Territo, A.; Woldu, S.; Schwartzmann, I.; Verri, P.; González-Pérez, L.; Cózar, J.M.; Miñana, B.; Medina, R.A.; de la Rosa-Kehrmann, F.; et al. Incidental Diagnosis of Bladder Cancer in a National Observational Study in Spain. Actas Urol. Esp. 2022, 47, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Kamecki, H.; Dębowska, M.; Nyk, Ł.; Przewor, A.; Demkow, T.; Sosnowski, R. The Clinical Features of Incidentally Diagnosed Urothelial Bladder Cancer: A Retrospective Data Analysis. Urol. Int. 2022, 106, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Nieder, A.M.; Lotan, Y.; Nuss, G.R.; Langston, J.P.; Vyas, S.; Manoharan, M.; Soloway, M.S. Are Patients with Hematuria Appropriately Referred to Urology? A Multi-Institutional Questionnaire Based Survey. Urol. Oncol. 2010, 28, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Wakai, K.; Utsumi, T.; Oka, R.; Endo, T.; Yano, M.; Kamijima, S.; Kamiya, N.; Hiruta, N.; Suzuki, H. Clinical Predictors for High-Grade Bladder Cancer before First-Time Transurethral Resection of the Bladder Tumor: A Retrospective Cohort Study. Jpn. J. Clin. Oncol. 2016, 46, 964–967. [Google Scholar] [CrossRef]
- Shapur, N.; Pode, D.; Katz, R.; Shapiro, A.; Yutkin, V.; Pizov, G.; Appelbaum, L.; Zorn, K.C.; Duvdevani, M.; Landau, E.H.; et al. Predicting the Risk of High-Grade Bladder Cancer Using Noninvasive Data. Urol. Int. 2011, 87, 319–324. [Google Scholar] [CrossRef]
- Warrick, J.I.; Knowles, M.A.; Yves, A.; van der Kwast, T.; Grignon, D.J.; Kristiansen, G.; Egevad, L.; Hartmann, A.; Cheng, L. Report from the International Society of Urological Pathology (ISUP) Consultation Conference on Molecular Pathology of Urogenital Cancers. II. Molecular Pathology of Bladder Cancer: Progress and Challenges. Am. J. Surg. Pathol. 2020, 44, e30–e46. [Google Scholar] [CrossRef]
- Lin, M.G.; Hong, Y.K.; Zhang, Y.; Lin, B.B.; He, X.J. Mechanism of LncRNA DUXAP8 in Promoting Proliferation of Bladder Cancer Cells by Regulating PTEN. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3370–3377. [Google Scholar] [CrossRef]
- Sylvester, R.J.; van der Meijden, A.P.M.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.W.; Kurth, K. Predicting Recurrence and Progression in Individual Patients with Stage Ta T1 Bladder Cancer Using EORTC Risk Tables: A Combined Analysis of 2596 Patients from Seven EORTC Trials. Eur. Urol. 2006, 49, 465–466, discussion 475–477. [Google Scholar] [CrossRef]
- Liu, S.; Hou, J.; Zhang, H.; Wu, Y.; Hu, M.; Zhang, L.; Xu, J.; Na, R.; Jiang, H.; Ding, Q. The Evaluation of the Risk Factors for Non-Muscle Invasive Bladder Cancer (NMIBC) Recurrence after Transurethral Resection (TURBt) in Chinese Population. PLoS ONE 2015, 10, e0123617. [Google Scholar] [CrossRef]
- Sylvester, R.J.; Rodríguez, O.; Hernández, V.; Turturica, D.; Bauerová, L.; Bruins, H.M.; Bründl, J.; van der Kwast, T.H.; Brisuda, A.; Rubio-Briones, J.; et al. European Association of Urology (EAU) Prognostic Factor Risk Groups for Non-Muscle-Invasive Bladder Cancer (NMIBC) Incorporating the WHO 2004/2016 and WHO 1973 Classification Systems for Grade: An Update from the EAU NMIBC Guidelines Panel. Eur. Urol. 2021, 79, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Meng, L.; Wang, X.; Diao, T.; Hu, M.; Wang, M.; Zhang, Y.; Liu, M. Predictive Nomogram and Risk Factors for Lymph Node Metastasis in Bladder Cancer. Front. Oncol. 2021, 11, 690324. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; He, Z.; Lu, Z.; Wu, W.; Chen, Y.; Wei, G.; Liu, Y. Application of Nomograms in the Prediction of Overall Survival and Cancer-Specific Survival in Patients with T1 High-Grade Bladder Cancer. Exp. Ther. Med. 2019, 18, 3405–3414. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Y.; He, W.; Yang, B.; Gou, X. Nomogram for Predicting Overall Survival of Patients with Bladder Cancer: A Population-Based Study. Int. J. Biol. Markers 2020, 35, 29–39. [Google Scholar] [CrossRef]
- Chen, D.; Luo, Z.; Ye, C.; Luo, Q.; Fan, W.; Chen, C.; Liu, G. Constructing and Validating Nomograms to Predict Risk and Prognostic Factors of Distant Metastasis in Urothelial Bladder Cancer Patients: A Population-Based Retrospective Study. BMC Urol. 2022, 22, 212. [Google Scholar] [CrossRef]
- Carrion, R.; Seigne, J. Surgical Management of Bladder Carcinoma. Cancer Control J. Moffitt Cancer Cent. 2002, 9, 284–292. [Google Scholar] [CrossRef]
- Kamecki, H.; Dębowska, M.; Poleszczuk, J.; Demkow, T.; Przewor, A.; Nyk, Ł.; Sosnowski, R. Incidental Diagnosis of Urothelial Bladder Cancer: Associations with Overall Survival. Cancers 2023, 15, 668. [Google Scholar] [CrossRef]
- Wurcel, V.; Cicchetti, A.; Garrison, L.; Kip, M.M.A.; Koffijberg, H.; Kolbe, A.; Leeflang, M.M.G.; Merlin, T.; Mestre-Ferrandiz, J.; Oortwijn, W.; et al. The Value of Diagnostic Information in Personalised Healthcare: A Comprehensive Concept to Facilitate Bringing This Technology into Healthcare Systems. Public Health Genom. 2019, 22, 8–15. [Google Scholar] [CrossRef]
- Zhang, J.; Gerst, S.; Lefkowitz, R.A.; Bach, A. Imaging of Bladder Cancer. Radiol. Clin. N. Am. 2007, 45, 183–205. [Google Scholar] [CrossRef]
- Gontero, P.; Compérat, E.; Dominguez Escrig, J.L.; Liedberg, F.; Mariappan, P.; Masson-Lecomte, A.; Mostafid, A.H.; van Rhijn, B.W.G.; Rouprêt, M.; Seisen, T.; et al. EAU Guidelines on Non-Muscle-Invasive Bladder Cancer (TaT1 and CIS); EAU Guidelines Office: Arnhem, The Netherlands, 2023; ISBN 978-94-92671-16-5. [Google Scholar]
- Krabbe, L.M.; Svatek, R.S.; Shariat, S.F.; Messing, E.; Lotan, Y. Bladder Cancer Risk: Use of the PLCO and NLST to Identify a Suitable Screening Cohort. Urol. Oncol. 2015, 33, 65.e19–65.e25. [Google Scholar] [CrossRef] [PubMed]
| Variable | Symptomatic Presentation | p-Value | |
|---|---|---|---|
| YES | NO | ||
| Baseline demographic data | |||
| Number of patients, n (%) | 378 (73.4) | 137 (26.6) | |
| Male | 265 (70.1) | 105 (76.7) | |
| Female | 113 (29.9) | 32 (23.3) | 0.145 1 |
| Age (years), median (IQR) | 71 (63–79) | 69 (62–75) | 0.043 2 |
| Smoking history, n (%) | 229 (60.6) | 78 (56.9) | 0.456 1 |
| Number of comorbidities, median (IQR) | 2 (1–3) | 1 (0–3) | 0.401 2 |
| Bladder cancer characteristics | |||
| Bladder cancer grade, n (%) | |||
| PUNLMP/low grade | 157 (41.5) | 97 (70.8) | |
| High-grade | 221 (58.5) | 40 (29.2) | <0.001 1 |
| Concomitant CIS, n (%) | 41 (10.8) | 5 (3.6) | 0.011 1 |
| Bladder cancer stage, n (%) | |||
| Ta | 180 (47.6) | 110 (80.3) | |
| T1 | 118 (31.2) | 21 (15.3) | |
| T2 | 80 (21.2) | 6 (4.4) | <0.001 1 |
| Morphological characteristics, median (IQR) | |||
| Number of tumors | 1 (1–3) | 1 (1–2) | 0.002 2 |
| Total tumor size (cm) | 3.5 (2.2–5) | 2 (1.15–3) | <0.001 2 |
| Dependent Variables | |||||
|---|---|---|---|---|---|
| Independent Variables | High Grade vs. Non-High-Grade | Concomitant CIS vs. no CIS | T2 Stage vs. Ta/T1 Stage | Number of Tumors | Tumor Size |
| OR (95% CI), p Value 1 | OR (95% CI), p Value 1 | OR (95% CI), p Value 1 | IRR (95% CI), p Value 2 | B Coefficient (95% CI), p Value 3 | |
| Sex | 1.62 (1.07–2.44), 0.022 | 2.12 (0.95–4.69), 0.065 | 0.68 (0.41–1.12), 0.129 | 0.99 (0.86–1.13), 0.857 | −0.24 (−0.72–0.25), 0.332 |
| Male | |||||
| Female (reference) | |||||
| Age | 1.04 (1.02–1.06), <0.001 | 1 (0.97–1.04), 0.891 | 1.01 (0.98–1.04), 0.524 | 1.01 (1.006–1.02), <0.001 | 0.05 (0.03–0.07), <0.001 |
| Smoking history | 0.87 (0.59–1.28), 0.470 | 0.91 (0.47–1.76), 0.778 | 0.63 (0.38–1.05), 0.076 | 0.96 (0.85–1.09), 0.571 | −0.32 (−0.78–0.14), 0.175 |
| Yes | |||||
| No (reference) | |||||
| Number of comorbidities | 0.91 (0.79–1.05), 0.186 | 0.88 (0.69–1.11), 0.282 | 0.96 (0.8–1.15), 0.668 | 0.99 (0.94–1.03), 0.542 | −0.07 (−0.23–0.09), 0.400 |
| Symptomatic presentation | 3.43 (2.22–5.29), <0.001 | 3.41 (1.31–8.88), 0.012 | 5.79 (2.45–13.71), <0.001 | 1.24 (1.06–1.43), 0.005 | 1.68 (1.19–2.18), <0.001 |
| Yes | |||||
| No (reference) | |||||
| Variable | Type of Hematuria | p-Value | |
|---|---|---|---|
| Gross | Microscopic | ||
| Bladder cancer grade, n (%) | |||
| PUNLMP/low grade | 143 (42.1) | 29 (58) | 0.034 1 |
| High-grade | 197 (57.9) | 21 (42) | |
| Concomitant CIS, n (%) | 32 (9.4) | 1 (2) | 0.079 1 |
| Bladder cancer stage, n (%) | |||
| Ta | 165 (48.5) | 37 (74) | |
| T1 | 107 (31.5) | 11 (22) | 0.002 1 |
| T2 | 68 (20) | 2 (4) | |
| Morphological characteristics, median (IQR) | |||
| Number of tumors | 1 (1–3) | 1 (1–2) | 0.369 2 |
| Total tumor size (cm) | 3.5 (2.35–5) | 2 (1.2–3.4) | <0.001 2 |
| Dependent Variables | |||||
|---|---|---|---|---|---|
| Independent Variables | High-Grade vs. Non-High-Grade | Concomitant CIS vs. no CIS | T2 Stage vs. Ta/T1 Stage | Number of Tumors | Tumor Size |
| OR (95% CI), p-Value 1 | OR (95% CI), p-Value 1 | OR (95% CI), p-Value 1 | IRR (95% CI), p-Value 2 | B coefficient (95% CI), p-Value 3 | |
| Sex | 1.65 (1.05–2.59), 0.032 | 2.47 (0.92–6.63), 0.072 | 0.76 (0.44–1.34), 0.349 | 1.02 (0.88–1.19), 0.140 | −0.2 (−0.8–0.39), 0.508 |
| Male | |||||
| Female (reference) | |||||
| Age | 1.04 (1.01–1.06), 0.003 | 0.99 (0.96–1.04) 0.892 | 1.01 (0.98–1.04), 0.423 | 1.02 (1.01–1.024), <0.001 | 0.06 (0.03–0.09), <0.001 |
| Smoking history | 0.93 (0.59–1.45), 0.733 | 0.91 (0.41–1.99), 0.814 | 0.86 (0.49–1.52), 0.605 | 0.94 (0.81–1.09), 0.438 | −0.26 (−0.84–0.32), 0.381 |
| Yes | |||||
| No (reference) | |||||
| Number of comorbidities | 0.88 (0.76–1.03), 0.107 | 0.82 (0.61–1.09), 0.821 | 0.92 (0.75–1.12), 0.404 | 0.97 (0.92–1.02), 0.241 | −0.165 (−0.36–0.03), 0.102 |
| Type of hematuria | 2.07 (1.12–3.84), 0.020 | 5.28 (0.7–39.72), 0.106 | 6.03 (1.42–25.49), 0.015 | 1.16 (0.94–1.44), 0.191 | 1.8 (0.99–2.6), <0.001 |
| Gross | |||||
| Microscopic (reference) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakus, D.; Šolić, I.; Jurić, I.; Borovac, J.A.; Šitum, M. The Impact of the Initial Clinical Presentation of Bladder Cancer on Histopathological and Morphological Tumor Characteristics. J. Clin. Med. 2023, 12, 4259. https://doi.org/10.3390/jcm12134259
Jakus D, Šolić I, Jurić I, Borovac JA, Šitum M. The Impact of the Initial Clinical Presentation of Bladder Cancer on Histopathological and Morphological Tumor Characteristics. Journal of Clinical Medicine. 2023; 12(13):4259. https://doi.org/10.3390/jcm12134259
Chicago/Turabian StyleJakus, Dora, Ivana Šolić, Ivan Jurić, Josip A. Borovac, and Marijan Šitum. 2023. "The Impact of the Initial Clinical Presentation of Bladder Cancer on Histopathological and Morphological Tumor Characteristics" Journal of Clinical Medicine 12, no. 13: 4259. https://doi.org/10.3390/jcm12134259
APA StyleJakus, D., Šolić, I., Jurić, I., Borovac, J. A., & Šitum, M. (2023). The Impact of the Initial Clinical Presentation of Bladder Cancer on Histopathological and Morphological Tumor Characteristics. Journal of Clinical Medicine, 12(13), 4259. https://doi.org/10.3390/jcm12134259