Associations of Traditionally Determined Left Ventricular Mass Indices and Hemodynamic and Non-Hemodynamic Components of Cardiac Remodeling with Diastolic and Systolic Function in Patients with Chronic Kidney Disease
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Methods
2.3. Data Analysis
3. Results
3.1. Recorded Baseline Characteristics in All, Dialysis and Non-Dialysis Patients
3.2. Central Pressures and Left Ventricular Structure and Function in All, Dialysis and Non-Dialysis Patients
3.3. Baseline Recorded Characteristics in Patients with and without Traditionally Determined Left Ventricular Hypertrophy and Inappropriate Left Ventricular Mass
3.4. Central Pressures and Left Ventricular Structure and Function in Patients with and without Traditionally Determined Left Ventricular Hypertrophy and Inappropriate Left Ventricular Mass
3.5. Associations of Left Ventricular Mass Parameters with Diastolic and Systolic Function
3.6. Confounder Adjusted and Mutually Independent Relationships of Left Ventricular Predicted and Inappropriate Excess left Ventricular Mass Indices with Traditionally Determined Left Ventricular Mass Indices
3.7. Performance of Traditionally Determined Left Ventricular Mass Indices, Inappropriate Left Ventricular Mass and Inappropriate Excess Left Ventricular Mass Indices in Identifying Patients with Reduced Ejection Fraction
4. Discussion
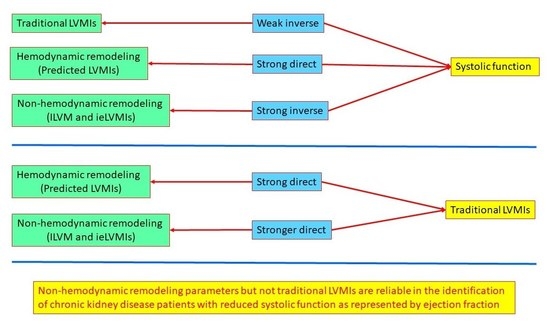
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foley, R.N.; Parfrey, P.S.; Harnett, J.D.; Kent, G.M.; Barre, P.E. The Prognostic Importance of Left Ventricular Geometry in Uremic Cardiomyopathy. J. Am. Soc. Nephrol. 1995, 5, 2024–2031. [Google Scholar] [CrossRef]
- Bao, J.-F.; Hu, P.P.; She, Q.-Y.; Zhang, D.; Mo, J.-J.; Li, A. A Bibliometric and Visualized Analysis of Uremic Cardiomyopathy From 1990 to 2021. Front. Cardiovasc. Med. 2022, 9, 908040. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shapiro, J.I. Evolving Concepts in The Pathogenesis of Uraemic Cardiomyopathy. Nat. Rev. Nephrol. 2019, 15, 159–175. [Google Scholar] [CrossRef]
- Garikapati, K.; Goh, D.; Khanna, S.; Echampathy, K. Uraemic Cardiomyopathy: A Review of The Current Literature. Clin. Med. Insights. Cardiol. 2021, 23, 1179546821998347. [Google Scholar] [CrossRef] [PubMed]
- Hickson, L.J.; Negrotto, S.N.; Onuigbo, M.; Scott, C.G.; Rule, A.D.; Norby, S.M.; Albright, R.C.; Casey, E.T.; Dillon, J.J.; Pellika, P.A.; et al. Echocardiography Criteria for Structural Heart Disease in Patients with End-Stage Renal Disease Initiating Haemodialysis. J. Am. Coll. Cardiol. 2016, 67, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.M.; Chao, C.J.; Tsai, M.T.; Lee, K.H.; Tseng, W.C.; Bin, P.J.; Lin, Y.P.; Hsu, C.Y.; Tarng, D.C. Echocardiographic Features of Left Ventricular Dysfunction and Outcomes in Chronic Kidney Disease. Heart 2022, 109, 1–9. [Google Scholar] [CrossRef]
- Lorell, B.H.; Carabello, B.A. Left Ventricular Hypertrophy. Pathogenesis, Detection, and Prognosis. Circulation 2000, 102, 470–479. [Google Scholar] [CrossRef]
- Bello, H.; Norton, G.R.; Peterson, V.R.; Libhaber, C.D.; Mmpopi, K.N.; Mthembu, N.; Masiu, M.; Da Silva Fernandes, D.; Bamaiyi, A.J.; Peters, F.; et al. Hemodynamic and functional correlates of Concentric vs. Eccentric LVH in a Community-Based Sample with Prevalent Volume-dependent Hypertension. Am. J. Hypertens. 2021, 34, 1300–1310. [Google Scholar] [CrossRef]
- Levy, D.; Garrison, R.J.; Savage, D.D.; Kannel, W.B.; Castelli, W.P. Prognostic Implications of Echocardiographically Determined Left Ventricular Mass in The Framingham Study. N. Engl. J. Med. 1910, 322, 1561–1566. [Google Scholar] [CrossRef]
- Koren, M.J.; Devereux, R.B.; Casale, P.N.; Savage, D.D.; Laragh, J.H. Relation of Left Ventricular Mass and Geometry to Morbidity and Mortality in Uncomplicated Essential Hypertension. Ann. Intern. Med. 1991, 114, 345–352. [Google Scholar] [CrossRef]
- Liao, Y.; Cooper, R.S.; Durazo-Arvizu, R.; Mensah, G.A.; Ghali, J.K. Prediction of Mortality Risk by Different Methods of Indexation of Left Ventricular Mass. J. Am. Coll. Cardiol. 1997, 29, 641–647. [Google Scholar] [CrossRef]
- Ghali, J.K.; Liao, Y.; Simmonsd, B.; Castaner, A.; Cao, G.; Cooper, R.S. The Prognostic Role of Left Ventricular Hypertrophy in Patients with or Without Coronary Artery Disease. Ann. Intern. Med. 1992, 117, 831–836. [Google Scholar] [CrossRef]
- De Simone, G.; Devereux, R.B.; Kimball, T.R.; Roman, M.J.; Contaldo, F.; Daniels, S.R. Interaction Between Body Size and Cardiac Workload: Influence on Left Ventricular Mass During Body Growth and Adulthood. Hypertension 1998, 31, 1077–1082. [Google Scholar] [CrossRef]
- Libhaber, C.D.; Norton, G.R.; Maseko, M.J.; Majane, O.H.; Millen, A.M.; Maunganidze, F.; Michel, F.S.; Brooksbank, R.; Libhabe, E.; Sareli, P.; et al. Relationship Between Inappropriate Left Ventricular Hypertrophy and Ejection Fraction Independent of Absolute or Indexed Mass in a Community Sample of African Ancestry. J. Hypertens. 2013, 31, 169–176. [Google Scholar] [CrossRef]
- Anstey, D.E.; Tanner, R.M.; Booth, J.N., III; Bress, A.P.; Diaz, K.M.; Sims, M.; Ogedegby, G.; Muntner, P.; Abdall, M. Inappropriate Left Ventricular Mass and Cardiovascular Disease Events and Mortality in Blacks: The Jackson Heart Study. J. Am. Heart Assoc. 2019, 8, e011897. [Google Scholar] [CrossRef]
- De Simone, G.; Verdecchia, P.; Pede, S.; Gorini, M.; Maggioni, A.P. Prognosis of Inappropriate Left Ventricular Mass in Hypertension: The MAVI Study. Hypertension 2002, 40, 470–476. [Google Scholar] [CrossRef]
- Palmieri, V.; de Simone, G.; Roman, M.J.; Schwartz, J.E.; Pickering, T.G.; Devereux, R.B. Ambulatrory Blood Pressure and Metabolic Abnormalities in Hypertensive Subjects with Inappropriately High Left Ventricular Mass. Hypertension 1999, 34, 1032–1040. [Google Scholar] [CrossRef]
- Mureddo, G.F.; Pasanisi, F.; Palmieri, V.; Celentano, A.; Contaldo, F.; de Simone, G. Appropriate or Inappropriate Left Ventricular Mass in The Presence or Absence of Prognostically Adverse Left Ventricular Hypertrophy. J. Hypertension 2001, 19, 1113–1119. [Google Scholar] [CrossRef]
- Palmieri, V.; Wachtell, K.; Bella, J.N.; Gerdts, E.; Papademitriou, V.; Nieminen, M.S.; Dahlof, B.; Roman, M.J.; Devereux, R.B. Usefulness of The Assessment of The Inappropriateness of Left Ventricular Mass to Detect Left Ventricular Systolic and Diastolic Abnormalities in The Absence of Echocardiographic Left Ventricular Hypertrophy: The LIFE Study. J. Hum. Hypertens. 2004, 18, 423–430. [Google Scholar] [CrossRef]
- Hung, C.S.; Ho, Y.L.; Chang, Y.Y.; Wu, V.C.; Wu, X.M.; Lee, J.K.; Chueh, S.C.; Lin, Y.H.; Changh, Y.S.; Yang, S.Y.; et al. Twenty-Four-Hour Urinary Aldosterone Predicts Inappropriate Left Ventricular Mass Index in Patients with Primary Aldosteronism. ScientificWorldJournal 2013, 2013, 294594. [Google Scholar] [CrossRef]
- Pan, C.-T.; Wu, X.-M.; Tsai, C.-H.; Chang, Y.-Y.; Chang, Y.-Y.; Chen, Z.-W.; Chang, C.-C.; Lee, B.-C.; Liao, C.-W.; Chen, Y.-L.; et al. Hemodynamic and Non-Hemodynamic Components of Cardiac Remodeling in Primary Aldosteronism. Front. Endocrinol. 2021, 12, 646097. [Google Scholar] [CrossRef] [PubMed]
- Chen, U.L.; Liao, C.W.; Wang, S.M.; Lai, T.S.; Huang, K.H.; Chang, C.C.; Lee, B.C.; Lu, C.C.; Chang, Y.R.; Chang, Y.Y.; et al. Diabetes Mellitus Is Associated with More Adverse Non-Hemodynamic Left Ventricular Remodeling and Less Recovery in Patients With Primary Aldosteronism. J. Investig. Med. 2023, 71, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, G.; Tarantini, L.; Frizzi, R.; Stefenelli, C.; Russo, T.-E.; Selmi, A.; Toller, C.; Furlanello, F.; de Simone, G. Chronic Kidney Disease Elicits Excessive Increase in Left Ventricullar Mass Growth in Patients at Increased Risk for Cardiovascular Events. J. Hypertens. 2011, 29, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-C.; Chang, J.-M.; Liu, W.-C.; Chen, Y.-Y.; Chen, L.-I.; Huang, J.-C.; Yang, T.-K.; Su, H.-M.; Chen, H.-C. The Ratio of Observed to Predicted Left Ventricular Mass Is Independently Associated with Increased Cardiovascular Events in Patients with Chronic Kidney Disease. Hypertens. Res. 2012, 35, 832–838. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Kitzman, D.W.; Palmieri, V.; Liu, J.E.; Oberman, A.; Hopkins, P.N.; Bella, J.N.; Rao, D.C.; Arnett, D.K.; Devereux, R.B. Association of inappropriate Left Ventricular Mass with Systolic and Diastolic Dysfunction: The HyperGEN Study. Am. J. Hypertens. 2004, 17, 828–833. [Google Scholar] [CrossRef]
- Gaash, W.H. Diagnosis and Treatment of Heart Failure Based on Left Ventricular Systolic or Diastolic Dysfunction. JAMA 1994, 271, 1276–1280. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Norton, G.R.; Robinson, C.; Woodiwiss, A.J.; Dessein, P.H. Potential Determinants of the E/e’ ratio in Non-Dialysis Compared with Dialysis. Nephrology 2021, 26, 988–998. [Google Scholar] [CrossRef]
- Tade, G.; Hsu, H.C.; Woodiwiss, A.J.; Peters, F.; Robinson, C.; Dlongolo, N.; Teckie, G.; Solomon, A.; Norton, G.R.; Dessein, P.H. Uric Acid, Ferritin, Parathyroid Hormone and Gamma-Glutamyl Transferase Concentrations are Associated with Uremic Cardiomyopathy Characteristics in Non-Dialysis and Dialysis Chronic Kidney Disease Patients. Int. J. Nephrol. Renovasc. Dis. 2022, 15, 353–369. [Google Scholar] [CrossRef]
- Woodiwiss, A.J.; Libhaber, C.; Libhaber, E.; Sareli, P.; Norton, G.R. Relationship Between On-Treatment Decreases in Inappropriate Versus Absolute or Indexed Left Ventricular Mass and Increases in Ejection Fraction in Hypertension. Hypertension 2012, 60, 810–817. [Google Scholar] [CrossRef]
- Sahn, D.J.; De Maria, A.; Kisslo, J.; Weynan, A. Recommendations Regarding Quantitin M-Mode Echocardiography: Results of A Survey of Echocardiographic Measurement. Circulation 1978, 58, 1072–1083. [Google Scholar] [CrossRef]
- De Simone, G.; Devereux, R.B.; Koren, M.J.; Mensah, G.A.; Casale, P.N.; Laragh, J.H. Midwall Left Ventricular Mechanics: An Independent Predictor of Cardiovascular Risk in Hypertension. Circulation 1996, 93, 259–265. [Google Scholar] [CrossRef]
- MacKinnon, D.P.; Fairchild, A.J.; Fritz, M.S. Mediation Analysis. Annu. Rev. Psychol. 2007, 58, 593–614. [Google Scholar] [CrossRef]
- Baron, R.M.; Kenny, D.A. The Moderator-Mediator Variable Distinction in Social Psychological Research: Conceptual, Strategic and Statistical Considerations. J. Personal. Soc. Psych. 1986, 51, 1173–1182. [Google Scholar] [CrossRef]
- Krull, J.L.; MacKinnon, D.P. Multilevel Modeling of Individual and Group Level Mediated Effects. Multivariate Behav. Res. 2001, 36, 249–277. [Google Scholar] [CrossRef]
- Normand, S.T. Some Old and New Statistical Tools for Outcomes Research. Circulation 2008, 118, 872–884. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Tade, G.; Norton, G.R.; Peters, F.; Robinson, C.; Dlongolo, N.; Teckie, G.; Woodiwiss, A.J.; Dessein, P.H. Aortic Stiffness and Pulsatile Pressures as Mediators of Chronic Kidney Disease Induced Impaired Diastolic Function. Int. J. Nephrol. Renovasc. Dis. 2022, 15, 27–40. [Google Scholar] [CrossRef]
- Lopez, B.; Gonzalez, A.; Querejeta, R.; Larman, M.; Diez, J. Alterations in the Pattern of Collagen Deposition May Contribute to the Deterioration of Systolic Function in Hypertensive Patients with Heart Failure. J. Am. Coll. Cardiol. 2006, 48, 89–96. [Google Scholar] [CrossRef]
- Shapiro, L.M.; Gibson, D.G. Patterns of Diastolic Dysfunction in Left Ventricular Hypertrophy. Br. Heart, J. 1988, 59, 438–445. [Google Scholar] [CrossRef]
- Mitsnefes, M.M.; Kimball, T.R.; Border, W.L.; Witt, S.A.; Glascock, B.J.; Khoury, P.R.; Daniels, S.R. Impaired Left Ventricular Diastolic Function in Children with Chronic Renal Failure. Kidney Int. 2004, 65, 1461–1466. [Google Scholar] [CrossRef]
- Hayashi, S.Y.; Rohana, M.; Lindholm, B.; Brodin, L.-A.; Lind, B.; Barany, P.; Alvestrand, A.; Seeberger, A. Left Ventricular Function in Patients with Chronic Kidney Disease Evaluated by Colour Tissue Doppler Velocity Imaging. Nephrol. Dial. Transplant 2006, 21, 125–132. [Google Scholar] [CrossRef]
- Aeschbacher, B.C.; Hutter, D.; Fuhrer, J.; Weidmann, P.; Delacretaz, E.; Allemann, Y. Diastolic Dysfunction Precedes Myocardial Hypertrophy in the Development of Hypertension. Am. J. Hypertens. 2001, 14, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Di Bello, V.; Taqlini, E.; Dell’Omo, G.; Giannini, C.; Donne, M.G.D.; Canale, M.L.; Nardi, C.; Palagi, C.; Dini, F.L.; Penno, G.; et al. Early Left Ventricular Mechanics Abnormalities in Prehypertension: A Two-dimensional Strain Echocardiography Study. Am. J. Hypertens. 2010, 23, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Messerli, F.H.; Rimoldi, S.F.; Bangalore, S. The Transition from Hypertension to Heart Failure. JACC: Heart Fail. 2017, 5, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Maizel, J.; Mentaverri, R.; Chillon, J.-M.; Six, I.; Giumelli, P.; Brazier, M.; Choukroun, G.; Tribouilloy, C.; Massy, Z.A.; et al. The Onset of Left Ventricular Diastolic Dysfunction in SHR Rats is not Related to Hypertrophy or Hypertension. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1524–H1532. [Google Scholar] [CrossRef]
- Winterberg, P.D.; Jiang, R.; Maxwell, J.T.; Wang, B.; Wagner, M.B. Myocardial Dysfunction Occurs Prior to Changes in Ventricular Geometry in Mice with Chronic Kidney Disease (CKD). Physio. Rep. 2016, 4, e12732. [Google Scholar] [CrossRef]
- Gandhi, S.K.; Powers, J.C.; Nomeir, A.-M.; Fowle, K.; Kitzman, D.W.; Rankin, K.M.; Little, W.C. The Pathogenesis of Acute Pulmonary Edema Associated with Hypertension. N. Engl. J. Med. 2001, 344, 17–22. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Wang, J.; Zhang, L.; Zhao, M.H. C-STRIDE (Chinese Cohort Study of Chronic Kidney Disease). J. Am. Heart Assoc. 2020, 9, e015359. [Google Scholar] [CrossRef]

| Characteristics | All Patients (n = 103) | Non-Dialysis (n = 62) | Dialysis (n = 41) | p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 57.3 (14.5) | 58.6 (14.1) | 55.3 (15.1) | 0.4 |
| Female sex (%) | 36.9 | 32.2 | 43.9 | 0.1 |
| Black (%) | 40.8 | 29.0 | 58.5 | 0.004 |
| Asian (%) | 29.1 | 33.9 | 21.9 | 0.1 |
| White (%) | 23.3 | 33.9 | 7.3 | 0.006 |
| Mixed (%) | 6.8 | 3.2 | 12.2 | 0.2 |
| CKD duration | 5.5 (4.1) | 6.0 (4.5) | 4.9 (3.3) | 0.2 |
| Anthropometry | ||||
| Body mass index (kg/m2) | 27.6 (5.6) | 27.8 (5.6) | 27.2 (5.9) | 0.5 |
| Weight (kg) | 78.7 (15.7) | 80.7 (16.0) | 75.7 (15.0) | 0.2 |
| Height (cm) | 169 (10) | 170 (9) | 168 (11) | 0.4 |
| Major traditional CV risk factors | ||||
| Hypertension (%) | 89.3 | 85.5 | 95.1 | 0.1 |
| Systolic BP (mmHg) | 141 (21) | 138 (20) | 146 (21) | 0.02 |
| Diastolic BP (mmHg) | 83 (12) | 81 (9) | 85 (15) | 0.1 |
| Peripheral PP (mmHg) | 58 (19) | 57 (17) | 61 (21) | 0.2 |
| Mean BP (mmHg) | 102 (12) | 100 (11) | 105 (14) | 0.02 |
| Heart rate (beats/min) | 75 (14) | 74 (15) | 76 (12) | 0.4 |
| Dyslipidemia (%) | 79.6 | 86.0 | 69.4 | 0.06 |
| Diabetes (%) | 34 | 33.9 | 34.2 | 0.9 |
| Smoking (%) | 2.9 | 4.8 | 0.0 | - |
| Non-traditional CV risk factors | ||||
| Dialysis duration (months) | - | - | 36 (12–48) | - |
| Estimated GFR (mL/min/1.73 m2) | - | 33 (17.9) | - | - |
| Phosphate (mmol/L) | 1.2 (1.2–1.6) | 1.2 (0.9–1.4) | 1.4 (0.9–1.7) | 0.1 |
| Haemoglobin (g/dL) | 11.6 (10.1–13.7) | 12.9(10.2–15.1) | 10.8 (9.8–12.0) | <0.0001 |
| Treatment | ||||
| Antihypertensive agent use (%) | 89.3 | 85.5 | 95.1 | 0.1 |
| Antihypertensives (n) | 2.1 (1.3) | 2.0 (1.3) | 2.3 (1.1) | 0.2 |
| ACEI/ARB use (%) | 79.0 | 78.7 | 79.5 | 0.9 |
| Calcium channel blocker use | 43.6 | 33.9 | 59.0 | 0.02 |
| Diuretic use | 30.4 | 32.8 | 26.8 | 0.7 |
| Beta blocker use | 43.0 | 36.1 | 53.9 | 0.08 |
| Alpha blocker use | 22.2 | 21.3 | 23.6 | 0.7 |
| Statin use (%) | 62.0 | 46.7 | 51.3 | 0.1 |
| ESA use (%) | 46.6 | 16.1 | 92.7 | <0.001 |
| Cardiovascular disease (%) | 19.4 | 20.9 | 17.1 | 0.8 |
| Characteristics | All Patients (n = 103) | Non-Dialysis (n = 62) | Dialysis (n = 41) | p-Value |
|---|---|---|---|---|
| Central systolic BP (mmHg) | 130 (19) | 127 (17) | 135 (21) | 0.01 |
| Central PP (mmHg) | 44 (33–54) | 42 (34–51) | 49 (32–61) | 0.1 |
| LVM (g) | 168 (133–230) | 164 (130–236) | 187 (144–229) | 0.2 |
| LVMI-BSA (g/m2) | 93 (72–117) | 86 (71–110) | 108 (80–120) | 0.02 |
| LVMI-ht1.7 (g/m1.7) | 68 (57–91) | 64 (56–89) | 77 (61–101) | 0.05 |
| LVH-ht1.7 (%) | 44 (45.4) | 21 (35.6) | 23 (60.5) | 0.01 |
| ILVM (%) | 134 (114–158) | 134 (114–150) | 142 (113–188) | 0.3 |
| ILVM increase (%) | 50 (53.8) | 32 (55.8) | 18 (51.4) | 0.8 |
| PLVMI-BSA (g/m2) | 71 (18) | 68 (17) | 74 (19) | 0.05 |
| PLVMI-ht1.7 (g/m1.7) | 54 (13) | 53 (13) | 56 (14) | 0.1 |
| IeLVMI-BSA (g/m2) | 20 (8–39) | 20 (8–33) | 30 (9–51) | 0.03 |
| IeLVMI-ht1.7 (g/m1.7) | 15 (6–30) | 15 (6–26) | 20 (7–41) | 0.05 |
| LV relative wall thickness | 0.36 (0.31–0.44) | 0.37 (0.32–0.45) | 0.33 (0.28–0.44) | 0.1 |
| Eccentric LVH (%) | 36.1 | 27.1 | 50.0 | 0.02 |
| Concentric LVH (%) | 9.3 | 8.5 | 10.5 | 0.7 |
| LVEDVI-BSA (ml/m2) | 64 (46–86) | 61 (45–79) | 77 (53–92) | 0.03 |
| LV e’ (cm/s) | 8.7 (2.7) | 9.0 (2.6) | 8.1 (2.8) | 0.01 |
| LV E/e’ | 10.0 (4.4) | 9.3 (4.2) | 11.1 (4.6) | 0.02 |
| LV E/e’ > 14 | 20 | 17.7 | 22.5 | 0.5 |
| LV lateral wall s’ (cm/s) | 8.7 (2.3) | 8.9 (2.3) | 8.2 (2.1) | 0.1 |
| LV midwall fractional shortening (%) | 19.6 (4.7) | 19.9 (4.7) | 19.1 (4.9) | 0.5 |
| LV ejection fraction (%) | 64 (14) | 65 (14) | 62 (14) | 0.1 |
| LV ejection fraction < 50% (%) | 17.2 | 16.7 | 17.9 | 0.5 |
| Stroke volume (mL/beat) | 70 (24) | 69 (24) | 72 (24) | 0.4 |
| Cardiac output (L/min) | 5.17 (1.92) | 4.95 (1.87) | 5.50 (1.98) | 0.2 |
| SW (gram-meters/beat) | 140 (98–182) | 135 (91–175) | 154 (104–194) | 0.2 |
| Characteristics | No LVH (n = 53) | LVH (n = 44) | p-Value | Appropriate LVM (n = 43) | Inappropriate LVM (n = 50) | p-Value |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (years) | 56.8 (14.4) | 57.0 (10.1) | 1.0 | 56.0 (14.4) | 57.4 (15.4) | 0.6 |
| Female sex (%) | 37.7 | 36.4 | 0.9 | 37.2 | 34.0 | 0.8 |
| Black (%) | 39.6 | 43.2 | 0.7 | 39.5 | 42.0 | 0.8 |
| Asian (%) | 34.0 | 25.0 | 0.3 | 30.2 | 32.0 | 0.8 |
| White (%) | 20.7 | 22.7 | 0.8 | 23.3 | 18.0 | 0.4 |
| Mixed (%) | 5.7 | 9.1 | 0.5 | 7.0 | 8.0 | 0.9 |
| CKD duration | 6.0 | 5.2 | 0.4 | 5.8 | 5.6 | 0.7 |
| Anthropometry | ||||||
| Body mass index (kg/m2) | 26.2 (4.9) | 28.3 (6.0) | 0.04 | 25.8 (4.0) | 28.3 (6.2) | 0.03 |
| Weight (kg) | 75.4 (13.8) | 80.8 (17.1) | 0.07 | 74.2 (12.2) | 81.0 (16.9) | 0.03 |
| Height (cm) | 170 (8) | 169 (12) | 0.6 | 170 (10) | 169 (11) | 0.9 |
| Major traditional CV risk factors | ||||||
| Hypertension (%) | 92.5 | 88.6 | 0.5 | 90.7 | 90.0 | 0.9 |
| Systolic BP (mmHg) | 136 (20) | 146 (21) | 0.03 | 143 (22) | 140 (20) | 0.5 |
| Diastolic BP (mmHg) | 80 (10) | 84 (13) | 0.1 | 82 (11) | 83 (12) | 0.5 |
| Peripheral PP (mmHg) | 56 (20) | 61 (18) | 0.1 | 61 (21) | 57 (17) | 0.2 |
| Mean BP (mmHg) | 99 (10) | 105 (14) | 0.03 | 102 (12) | 102 (13) | 1.0 |
| Heart rate (beats/min) | 75 (16) | 75 (13) | 0.8 | 74 (15) | 75 (15) | 0.7 |
| Dyslipidemia (%) | 73.9 | 82.9 | 0.3 | 76.9 | 79.5 | 0.9 |
| Diabetes (%) | 34.0 | 34.1 | 1.0 | 27.9 | 38.0 | 0.4 |
| Smoking (%) | 3.8 | 2.3 | 0.8 | 2.3 | 4.0 | 0.6 |
| Non-traditional CV risk factors | ||||||
| Dialysis duration (months) | 48 (24–48) | 36 (21–39) | 0.4 | 36 (18–48) | 36 (18–36) | 0.5 |
| Estimated GFR (ml/min/1.73 m2) | 34 (19) | 35 (25) | 0.3 | 37 (23) | 33 (20) | 0.9 |
| Phosphate (mmol/l) | 1.2 (1.0–1.4) | 1.4 (0.9–1.6) | 0.2 | 1.2 (0.9–1.6) | 1.3 (0.9–1.5) | 0.8 |
| Haemoglobin (g/dl) | 12.2 (10.5–13.7) | 10.9 (9.6–13.4) | 0.08 | 11.6 (10.2–13.8) | 11.4 (9.7–13.7) | 0.5 |
| Treatment | ||||||
| Antihypertensive agent use (%) | 92.4 | 88.6 | 0.6 | 90.7 | 90.0 | 0.9 |
| Antihypertensives (n) | 1.9 (1.0) | 2.5 (1.4) | 0.01 | 2.0 (1.0) | 2.3 (1.4) | 0.3 |
| ACEI/ARB use (%) | 82.7 | 76.2 | 0.4 | 83.3 | 77.1 | 0.5 |
| Calcium channel blocker use | 34.0 | 61.9 | 0.007 | 41.9 | 47.9 | 0.6 |
| Diuretic use | 26.9 | 31.8 | 0.7 | 28.6 | 28.0 | 0.,9 |
| Beta blocker use | 18.9 | 59.5 | 0.002 | 26.2 | 56.3 | 0.005 |
| Alpha blocker use | 17.7 | 28.6 | 0.2 | 19.0 | 25.5 | 0.6 |
| Statin use (%) | 59.6 | 61.9 | 0.8 | 59.5 | 60.4 | 1.0 |
| ESA (%) | 34.0 | 61.4 | 0.008 | 46.5 | 44.0 | 0.8 |
| Cardiovascular disease (%) | 11.3 | 25.0 | 0.03 | 11.6 | 22.0 | 0.4 |
| Characteristics | No LVH (n = 53) | LVH (n = 44) | p-Value | Appropriate LVM (n = 43) | Inappropriate LVM (n = 50) | p-Value |
|---|---|---|---|---|---|---|
| Central systolic BP (mmHg) | 126 (18) | 134 (19) | 0.05 | 131 (19) | 128 (18) | 0.3 |
| Central PP (mmHg) | 41 (33–51) | 48 (34–61) | 0.06 | 44 (35–54) | 43 (33–53) | 0.4 |
| LVM (g) | 144 (155–167) | 232 (191–284) | <0.001 | 148 (112–176) | 208 (160–269) | <0.001 |
| LVMI-BSA (g/m2) | 76 (64–89) | 118 (108–141) | <0.001 | 80 (64–93) | 112 (84–130) | <0.001 |
| LVMI-ht1.7 (g/m1.7) | 59 (50–66) | 94 (81–111) | <0.001 | 60 (46–72) | 89 (66–109) | <0.001 |
| LVH-ht1.7 (%) | 0 | 100 | - | 18.6 | 68.0 | <0.001 |
| ILVM (%) | 117 (104–135) | 150 (134–201) | <0.001 | 113 (102–120) | 154 (142–194) | <0.001 |
| ILVM increase (%) | 31.4 | 80.9 | <0.001 | 0 | 100 | - |
| PLVMI-BSA (g/m2) | 66 (18) | 77 (18) | 0.003 | 75 (18) | 67 (18) | 0.03 |
| PLVMI-ht1.7 (g/m1.7) | 49 (12) | 60 (13) | <0.001 | 56 (12) | 52 (14) | 0.2 |
| IeLVMI-BSA (g/m2) | 11 (3–19) | 40 (25–63) | <0.001 | 8 (2–12) | 38 (25–59) | <0.001 |
| IeLVMI-ht1.7 (g/m1.7) | 8 (2–15) | 32 (20–56) | <0.001 | 6 (1–9) | 29 (21–52) | <0.001 |
| LV relative wall thickness | 0.39 (0.32–0.44) | 0.34 (0.29–0.44) | 0.5 | 0.33 (0.29–0.41) | 0.37 (0.33–0.46) | 0.01 |
| Eccentric LVH (%) | 0 | 79.5 | - | 18.6 | 54.0 | <0.001 |
| Concentric LVH (%) | 0 | 20.4 | - | 0 | 14.0 | - |
| LVEDVI-BSA (ml/m2) | 53 (44–67) | 85 (71–108) | <0.001 | 64 (45–80) | 72 (47–100) | <0.001 |
| LV e’ (cm/s) | 9.0 (2.8) | 8.6 (2.7) | 0.4 | 9.1 (2.4) | 8.6 (2.9) | 0.5 |
| LV E/e’ | 9.2 (4.1) | 10.7 (4.5) | 0.08 | 9.5 (4.0) | 10.3 (4.8) | 0.4 |
| LV E/e’ > 14 (%) | 15.1 | 25.0 | 0.2 | 11.6 | 28.0 | 0.07 |
| LV lateral wall s’ (cm/s) | 8.8 (2.3) | 8.5 (2.3) | 0.5 | 8.4 (7.6–10.1) | 8.5 (7.1–10.1) | 0.7 |
| LV midwall fractional shortening (%) | 20.1 (4.6) | 18.9 (5.0) | 0.3 | 21.1 (4.3) | 18.4 (4.9) | 0.02 |
| LV ejection fraction (%) | 67 (13) | 61 (14) | 0.03 | 70 (10) | 60 (15) | <0.001 |
| LV ejection fraction < 50% (%) | 11.3 | 22.7 | 0.08 | 4.7 | 28.0 | 0.01 |
| Stroke volume (mL/beat) | 62 (21) | 80 (24) | <0.001 | 74 (22) | 68 (25) | 0.2 |
| Cardiac output (L/min) | 4.60 (1.64) | 5.95 (1.98) | 0.001 | 5.45 (1.84) | 4.97 (1.97) | 0.2 |
| SW (gram-meters/beat) | 111 (88–155) | 165 (128–206) | <0.001 | 151 (104–184) | 129 (91–180) | 0.2 |
| LVM Parameter | E’ | E/e’ | Lateral s’ | Midwall Fractional Shortening | Ejection Fraction | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Partial R | p-Value | Partial R | p-Value | Partial R | p-Value | Partial R | p-Value | Partial R | p-Value | |
| Log LVMI-BSA | −0.054 | 0.6 | 0.172 | 0.09 | −0.093 | 0.4 | −0.121 | 0.3 | −0.296 | 0.003 |
| Log LVMI-ht1.7 | −0.091 | 0.4 | 0.186 | 0.07 | −0.116 | 0.3 | −0.172 | 0.1 | −0.294 | 0.003 |
| Log ILVM | −0.022 | 0.8 | 0.066 | 0.5 | −0.049 | 0.6 | −0.413 | <0.001 | −0.461 | <0.001 |
| PLVMI-BSA | −0.117 | 0.3 | 0.156 | 0.1 | −0.108 | 0.3 | 0.235 | 0.05 | 0.121 | 0.2 |
| PLVMI-ht1.7 | −0.188 | 0.07 | 0.196 | 0.06 | −0.148 | 0.2 | 0.158 | 0.2 | 0.114 | 0.3 |
| Log ieLVMI-BSA | −0.142 | 0.2 | 0.196 | 0.07 | −0.127 | 0.2 | −0.217 | 0.09 | −0.409 | <0.001 |
| Log ieLVMI-ht1.7 | −0.159 | 0.1 | 0.203 | 0.06 | −0.137 | 0.2 | −0.230 | 0.07 | −0.402 | <0.001 |
| LVM Parameter | E’ | E/e’ | Lateral s’ | Midwall Fractional Shortening | Ejection Fraction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Partial R | p-Value | Model R2 | Partial R | p-Value | Model R2 | Partial R | p-Value | Model R2 | Partial R | p-Value | Model R2 | Partial R | p-Value | Model R2 | |
| Log LVMI-BSA | 0.059 | 0.6 | 0.272 | 0.078 | 0.5 | 0.252 | −0.051 | 0.6 | 0.147 | −0.165 | 0.2 | 0.188 | −0.206 | 0.05 | 0.205 |
| Log LVMI-BSA a | 0.094 | 0.4 | 0.319 | 0.086 | 0.4 | 0.273 | −0.040 | 0.7 | 0.168 | 0.253 | 0.05 | 0.374 | 0.280 | 0.01 | 0.421 |
| Log LVMI-BSA b | 0.122 | 0.3 | 0.319 | 0.103 | 0.4 | 0.282 | −0.035 | 0.8 | 0.166 | 0.097 | 0.5 | 0.209 | 0.221 | 0.05 | 0.368 |
| Log LVMI-BSA c | 0.008 | 0.9 | 0.315 | 0.084 | 0.4 | 0.273 | −0.063 | 0.6 | 0.168 | −0.349 | 0.006 | 0.362 | −0.407 | <0.001 | 0.413 |
| Log LVMI-ht1.7 | 0.019 | 0.9 | 0.270 | 0.102 | 0.4 | 0.255 | −0.089 | 0.4 | 0.162 | −0.188 | 0.1 | 0.196 | −0.216 | 0.04 | 0.211 |
| Log LVMI-ht1.7 a | 0.012 | 0.9 | 0.316 | 0.125 | 0.3 | 0.279 | −0.075 | 0.5 | 0.179 | 0.246 | 0.06 | 0.373 | 0.317 | 0.004 | 0.455 |
| Log LVMI-ht1.7 d | 0.046 | 0.7 | 0.315 | 0.152 | 0.2 | 0.294 | −0.075 | 0.5 | 0.176 | 0.080 | 0.6 | 0.209 | 0.266 | 0.02 | 0.406 |
| Log LVM-ht1.7 e | −0.006 | 1.0 | 0.316 | 0.083 | 0.5 | 0.279 | −0.091 | 0.4 | 0.179 | −0.393 | 0.002 | 0.362 | −0.470 | <0.001 | 0.451 |
| Log ILVM | −0.021 | 0.8 | 0.316 | 0.055 | 0.6 | 0.267 | −0.074 | 0.5 | 0.174 | −0.450 | <0.001 | 0.332 | −0.512 | <0.001 | 0.394 |
| Log ILVM f | −0.076 | 0.5 | 0.321 | −0.033 | 0.8 | 0.273 | −0.031 | 0.8 | 0.174 | −0.494 | <0.001 | 0.386 | −0.561 | <0.001 | 0.461 |
| Log ILVM g | −0.023 | 0.8 | 0.316 | 0.125 | 0.3 | 0.279 | 0.009 | 0.9 | 0.179 | −0.470 | <0.001 | 0.373 | −0.552 | <0.001 | 0.455 |
| Log ILVM h | −0.103 | 0.4 | 0.326 | −0.009 | 0.9 | 0.270 | −0.076 | 0.5 | 0.175 | −0.514 | <0.001 | 0.396 | −0.561 | <0.001 | 0.460 |
| PLVMI-BSA | 0.071 | 0.5 | 0.315 | 0.060 | 0.6 | 0.276 | −0.026 | 0.8 | 0.164 | 0.367 | 0.004 | 0.274 | 0.383 | <0.001 | 0.296 |
| PLVMI-ht1.7 | −0.007 | 0.9 | 0.316 | 0.108 | 0.3 | 0.274 | −0.052 | 0.6 | 0.172 | 0.315 | 0.01 | 0.246 | 0.378 | <0.001 | 0.296 |
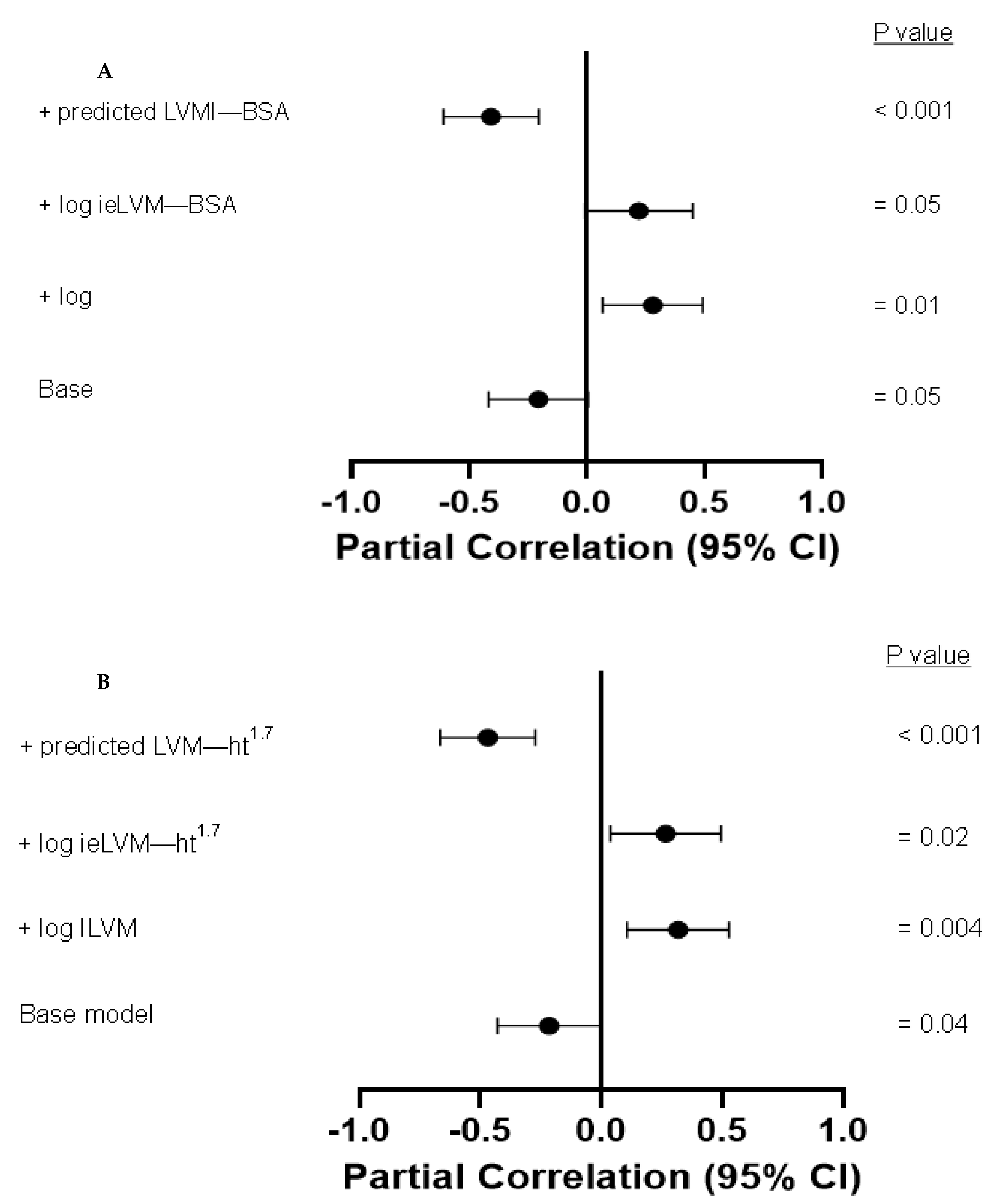
| Log ieLVMI-BSA | −0.037 | 0.7 | 0.309 | 0.126 | 0.3 | 0.274 | −0.078 | 0.5 | 0.165 | −0.238 | 0.08 | 0.201 | −0.373 | <0.001 | 0.335 |
| Log ieLVMI-ht1.7 | −0.048 | 0.7 | 0.313 | 0.132 | 0.3 | 0.278 | −0.095 | 0.4 | 0.171 | −0.245 | 0.07 | 0.204 | −0.389 | <0.001 | 0.361 |
| Model | Partial R | p-Value | Model R2 |
|---|---|---|---|
| Model 1 | |||
| PLVMI-BSA | 0.373 | 0.002 | |
| Log ieLVMI-BSA | −0.331 | <0.001 | 0.415 |
| Model 2 | |||
| PLVMI-ht1.7 | 0.458 | <0.001 | |
| Log ieLVMI-ht1.7 | −0.328 | <0.001 | 0.454 |
| LVM Parameter | Midwall Fractional Shortening | Ejection Fraction | ||||
|---|---|---|---|---|---|---|
| Partial R | p-Value | Model R2 | Partial R | p-Value | Model R2 | |
| Log LVMI-BSA | −0.235 | 0.09 | 0.258 | −0.285 | 0.01 | 0.199 |
| Log LVMI-BSA a | 0.238 | 0.09 | 0.439 | 0.254 | 0.03 | 0.407 |
| Log LVMI-BSA b | 0.057 | 0.7 | 0.273 | 0.149 | 0.3 | 0.381 |
| Log LVM-BSA c | −0.376 | 0.006 | 0.425 | −0.447 | <0.001 | 0.397 |
| Log LVMI-ht1.7 | −0.240 | 0.08 | 0.260 | −0.276 | 0.02 | 0.195 |
| Log LVMI-ht 1.7 a | 0.280 | 0.04 | 0.459 | 0.307 | 0.01 | 0.430 |
| Log LVMI-ht1.7 d | 0.081 | 0.6 | 0.280 | 0.205 | 0.1 | 0.395 |
| Log LVMI-ht1.7 e | −0.425 | 0.002 | 0.446 | −0.489 | <0.001 | 0.421 |
| Log ILVM | −0.505 | <0.001 | 0.413 | −0.524 | <0.001 | 0.371 |
| Log ILVM f | −0.531 | <0.001 | 0.465 | −0.529 | <0.001 | 0.427 |
| Log ILVM g | −0.520 | <0.001 | 0.459 | −0.536 | <0.001 | 0.430 |
| Log ILVM h | −0.452 | <0.001 | 0.468 | −0.522 | <0.001 | 0.421 |
| PLVMI-BSA | 0.389 | 0.004 | 0.331 | 0.362 | 0.002 | 0.245 |
| PLVMI-ht1.7 | 0.376 | 0.006 | 0.323 | 0.350 | 0.004 | 0.239 |
| Log ieLVMI-BSA | −0.284 | 0.05 | 0.270 | −0.453 | <0.001 | 0.367 |
| Log ieLVMI-ht1.7 | −0.291 | 0.05 | 0.275 | −0.452 | <0.001 | 0.368 |
| Model | Standardized β (5% to 95% CI) | p-Value | Model R2 |
|---|---|---|---|
| Model 1 a | |||
| Predicted LVMI-BSA | 0.561 (0.470 to 0.651) | <0.001 | |
| Log ieLVMI-BSA | 0.666 (0.594 to 0.738) | <0.001 | 0.914 |
| Model 2 b | |||
| Predicted LVMI-ht1.7 | 0.544 (0.458 to 0.630) | <0.001 | |
| Log ieLVMI-ht1.7 | 0.670 (0.599 to 0.741) | <0.001 | 0.917 |
| Model | Standardized β (5% to 95% CI) | p-Value | Model R2 |
|---|---|---|---|
| Model 1 a | |||
| Predicted LVMI-BSA | 0.561 (0.470 to 0.651) | <0.001 | |
| Log ieLVMI-BSA | 0.666 (0.594 to 0.738) | <0.001 | 0.914 |
| Model 2 b | |||
| Predicted LVMI-ht1.7 | 0.544 (0.458 to 0.630) | <0.001 | |
| Log ieLVMI-ht1.7 | 0.670 (0.599 to 0.741) | <0.001 | 0.917 |
| LVM Parameter | Cut– | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| LVMI-BSA (g/m2) | 95 | 80 | 61 |
| 117 | 69 | 80 | |
| LVMI-ht1.7 (g/m1.7) | 68 | 80 | 56 |
| 92 | 44 | 80 | |
| ILVM (%) | 144 | 80 | 73 |
| 150 | 69 | 80 | |
| IeLVMI-BSA (g/m2) | 30 | 80 | 73 |
| 38 | 63 | 80 | |
| IeLVMI-ht1.7 (g/m1.7) | 22 | 80 | 71 |
| 30 | 63 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, H.-C.; Tade, G.; Robinson, C.; Dlongolo, N.; Teckie, G.; Solomon, A.; Woodiwiss, A.J.; Dessein, P.H. Associations of Traditionally Determined Left Ventricular Mass Indices and Hemodynamic and Non-Hemodynamic Components of Cardiac Remodeling with Diastolic and Systolic Function in Patients with Chronic Kidney Disease. J. Clin. Med. 2023, 12, 4211. https://doi.org/10.3390/jcm12134211
Hsu H-C, Tade G, Robinson C, Dlongolo N, Teckie G, Solomon A, Woodiwiss AJ, Dessein PH. Associations of Traditionally Determined Left Ventricular Mass Indices and Hemodynamic and Non-Hemodynamic Components of Cardiac Remodeling with Diastolic and Systolic Function in Patients with Chronic Kidney Disease. Journal of Clinical Medicine. 2023; 12(13):4211. https://doi.org/10.3390/jcm12134211
Chicago/Turabian StyleHsu, Hon-Chun, Grace Tade, Chanel Robinson, Noluntu Dlongolo, Gloria Teckie, Ahmed Solomon, Angela Jill Woodiwiss, and Patrick Hector Dessein. 2023. "Associations of Traditionally Determined Left Ventricular Mass Indices and Hemodynamic and Non-Hemodynamic Components of Cardiac Remodeling with Diastolic and Systolic Function in Patients with Chronic Kidney Disease" Journal of Clinical Medicine 12, no. 13: 4211. https://doi.org/10.3390/jcm12134211
APA StyleHsu, H.-C., Tade, G., Robinson, C., Dlongolo, N., Teckie, G., Solomon, A., Woodiwiss, A. J., & Dessein, P. H. (2023). Associations of Traditionally Determined Left Ventricular Mass Indices and Hemodynamic and Non-Hemodynamic Components of Cardiac Remodeling with Diastolic and Systolic Function in Patients with Chronic Kidney Disease. Journal of Clinical Medicine, 12(13), 4211. https://doi.org/10.3390/jcm12134211