Increased Adipocyte Hypertrophy in Patients with Nascent Metabolic Syndrome
Abstract
1. Introduction
2. Patients and Methods
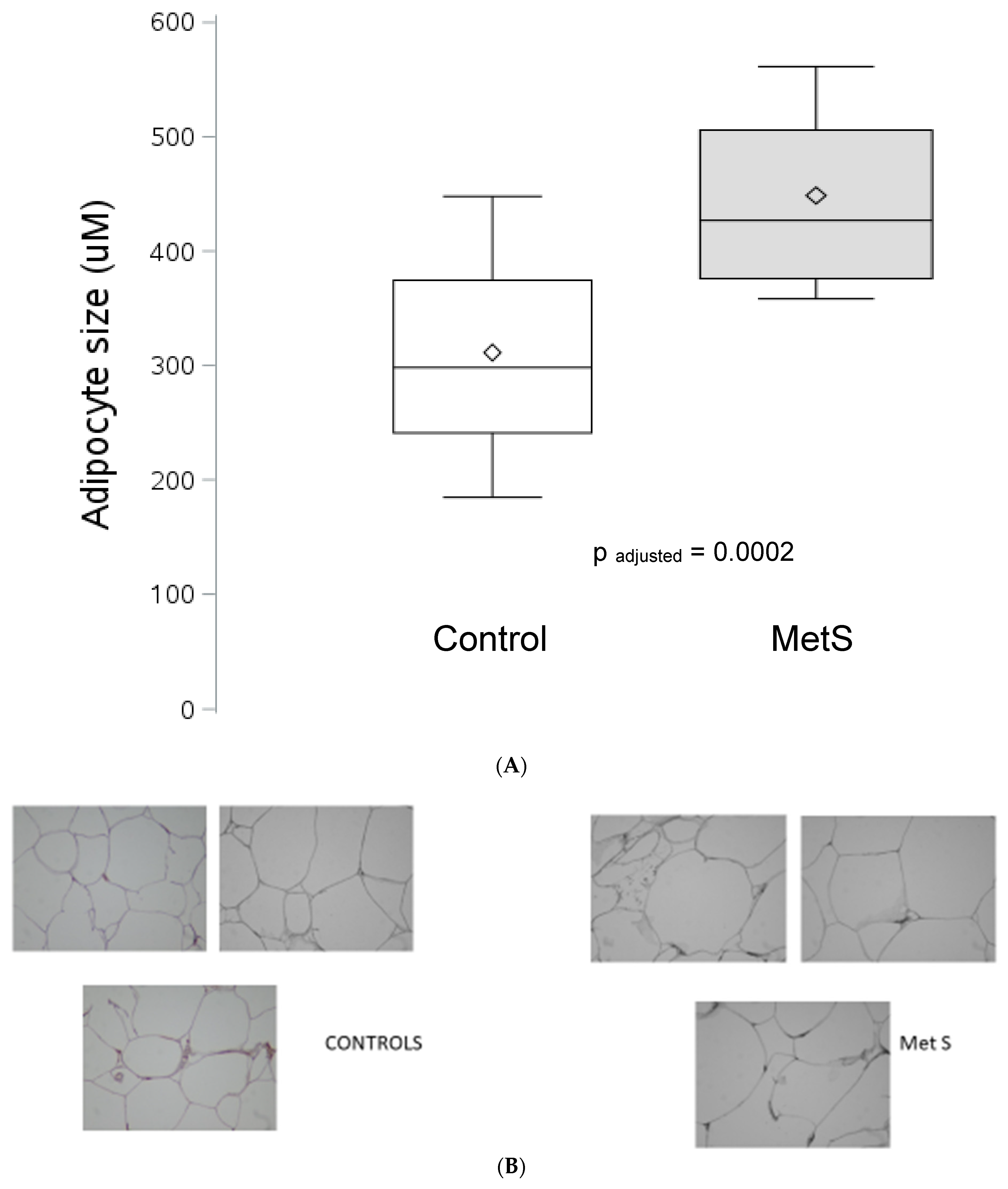
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, R.Z.; Richard, G.; Gévry, N.; Tchernof, A.; Carpentier, A.C. Fat Cell Size: Measurement Methods, Pathophysiological Origins, and Relationships with Metabolic Dysregulations. Endocr. Rev. 2022, 43, 35–60. [Google Scholar] [CrossRef] [PubMed]
- Stenkula, K.G.; Erlanson-Albertsson, C. Adipose cell size: Importance in health and disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R284–R295. [Google Scholar] [CrossRef] [PubMed]
- Laforest, S.; Labrecque, J.; Michaud, A.; Cianflone, K.; Tchernof, A. Adipocyte size as a determinant of metabolic disease and adipose tissue dysfunction. Crit. Rev. Clin. Lab. Sci. 2015, 52, 301–313. [Google Scholar] [CrossRef]
- Laforest, S.; Michaud, A.; Paris, G.; Pelletier, M.; Vidal, H.; Géloën, A.; Tchernof, A. Comparative analysis of three human adipocyte size measurement methods and their relevance for Cardiometabolic risk. Obesity 2017, 25, 122–131. [Google Scholar] [CrossRef]
- Gutiérrez-Cuevas, J.; Santos, A.; Armendariz-Borunda, J. Pathophysiological Molecular Mechanisms of Obesity: A Link between MAFLD and NASH with Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 11629. [Google Scholar] [CrossRef] [PubMed]
- Mundi, M.S.; Karpyak, M.V.; Koutsari, C.; Votruba, S.B.; O’Brien, P.C.; Jensen, M.D. Body fat distribution, adipocyte size, and metabolic characteristics of nondiabetic adults. J. Clin. Endocrinol. Metab. 2010, 95, 67–73. [Google Scholar] [CrossRef]
- Rydén, M.; Petrus, P.; Andersson, D.P.; Medina-Gómez, G.; Escasany, E.; Corrales Cordón, P.; Dahlman, I.; Kulyté, A.; Arner, P. Insulin action is severely impaired in adipocytes of apparently healthy overweight and obese subjects. J. Intern. Med. 2019, 285, 578–588. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Sherman, A.; Tsao, P.; Gonzalez, O.; Yee, G.; Lamendola, C.; Reaven, G.M.; Cushman, S.W. Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia 2007, 50, 1707–1715. [Google Scholar] [CrossRef]
- McLaughlin, T.; Deng, A.; Yee, G.; Lamendola, C.; Reaven, G.; Tsao, P.S.; Cushman, S.W.; Sherman, A. Inflammation in subcutaneous adipose tissue: Relationship to adipose cell size. Diabetologia 2010, 53, 369–377. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute: American Heart Association: World Heart Federation; International Atherosclerosis Society; and International Association for the study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Samson, S.L.; Garber, A.J. Metabolic syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Tittelbach, T.J.; Berman, D.M.; Nicklas, B.J.; Ryan, A.S.; Goldberg, A.P. Racial differences in adipocyte size and relationship to the metabolic syndrome in obese women. Obes. Res. 2004, 12, 990–998. [Google Scholar] [CrossRef]
- Langkilde, A.; Tavenier, J.; Danielsen, A.V.; Eugen-Olsen, J.; Therkildsen, C.; Jensen, F.K.; Henriksen, J.H.; Langberg, H.; Steiniche, T.; Petersen, J.; et al. Histological and Molecular Adipose Tissue Changes are Related to Metabolic Syndrome rather than Lipodystrophy in Human Immunodeficiency Virus-Infected Patients: A Cross-Sectional Study. J. Infect. Dis. 2018, 218, 1090–1098. [Google Scholar] [CrossRef]
- Jialal, I.; Devaraj, S.; Adams-Huet, B.; Chen, X.; Kaur, H. Increased cellular and circulating biomarkers of oxidative stress in nascent metabolic syndrome. J. Clin. Endocrinol. Metab. 2012, 97, E1844–E1850. [Google Scholar] [CrossRef]
- Bremer, A.A.; Devaraj, S.; Afify, A.; Jialal, I. Adipose tissue dysregulation in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E1782–E1788. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I.; Huet, B.A.; Kaur, H.; Chien, A.; Devaraj, S. Increased toll-like receptor activity in patients with metabolic syndrome. Diabetes Care 2012, 35, 900–904. [Google Scholar] [CrossRef]
- Reddy, P.; Lent-Schochet, D.; Ramakrishnan, N.; McLaughlin, M.; Jialal, I. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin. Chim. Acta 2019, 496, 35–44. [Google Scholar] [CrossRef]
- Jialal, I.; Adams-Huet, B.; Major, A.; Devaraj, S. Increased fibrosis and angiogenesis in subcutaneous gluteal adipose tissue in nascent metabolic syndrome. Diabetes Metab. 2017, 43, 364–367. [Google Scholar] [CrossRef]
- Pahwa, R.; Singh, A.; Adams-Huet, B.; Devaraj, S.; Jialal, I. Increased inflammasome activity in subcutaneous adipose tissue of patients with metabolic syndrome. Diabetes Metab. Res. Rev. 2021, 37, e3383. [Google Scholar] [CrossRef]
- McLaughlin, T.; Abbasi, F.; Cheal, K.; McLaughlin, T.; Abbasi, F.; Cheal, K.; Chu, J.; Lamendola, C.; Reaven, G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann. Intern. Med. 2003, 139, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Villaret, A.; Galitzky, J.; Decaunes, P.; Estève, D.; Marques, M.A.; Sengenès, C.; Chiotasso, P.; Tchkonia, T.; Lafontan, M.; Kirkland, J.L.; et al. Adipose tissue endothelial cells from obese human subjects: Differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes 2010, 59, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, A.Y.; Ledoux, S.; Quéguiner, I.; Caldérari, S.; Mechler, C.; Msika, S.; Corvol, P.; Larger, E. Link between adipose tissue angiogenesis and fat accumulation in severely obese subjects. J. Clin. Endocrinol. Metab. 2012, 97, E775–E780. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, A.Y.; Ledoux, S.; Larger, E. Adipose tissue angiogenesis in obesity. Thromb. Haemost. 2013, 110, 661–668. [Google Scholar] [CrossRef]
- Rutkowski, J.M.; Davis, K.E.; Scherer, P.E. Mechanisms of obesity and related pathologies; the macro-and microcirculation of adipose tissue. FEBS J. 2009, 276, 5738–5746. [Google Scholar] [CrossRef]
- Gurung, P.; Moussa, K.; Adams-Huet, B.; Devaraj, S.; Jialal, I. Increased mast cell abundance in adipose tissue of metabolic syndrome: Relevance to the proinflammatory state and increased adipose tissue fibrosis. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E504–E509. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Dasu, M.R.; Jialal, I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E145–E154. [Google Scholar] [CrossRef]
- Jialal, I.; Kaur, H.; Devaraj, S. Toll-like receptor status in obesity and metabolic syndrome: A translational perspective. J. Clin. Endocrinol. Metab. 2014, 99, 39–48. [Google Scholar] [CrossRef]
- Jialal, I.; Devaraj, S.; Bettaieb, A.; Haj, F.; Adams-Huet, B. Increased adipose tissue secretion of Fetuin-A, lipopolysaccharide-binding protein and high-mobility group box protein 1 in metabolic syndrome. Atherosclerosis 2015, 241, 130–137. [Google Scholar] [CrossRef]
| Controls, n = 19 | MetS, n = 20 | p-Value a | |
|---|---|---|---|
| Sex, Female/Male | 17/2 | 16/4 | 0.66 |
| Age, yr | 50 (40–60) | 55 (48–61) | 0.27 |
| BMI, kg/m2 | 28.8 (26.4–34.5) | 35.4 (32.3–39.3) | 0.006 |
| Waist circumference, cm | 87.6 (81.3–109.2) | 106.0 (97.2–122.6) | 0.0003 |
| Weight, kg | 82.7 (65.0–97.5) | 98.4 (81.7–120.0) | 0.01 |
| BP-systolic, mmHg | 120 (110–132) | 128 (122–135) | 0.14 |
| BP-diastolic, mmHg | 75 (69–82) | 78 (75–85) | 0.35 |
| Glucose, mg/dL | 88 (85–93) | 100 (93–113) | 0.0002 |
| Total cholesterol, mg/dL | 193 (162–205) | 190 (167–205) | 0.76 |
| HDL-cholesterol, mg/dL | 57 (47–68) | 42 (37–48) | 0.0006 |
| Triglycerides, mg/dL | 75 (49–109) | 112 (94–160) | 0.001 |
| HOMA-IR | 1.3 (1.1–2.9) | 4.0 (2.4–5.8) | 0.0003 |
| hsCRP, mg/L | 1.7 (0.4–4.0) | 4.5 (2.2–6.0) | 0.008 |
| FFA, mmol/L | 0.34 (0.18–0.44) | 0.83 (0.73–0.88) | <0.0001 |
| Rho Coefficient | p-Values | |
|---|---|---|
| Waist Circumference, cm | 0.20 | 0.22 |
| Plasma Glucose, mg/dL | 0.48 | 0.002 |
| Plasma HDL-C, mg/dL | −0.48 | 0.002 |
| Plasma TG, mg/dL | 0.31 | 0.06 |
| Plasma hsCRP, mg/L | 0.12 | 0.47 |
| HOMA-IR | 0.13 | 0.48 |
| Plasma TG:HDL-C Ratio | 0.40 | 0.01 |
| Plasma Leptin, ng/mL | 0.12 | 0.48 |
| Plasma Adiponectin, ug/mL | 0.31 | 0.07 |
| Monocyte TLR-4 (MFI/105 cells) | 0.47 | 0.01 |
| PlasmaEndotoxin (EndotoxinU/mL) | 0.68 | 0.002 |
| Plasma FFA, mmol/L | 0.63 | 0.006 |
| SAT-CD 31 (RAU) | 0.48 | 0.01 |
| SAT-VEGF (RAU) | 0.41 | 0.03 |
| SAT-Collagen (RAU) | 0.41 | 0.03 |
| SAT-SIRIUS RED stain (RAU) | 0.49 | 0.008 |
| SAT-Caspase 1 (RAU) | 0.40 | 0.03 |
| SAT-Interleukin-1 (RAU) | 0.39 | 0.03 |
| SAT-CD68 (RAU) | 0.01 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jialal, I.; Adams-Huet, B.; Devaraj, S. Increased Adipocyte Hypertrophy in Patients with Nascent Metabolic Syndrome. J. Clin. Med. 2023, 12, 4247. https://doi.org/10.3390/jcm12134247
Jialal I, Adams-Huet B, Devaraj S. Increased Adipocyte Hypertrophy in Patients with Nascent Metabolic Syndrome. Journal of Clinical Medicine. 2023; 12(13):4247. https://doi.org/10.3390/jcm12134247
Chicago/Turabian StyleJialal, Ishwarlal, Beverley Adams-Huet, and Sridevi Devaraj. 2023. "Increased Adipocyte Hypertrophy in Patients with Nascent Metabolic Syndrome" Journal of Clinical Medicine 12, no. 13: 4247. https://doi.org/10.3390/jcm12134247
APA StyleJialal, I., Adams-Huet, B., & Devaraj, S. (2023). Increased Adipocyte Hypertrophy in Patients with Nascent Metabolic Syndrome. Journal of Clinical Medicine, 12(13), 4247. https://doi.org/10.3390/jcm12134247







