Treatment of COVID-19 during the Acute Phase in Hospitalized Patients Decreases Post-Acute Sequelae of COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
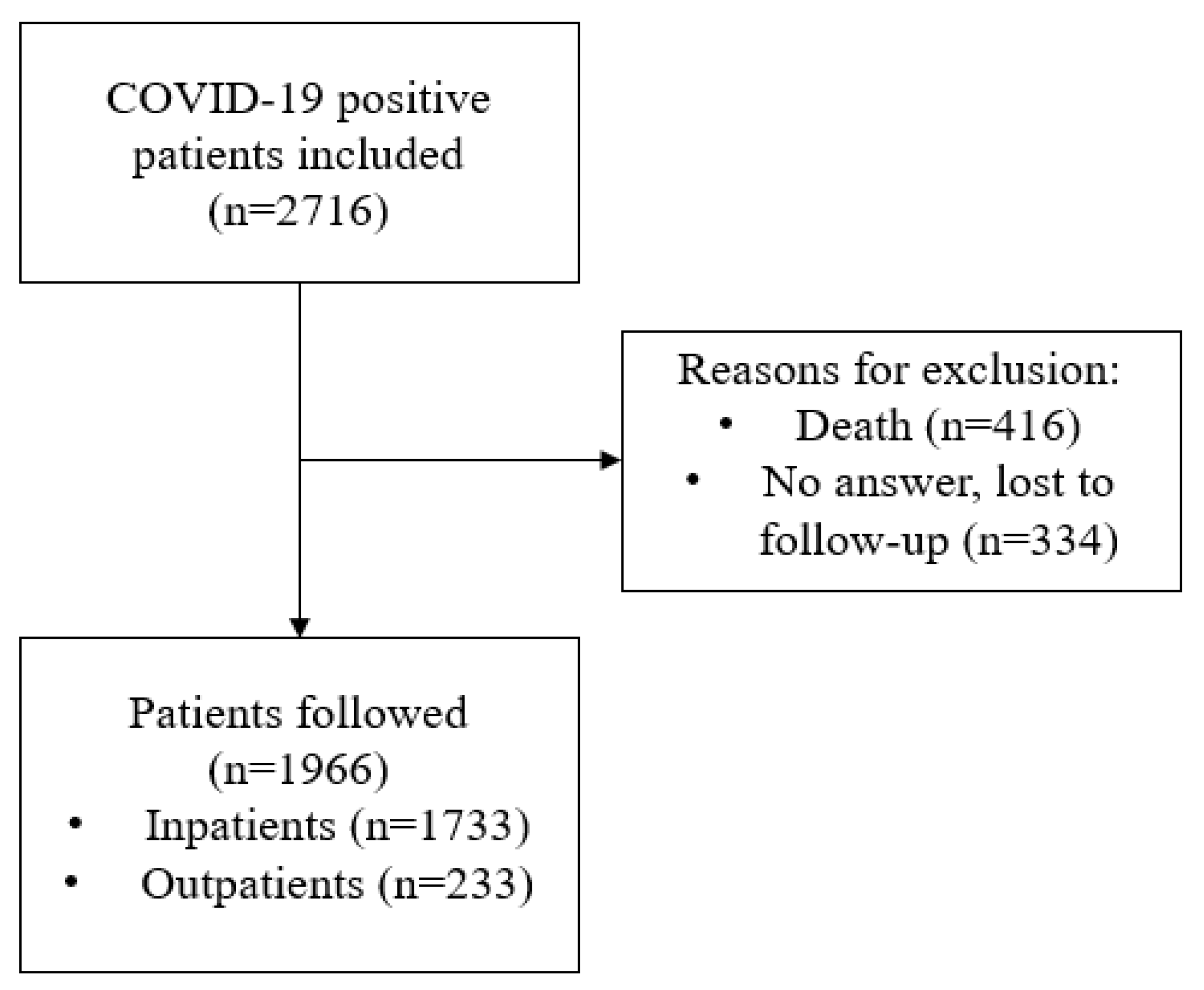
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. COVID-19 Severity Groups
2.4. Treatment Received for SARS-CoV-2 Infection
2.5. Symptom Assessment
2.6. Blood Sampling
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. PASC in Hospitalized Patients
3.2.1. Clinical Data
3.2.2. Laboratory Data
3.2.3. PASC Prediction
3.3. PASC in Outpatients
3.4. Treatment Received
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. MAR Post-COVID-19 Unit
References
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Menges, D.; Ballouz, T.; Anagnostopoulos, A.; Aschmann, H.E.; Domenghino, A.; Fehr, J.S.; Puhan, M.A. Burden of post-COVID-19 syndrome and implications for healthcare service planning: A population-based cohort study. PLoS ONE 2021, 16, e0254523. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Pareek, M. Toward a Universal Definition of Post-COVID-19 Condition-How Do We Proceed? JAMA Netw. Open 2023, 6, e235779. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nature reviews. Microbiology 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Logue, J.K.; Franko, N.M.; McCulloch, D.J.; McDonald, D.; Magedson, A.; Wolf, C.R.; Chu, H.Y. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw. Open 2021, 4, e210830. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F.; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef]
- Cervia, C.; Zurbuchen, Y.; Taeschler, P.; Ballouz, T.; Menges, D.; Hasler, S.; Adamo, S.; Raeber, M.E.; Bächli, E.; Rudiger, A.; et al. Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome. Nat. Commun. 2022, 13, 446. [Google Scholar] [CrossRef]
- Tleyjeh, I.M.; Saddik, B.; AlSwaidan, N.; AlAnazi, A.; Ramakrishnan, R.K.; Alhazmi, D.; Aloufi, A.; AlSumait, F.; Berbari, E.; Halwani, R. Prevalence and predictors of Post-Acute COVID-19 Syndrome (PACS) after hospital discharge: A cohort study with 4 months median follow-up. PLoS ONE 2021, 16, e0260568. [Google Scholar] [CrossRef]
- Xie, Y.; Choi, T.; Al-Aly, Z. Molnupiravir and risk of post-acute sequelae of COVID-19: Cohort study. BMJ 2023, 381, e074572. [Google Scholar] [CrossRef]
- Xie, Y.; Choi, T.; Al-Aly, Z. Association of Treatment With Nirmatrelvir and the Risk of Post-COVID-19 Condition. JAMA Intern. Med. 2023, 183, 554–564. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Sibila, O.; Molina-Molina, M.; Valenzuela, C.; Ríos-Cortés, A.; Arbillaga-Etxarri, A.; Torralba García, Y.; Díaz-Pérez, D.; Landete, P.; Mediano, O.; Tomás López, L.; et al. Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) Consensus for Post-COVID-19 Clinical Follow-Up. Open Respir. Arch. 2020, 2, 278–283. [Google Scholar] [CrossRef]
- Arribas, J.R.; Carolina Garcia-Vidal, C.; Galán Montemayor, J.C.; Paño, J.R.; Rodríguez Baño, J. Recomendaciones SEIMC Para El Manejo Clínico de Pacientes Con COVID-19. Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica 2:1–2. 2020. Available online: https://seimc.org/contenidos/documentoscientificos/recomendaciones/seimc-rc-2020-COVID19-manejoclinico.pdf (accessed on 1 May 2023).
- Lamontagne, F.; Agarwal, A.; Rochwerg, B.; Siemieniuk, R.A.; Agoritsas, T.; Askie, L.; Lytvyn, L.; Leo, Y.S.; Macdonald, H.; Zeng, L.; et al. A living WHO guideline on drugs for COVID-19. BMJ 2020, 370, m3379. [Google Scholar] [CrossRef] [PubMed]
- Seeßle, J.; Waterboer, T.; Hippchen, T.; Simon, J.; Kirchner, M.; Lim, A.; Müller, B.; Merle, U. Persistent Symptoms in Adult Patients 1 Year After Coronavirus Disease 2019 (COVID-19): A Prospective Cohort Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 74, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zheng, B.; Daines, L.; Sheikh, A. Long-Term Sequelae of COVID-19: A Systematic Review and Meta-Analysis of One-Year Follow-Up Studies on Post-COVID Symptoms. Pathogens 2022, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, M.; Szarpak, L.; Rafique, Z.; Peacock, F.W.; Pruc, M.; Szwed, P.; Chirico, F.; Navolokina, A.; Ladny, J.R.; Denegri, A. Prediction Value of KREBS Von Den Lungen-6 (KL-6) Biomarker in COVID-19 Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 6600. [Google Scholar] [CrossRef] [PubMed]
- Fialek, B.; De Roquetaillade, C.; Pruc, M.; Navolokina, A.; Chirico, F.; Ladny, J.R.; Peacock, F.W.; Szarpak, L. Systematic review with meta-analysis of mid-regional pro-adrenomedullin (MR-proadm) as a prognostic marker in COVID-19-hospitalized patients. Ann. Med. 2023, 55, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, M.; Ładny, J.; Rafique, Z.; Peacock, F.; Pruc, M.; Gasecka, A.; Szwed, P.; Jankowski, L.; Chmielewski, J.; Panasiuk, L.; et al. Prediction value of soluble urokinase plasminogen activator receptor (suPAR) in COVID-19 patients—A systematic review and meta-analysis. Ann. Agric. Environ. Med. AAEM 2023, 30, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Cimellaro, A.; Addesi, D.; Cavallo, M.; Spagnolo, F.; Suraci, E.; Cordaro, R.; Spinelli, I.; Passafaro, F.; Colosimo, M.; Pintaudi, M.; et al. Monoclonal Antibodies and Antivirals against SARS-CoV-2 Reduce the Risk of Long COVID: A Retrospective Propensity Score-Matched Case-Control Study. Biomedicines 2022, 10, 3135. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- NIH Therapeutic Management of Adults with COVID-19. Available online: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalizedadults--therapeutic-management/ (accessed on 1 December 2022).
- NICE. Guideline [NG191]. COVID-19 Rapid Guideline: Managing COVID-19. Available online: https://app.magicapp.org/#/guideline/L4Qb5n/section/ERYAXn (accessed on 31 December 2022).
- Bartoletti, M.; Azap, O.; Barac, A.; Bussini, L.; Ergonul, O.; Krause, R.; Paño-Pardo, J.R.; Power, N.R.; Sibani, M.; Szabo, B.G.; et al. ESCMID COVID-19 living guidelines: Drug treatment and clinical management. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2022, 28, 222–238. [Google Scholar] [CrossRef] [PubMed]
- IDSA. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Available online: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatmentand-management/ (accessed on 31 December 2022).
- Garcia-Vidal, C.; Puerta-Alcalde, P.; Mateu, A.; Cuesta-Chasco, G.; Meira, F.; Lopera, C.; Monzo, P.; Santos-Bravo, M.; Duenas, G.; Chumbita, M.; et al. Prolonged viral replication in patients with hematologic malignancies hospitalized with COVID-19. Haematologica 2022, 107, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- Niknam, Z.; Jafari, A.; Golchin, A.; Danesh Pouya, F.; Nemati, M.; Rezaei-Tavirani, M.; Rasmi, Y. Potential therapeutic options for COVID-19: An update on current evidence. Eur. J. Med. Res. 2022, 27, 6. [Google Scholar] [CrossRef]
- Toussirot, E.; Marotte, H.; Mulleman, D.; Cormier, G.; Coury, F.; Gaudin, P.; Dernis, E.; Bonnet, C.; Damade, R.; Grauer, J.L.; et al. Increased high molecular weight adiponectin and lean mass during tocilizumab treatment in patients with rheumatoid arthritis: A 12-month multicentre study. Arthritis Res. Ther. 2020, 22, 224. [Google Scholar] [CrossRef]
- Castillero, E.; Alamdari, N.; Aversa, Z.; Gurav, A.; Hasselgren, P.O. PPARβ/δ regulates glucocorticoid- and sepsis-induced FOXO1 activation and muscle wasting. PLoS ONE 2013, 8, e59726. [Google Scholar] [CrossRef]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Schakman, O.; Kalista, S.; Barbé, C.; Loumaye, A.; Thissen, J.P. Glucocorticoid-induced skeletal muscle atrophy. Int. J. Biochem. Cell Biol. 2013, 45, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Hasan, L.K.; Deadwiler, B.; Haratian, A.; Bolia, I.K.; Weber, A.E.; Petrigliano, F.A. Effects of COVID-19 on the Musculoskeletal System: Clinician’s Guide. Orthop. Res. Rev. 2021, 13, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, C.; Collins, L.F.; Malani, P. Long-term Health Consequences of COVID-19. JAMA 2020, 324, 1723–1724. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee for the COMEBAC Study Group; Morin, L.; Savale, L.; Pham, T.; Colle, R.; Figueiredo, S.; Harrois, A.; Gasnier, M.; Lecoq, A.L.; Meyrignac, O.; et al. Four-Month Clinical Status of a Cohort of Patients After Hospitalization for COVID-19. JAMA 2021, 325, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, X.; Gu, X.; Zhang, H.; Ren, L.; Guo, L.; Liu, M.; Wang, Y.; Cui, D.; Wang, Y.; et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: A longitudinal cohort study. Lancet Respir. Med. 2022, 10, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, H.; Zhou, Y.; Wu, X.; Zhao, Y.; Lu, Y.; Tan, W.; Yuan, M.; Ding, X.; Zou, J.; et al. Risk factors associated with disease severity and length of hospital stay in COVID-19 patients. J. Infect. 2020, 81, e95–e97. [Google Scholar] [CrossRef]
- Wong, A.W.; Shah, A.S.; Johnston, J.C.; Carlsten, C.; Ryerson, C.J. Patient-reported outcome measures after COVID-19: A prospective cohort study. Eur. Respir. J. 2020, 56, 2003276. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Shang, Y.M.; Song, W.B.; Li, Q.Q.; Xie, H.; Xu, Q.F.; Jia, J.L.; Li, L.M.; Mao, H.L.; Zhou, X.M.; et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. eClinicalMedicine 2020, 25, 100463. [Google Scholar] [CrossRef]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing TK, P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk Factors Associated With Post-COVID-19 Condition: A Systematic Review and Meta-analysis. JAMA Int. Med. 2023, 183, e230750. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Shi, X.; Zhu, B.; Zhao, W.; Li, X.; Zou, X.; Liu, G.; Han, X. COVID-19 vaccine uptake, reasons, and associated factors among older adults in Shenzhen, China. Hum. Vaccines Immunother. 2023, 19, 2196914. [Google Scholar] [CrossRef]
- Koller, A.; Szebeni, J. COVID-19 vaccines elicit effective IgG responses in an elderly thymus cancer patient with chemotherapy. Hum. Vaccines Immunother. 2023, 19, 2188035. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid-mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of post-acute COVID-19 in primary care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef]
- Han, X.; Fan, Y.; Alwalid, O.; Li, N.; Jia, X.; Yuan, M.; Li, Y.; Cao, Y.; Gu, J.; Wu, H.; et al. Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia. Radiology 2021, 299, E177–E186. [Google Scholar] [CrossRef]
- Akdis, C.A.; Arkwright, P.D.; Brüggen, M.C.; Busse, W.; Gadina, M.; Guttman-Yassky, E.; Kabashima, K.; Mitamura, Y.; Vian, L.; Wu, J.; et al. Type 2 immunity in the skin and lungs. Allergy 2020, 75, 1582–1605. [Google Scholar] [CrossRef]
- Maurya, R.; Sebastian, P.; Namdeo, M.; Devender, M.; Gertler, A. COVID-19 Severity in Obesity: Leptin and Inflammatory Cytokine Interplay in the Link Between High Morbidity and Mortality. Front. Immunol. 2021, 12, 649359. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Liu, Z.; Tang, L.; Li, L.; Gan, Q.; Shi, H.; Jiao, Q.; Guan, Y.; Xie, M.; He, X.; et al. Longitudinal clinical and radiographic evaluation reveals interleukin-6 as an indicator of persistent pulmonary injury in COVID-19. Int. J. Med. Sci. 2021, 18, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Fialek, B.; Pruc, M.; Smereka, J.; Jas, R.; Rahnama-Hezavah, M.; Denegri, A.; Szarpak, A.; Jaguszewski, M.J.; Peacock, F.W.; Szarpak, L. Diagnostic value of lactate dehydrogenase in COVID-19: A systematic review and meta-analysis. Cardiol. J. 2022, 29, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Szarpak, L.; Ruetzler, K.; Safiejko, K.; Hampel, M.; Pruc, M.; Kanczuga-Koda, L.; Filipiak, K.J.; Jaguszewski, M.J. Lactate dehydrogenase level as a COVID-19 severity marker. Am. J. Emerg. Med. 2021, 45, 638–639. [Google Scholar] [CrossRef] [PubMed]
- Ruetzler, K.; Szarpak, Ł.; Ładny, J.R.; Gąsecka, A.; Gilis-Malinowska, N.; Pruc, M.; Smereka, J.; Nowak, B.; Filipiak, K.J.; Jaguszewski, M.J. D-dimer levels predict COVID-19 severity and mortality. Kardiol. Pol. 2021, 79, 217–218. [Google Scholar] [CrossRef]
- Teuwen, L.A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 2020, 20, 389–391. [Google Scholar] [CrossRef]
- Anka, A.U.; Tahir, M.I.; Abubakar, S.D.; Alsabbagh, M.; Zian, Z.; Hamedifar, H.; Sabzevari, A.; Azizi, G. Coronavirus disease 2019 (COVID-19): An overview of the immunopathology, serological diagnosis and management. Scand. J. Immunol. 2021, 93, e12998. [Google Scholar] [CrossRef]
- Roca, E.; Ventura, L.; Zattra, C.M.; Lombardi, C. EOSINOPENIA: An early, effective and relevant COVID-19 biomarker? QJM Mon. J. Assoc. Physicians 2021, 114, 68–69. [Google Scholar] [CrossRef]
- Cazzaniga, M.; Fumagalli, L.A.M.; D’angelo, L.; Cerino, M.; Bonfanti, G.; Fumagalli, R.M.; Schiavo, G.; Lorini, C.; Lainu, E.; Terragni, S.; et al. Eosinopenia is a reliable marker of severe disease and unfavourable outcome in patients with COVID-19 pneumonia. Int. J. Clin. Pract. 2021, 75, e14047. [Google Scholar] [CrossRef]
- Lourda, M.; Dzidic, M.; Hertwig, L.; Bergsten, H.; Palma Medina, L.M.; Sinha, I.; Kvedaraite, E.; Chen, P.; Muvva, J.R.; Gorin, J.B.; et al. High-dimensional profiling reveals phenotypic heterogeneity and disease-specific alterations of granulocytes in COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2109123118. [Google Scholar] [CrossRef]
- Koenderman, L.; Siemers, M.J.; van Aalst, C.; Bongers, S.H.; Spijkerman, R.; Bindels, B.J.J.; Giustarini, G.; van Goor, H.M.R.; Kaasjager, K.A.H.; Vrisekoop, N. The Systemic Immune Response in COVID-19 Is Associated with a Shift to Formyl-Peptide Unresponsive Eosinophils. Cells 2021, 10, 1109. [Google Scholar] [CrossRef] [PubMed]
- Diny, N.L.; Rose, N.R.; Čiháková, D. Eosinophils in Autoimmune Diseases. Front. Immunol. 2017, 8, 484. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.C.; Humbert, M.; Buggert, M. The known unknowns of T cell immunity to COVID-19. Sci. Immunol. 2020, 5, eabe8063. [Google Scholar] [CrossRef] [PubMed]
- Haslam, A.; Prasad, V. Comparability of Control and Comparison Groups in Studies Assessing Long COVID. Am. J. Med. 2023. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]

| All Patients n = 1966 | Mild n = 1081 (54.9%) (No Supplemental Oxygen FiO2 21%) | Moderate n = 542 (27.6%) (Supplemental Oxygen FiO2 < 40%) | Severe n = 343 (17.5%) (FiO2 > 40%, HFNC, NIV, IMV) | p Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years), mean (SD) | 56.4 (17.1) | 51 (16.5) | 63 (15.8) | 64 (14.0) | <0.001 |
| Females, n (%) | 974 (49.5) | 616 (56.9) | 228 (42.1) | 130 (37.9) | <0.001 |
| Comorbidities, n (%) | |||||
| Arterial hypertension | 578 (29.4) | 181 (16.7) | 235 (43.3) | 162 (47.2) | <0.001 |
| Psychiatric disorders | 379 (19.2) | 182 (16.8) | 117 (21.6) | 80 (23.3) | 0.348 |
| Diabetes Mellitus | 258 (13.1) | 81 (7.5) | 108 (19.9) | 69 (20.1) | <0.001 |
| Obesity | 131 (6.6) | 36 (3.3) | 49 (9.0) | 46 (13.4) | <0.001 |
| Kidney failure | 166 (8.4) | 43 (3.9) | 60 (11.1) | 63 (18.3) | <0.001 |
| COPD | 122 (6.2) | 36 (3.3) | 52 (9.6) | 34 (9.9) | <0.001 |
| Asthma | 120 (6.1) | 64 (5.9) | 38 (7.0) | 18 (5.2) | 0.492 |
| Heart failure | 61 (3.1) | 11 (1.0) | 28 (5.2) | 22 (6.4) | <0.001 |
| Neoplasia | 46 (2.3) | 14 (1.3) | 20 (3.7) | 12 (3.5) | 0.003 |
| Solid organ transplant | 11 (1.0) | 1 (0.9) | 4 (0.7) | 6 (1.7) | 0.004 |
| Persisting symptoms, n (%) | 728 (37.0) | 370 (34.2) | 164 (30.2) | 194 (56.6) | <0.001 |
| Dyspnea | 472 (24.0) | 230 (21.3) | 108 (19.9) | 134 (39.1) | <0.001 |
| Fatigue | 391 (19.8) | 208 (19.2) | 88 (16.1) | 95 (27.7) | <0.001 |
| Myalgia | 237 (12.0) | 126 (11.6) | 47 (8.7) | 64 (18.6) | <0.001 |
| Anxiety symptoms | 227 (11.5) | 139 (12.8) | 48 (8.8) | 40 (11.6) | 0.035 |
| Depressive symptoms | 198 (10.0) | 115 (10.6) | 44 (8.1) | 39 (11.3) | 0.081 |
| Memory loss | 177 (8.9) | 103 (9.5) | 39 (7.2) | 35 (10.2) | 0.087 |
| Motor dysfunction | 162 (8.2) | 72 (6.6) | 32 (5.9) | 58 (17.0) | 0.001 |
| Palpitations | 129 (6.5) | 84 (7.7) | 21 (3.9) | 24 (7.0) | 0.008 |
| Hair loss | 124 (6.3) | 64 (5.9) | 22 (4.1) | 38 (11.1) | <0.001 |
| Anosmia/hyposmia | 121 (6.2) | 79 (7.3) | 25 (4.6) | 17 (5.0) | 0.040 |
| Headache | 119 (6.0) | 86 (8.0) | 16 (3.0) | 14 (4.1) | <0.001 |
| Persistent cough | 117 (5.9) | 67 (6.2) | 20 (3.7) | 30 (8.7) | 0.004 |
| Ageusia/hypogeusia | 106 (5.4) | 65 (6.0) | 21 (3.9) | 20 (5.8) | 0.083 |
| Hypoesthesia | 103 (5.2) | 55 (5.1) | 15 (2.8) | 33 (9.6) | <0.001 |
| Dizziness/instability | 91 (4.6) | 46 (4.2) | 20 (3.7) | 25 (7.3) | 0.015 |
| Audition loss | 73 (3.7) | 42 (3.8) | 15 (2.8) | 16 (4.6) | 0.120 |
| Reduced mobility | 61 (3.1) | 26 (2.4) | 17 (3.1) | 18 (5.2) | 0.015 |
| Chest pain | 56 (2.8) | 29 (2.7) | 14 (2.6) | 13 (3.8) | 0.168 |
| Dysphagia | 52 (2.6) | 24 (2.2) | 14 (2.6) | 14 (4.1) | 0.080 |
| Skin alterations/mouth ulcers | 68 (3.5) | 39 (3.6) | 13 (2.4) | 16 (4.6) | 0.076 |
| Orthopnea | 38 (1.9) | 19 (1.8) | 10 (1.8) | 9 (2.6) | 0.189 |
| Fever | 30 (1.5) | 24 (2.2) | 3 (0.6) | 3 (0.9) | 0.013 |
| Without PASC (n = 1389) | With PASC (n = 344) | p Value | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean (SD) | 56.5 (17.5) | 58.6 (15.6) | 0.047 |
| Females, n (%) | 624 (44.9) | 208 (60.5) | <0.001 |
| Comorbidities, n (%) | |||
| Arterial hypertension | 440 (31.7) | 126 (36.6) | 0.135 |
| Psychiatric disorders | 293 (21.1) | 83 (24.1) | 0.278 |
| Diabetes Mellitus | 202 (14.5) | 52 (15.1) | 0.835 |
| Obesity | 91 (6.6) | 39 (11.3) | 0.004 |
| Kidney failure | 130 (9.4) | 33 (9.6) | 0.978 |
| COPD | 91 (6.6) | 30 (8.7) | 0.179 |
| Asthma | 78 (5.6) | 38 (11.0) | <0.001 |
| Heart failure | 46 (3.3) | 14 (4.1) | 0.593 |
| Neoplasia | 38 (2.7) | 7 (2.0) | 0.459 |
| Solid organ transplant | 6 (0.4) | 5 (1.5) | 0.058 |
| Disease severity, n (%) | <0.001 | ||
| Mild | 754 (53.7) | 143 (41.0) | |
| Moderate | 426 (30.3) | 97 (27.8) | |
| Severe | 224 (16.0) | 109 (31.2) | |
| Persisting symptoms, n (%) | |||
| Dyspnea | 164 (12.4) | 187 (56.7) | <0.001 |
| Fatigue | 108 (8.2) | 175 (53.0) | <0.001 |
| Myalgia | 56 (4.2) | 107 (32.4) | <0.001 |
| Persistent cough | 41 (3.1) | 43 (13.0) | <0.001 |
| Anxiety symptoms | 70 (5.3) | 89 (27.0) | <0.001 |
| Depressive symptoms | 61 (4.6) | 74 (24.0) | <0.001 |
| Memory loss | 65 (4.9) | 56 (17.0) | <0.001 |
| Hair loss | 57 (4.3) | 28 (8.5) | <0.002 |
| Headache | 34 (2.6) | 44 (13.3) | <0.001 |
| Hypoesthesia | 32 (2.4) | 36 (10.9) | <0.001 |
| Ageusia/hypogeusia | 39 (2.9) | 31 (9.4) | <0.001 |
| Anosmia/hyposmia | 46 (3.5) | 38 (11.5) | <0.001 |
| Motor dysfunction | 63 (4.8) | 59 (17.6) | <0.001 |
| Dysphagia | 21 (1.6) | 17 (5.1) | <0.001 |
| Chest pain | 24 (1.8) | 14 (4.2) | 0.009 |
| Audition loss | 24 (1.8) | 26 (7.8) | <0.001 |
| Dizziness/instability | 33 (2.5) | 34 (10.1) | <0.001 |
| Reduced mobility | 26 (1.9) | 26 (7.8) | <0.001 |
| Orthopnea | 20 (1.5) | 11 (3.3) | 0.003 |
| Palpitations | 34 (2.5) | 53 (15.8) | <0.001 |
| Skin alterations/mouth ulcers | 24 (1.8) | 13 (3.9) | 0.020 |
| Fever | 7 (0.5) | 8 (2.4) | <0.001 |
| Blood Test Data, Mean (SD) * | Without PASC (n = 1159) | With PASC (n = 286) | p Value |
|---|---|---|---|
| Baseline value ** | |||
| Glucose (mg/dL) | 124.77 (53.32) | 127.68 (54.98) | 0.414 |
| Creatinine (mg/dL) | 0.97 (0.53) | 0.99 (0.87) | 0.526 |
| LDH (U/L) | 282.79 (110.88) | 299.66 (125.97) | 0.029 |
| Ferritin (ng/mL), median (p25–p75) | 696.50 (367.00–1180.50) | 764.53 (261.00–1203.50) | 0.743 |
| IL-6 (pg/mL), median (p25–p75) | 23.63 (7.60–57.62) | 26.50 (11.40–75.42) | 0.008 |
| Procalcitonin (ng/mL) | 0.70 (5.14) | 0.33 (1.54) | 0.400 |
| CRP (mg/dL) | 7.79 (7.82) | 7.77 (7.63) | 0.968 |
| Total proteins (g/dL) | 6.48 (0.61) | 6.42 (0.59) | 0.178 |
| Total Lymphocytes (×103/μL) | 1.23 (0.84) | 1.08 (0.55) | 0.020 |
| Lymphocytes T (×103/μL) | 1.28 (0.85) | 1.20 (0.61) | 0.130 |
| CD4-T Lymphocyte (×103/μL) | 525.22 (320.77) | 477.34 (292.01) | 0.065 |
| CD8-T Lymphocyte (×103/μL) | 286.37 (213.68) | 253.05 (179.30) | 0.051 |
| Coefficient CD4/CD8 | 2.34 (1.60) | 2.39 (1.92) | 0.719 |
| Neutrophils (×103/μL) | 5.32 (3.22) | 5.37 (2.93) | 0.811 |
| Eosinophils (×103/μL) | 0.05 (0.09) | 0.04 (0.08) | 0.449 |
| D-dimer (μg/L), median (p25–p75) | 630 (390–1050) | 600 (400–1040) | 0.612 |
| Platelets (×103/μL) | 221.62 (85.71) | 236.62 (93.01) | 0.010 |
| Peak value ** | |||
| Glucose (mg/dL) | 116.20 (58.32) | 116.90 (57.92) | 0.861 |
| Creatinine (mg/dL) | 1.04 (0.70) | 1.11 (1.02) | 0.169 |
| LDH (U/L) | 304.75 (123.48) | 332.70 (144.98) | 0.001 |
| Ferritin (ng/mL), median (p25–p75) | 723 (393–972) | 693 (308–951) | 0.688 |
| IL-6 (pg/mL), median (p25–p75) | 16.60 (4.80–53.40) | 25.40 (6.50–71) | 0.120 |
| Procalcitonin (ng/mL) | 0.72 (4.41) | 0.67 (1.83) | 0.905 |
| CRP (mg/dL) | 6.31 (5.72) | 6.28 (5.43) | 0.924 |
| Total proteins (g/dL) | 6.63 (0.57) | 6.59 (0.51) | 0.334 |
| Total Lymphocytes (×103/μL) | 1.31 (1.04) | 1.16 (0.60) | 0.050 |
| Lymphocytes T (×103/μL) | 1.82 (1.02) | 1.97 (0.91) | 0.019 |
| CD4-T Lymphocyte (×103/μL) | 554.12 (340.29) | 513.64 (329.85) | 0.137 |
| CD8-T Lymphocyte (×103/μL) | 300.81 (222.68) | 272.28 (187.62) | 0.101 |
| Coefficient CD4/CD8 | 2.41 (1.62) | 2.48 (1.98) | 0.625 |
| Neutrophils (×103/μL) | 611.07 (1658.34) | 990.23 (2753.85) | 0.029 |
| Eosinophils (×103/μL) | 0.13 (0.19) | 0.19 (0.28) | <0.001 |
| D-dimer (μg/L), median (p25–p75) | 710 (440–950) | 740 (460–915) | 0.814 |
| Platelets (×103/μL) | 325.19 (141.74) | 365.57 (150.91) | <0.001 |
| n = 1432 | OR (95% CI) | p-Value |
|---|---|---|
| Age | 1.00 (0.99, 1.01) | 0.862 |
| Gender | ||
| Male | 1.00 | |
| Female | 2.01 (1.51, 2.67) | <0.001 |
| Asthma | ||
| No | 1.00 | |
| Yes | 2.04 (1.30, 3.20) | 0.002 |
| Obesity | ||
| No | 1.00 | |
| Yes | 1.35 (0.88, 2.08) | 0.170 |
| Psychiatric disorders | ||
| No | 1.00 | |
| Yes | 1.11 (0.81, 1.51) | 0.528 |
| COVID-19 severity | ||
| Mild | 1.00 | |
| Moderate | 1.38 (0.97, 1.96) | 0.074 |
| Severe | 2.48 (1.63, 3.79) | <0.001 |
| Urea (peak), (mg/dL) | ||
| Urea (peak) < 49 | 1.00 | |
| Urea (peak) ≥49 | 1.02 (0.71, 1.46) | 0.915 |
| Lymphocytes T (peak), (×103/μL) | ||
| Lymphocytes T (peak) < 3 | 1.00 | |
| Lymphocytes T (peak) ≥ 3 | 1.09 (0.69, 1.74) | 0.715 |
| Eosinophils T (peak), (×103/μL) | ||
| Eosinophils T (peak) < 0.5 | 1.00 | |
| Eosinophils T (peak) ≥ 0.5 | 1.75 (0.99, 3.12) | 0.056 |
| Neutrophils (peak), (×103/μL) | ||
| Neutrophils (peak) < 7 | 1.00 | |
| Neutrophils (peak) ≥ 7 | 1.37 (0.98, 1.92) | 0.068 |
| Platelets (peak), (×103/μL) | ||
| Platelets (peak) < 410 | 1.00 | |
| Platelets (peak) ≥ 410 | 1.15 (0.84, 1.57) | 0.382 |
| Without PASC (n = 112) | With PASC (n = 121) | p Value | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean (SD) | 52.30 (15.86) | 52.26 (15.05) | 0.981 |
| Females, n (%) Males, n (%) | 52 (46.4%) 60 (53.5%) | 90 (74.4%) 31 (25.6%) | <0.001 <0.001 |
| Comorbidities, n (%) | |||
| Arterial hypertension | 7 (6.3%) | 5 (4.1%) | 0.465 |
| Psychiatric disorders | 19 (18.1%) | 44 (38.9%) | 0.001 |
| Diabetes Mellitus | 2 (1.8%) | 2 (1.7%) | 0.938 |
| Obesity | 0 (0.0%) | 1 (12.5%) | 0.191 |
| Kidney failure | 2 (15.4%) | 1 (12.5%) | 0.854 |
| COPD | 1 (0.9%) | 0 (0.0%) | 0.298 |
| Asthma | 2 (15.4%) | 2 (25.0%) | 0.586 |
| Heart failure | 1 (7.7%) | 0 (0.0%) | 0.421 |
| Neoplasia | 1 (0.9%) | 0 (0.0%) | 0.298 |
| Solid organ transplant | 0 (0%) | 0 (0%) | |
| Persisting symptoms, n (%) | |||
| Dyspnea | 43 (41.0%) | 78 (69.0%) | <0.001 |
| Fatigue | 27 (25.7%) | 81 (71.7%) | <0.001 |
| Myalgia | 18 (17.1%) | 56 (49.6%) | <0.001 |
| Persistent cough | 10 (9.5%) | 23 (20.4%) | 0.026 |
| Anxiety symptoms | 14 (13.3%) | 54 (47.8%) | <0.001 |
| Depressive symptoms | 19 (18.1%) | 44 (38.9%) | 0.001 |
| Memory loss | 14 (13.3%) | 42 (37.2%) | <0.001 |
| Hair loss | 20 (19.0%) | 19 (16.8%) | 0.667 |
| Headache | 10 (9.5%) | 31 (27.4%) | 0.001 |
| Hypoesthesia | 11 (10.5%) | 24 (21.2%) | 0.031 |
| Ageusia/hypogeusia | 18 (17.1%) | 18 (15.9%) | 0.809 |
| Anosmia/hyposmia | 19 (18.1%) | 18 (15.9%) | 0.670 |
| Motor dysfunction | 19 (18.1%) | 21 (18.6%) | 0.926 |
| Dysphagia | 5 (4.8%) | 9 (8.0%) | 0.335 |
| Chest pain | 6 (5.7%) | 12 (10.6%) | 0.189 |
| Audition loss | 12 (11.4%) | 12 (10.6%) | 0.849 |
| Dizziness/instability | 6 (5.7%) | 19 (16.8%) | 0.010 |
| Reduced mobility | 1 (1.0%) | 9 (8.0%) | 0.013 |
| Orthopnea | 1 (1.0%) | 6 (5.3%) | 0.068 |
| Palpitations | 11 (10.5%) | 34 (30.1%) | <0.001 |
| Skin alterations/mouth ulcers | 12 (11.4%) | 19 (16.8%) | 0.255 |
| Fever | 1 (1.0%) | 15 (13.3%) | <0.001 |
| Moderate Disease | Severe Disease | ||||
|---|---|---|---|---|---|
| Treatments | n | p50 (p25–p75) * | n | p50 (p25–p75) | |
| Remdesivir | No | 472 | 262 (217, 304) | 296 | 284 (216, 336) |
| Yes | 43 | 109 (45, 143) | 36 | 187 (131, 188) | |
| Tocilizumab or Sarilumab | No | 466 | 256 (143, 303) | 193 | 292 (216, 336) |
| Yes | 49 | 252 (150, 298) | 139 | 269 (175, 325) | |
| Methylprednisolone or Prednisone | No | 395 | 256 (138, 300) | 126 | 255 (158, 312) |
| Yes | 120 | 252 (153, 308) | 206 | 294 (220, 336) | |
| Dexamethasone | No | 285 | 271 (236, 308) | 87 | 309 (239, 341) |
| Yes | 230 | 133 (63, 298) | 245 | 266 (158, 330) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badenes Bonet, D.; Caguana Vélez, O.A.; Duran Jordà, X.; Comas Serrano, M.; Posso Rivera, M.; Admetlló, M.; Herranz Blasco, A.; Cuadrado Godia, E.; Marco Navarro, E.; Martin Ezquerra, G.; et al. Treatment of COVID-19 during the Acute Phase in Hospitalized Patients Decreases Post-Acute Sequelae of COVID-19. J. Clin. Med. 2023, 12, 4158. https://doi.org/10.3390/jcm12124158
Badenes Bonet D, Caguana Vélez OA, Duran Jordà X, Comas Serrano M, Posso Rivera M, Admetlló M, Herranz Blasco A, Cuadrado Godia E, Marco Navarro E, Martin Ezquerra G, et al. Treatment of COVID-19 during the Acute Phase in Hospitalized Patients Decreases Post-Acute Sequelae of COVID-19. Journal of Clinical Medicine. 2023; 12(12):4158. https://doi.org/10.3390/jcm12124158
Chicago/Turabian StyleBadenes Bonet, Diana, Oswaldo Antonio Caguana Vélez, Xavier Duran Jordà, Merce Comas Serrano, Margarita Posso Rivera, Mireia Admetlló, Anna Herranz Blasco, Elisa Cuadrado Godia, Ester Marco Navarro, Gemma Martin Ezquerra, and et al. 2023. "Treatment of COVID-19 during the Acute Phase in Hospitalized Patients Decreases Post-Acute Sequelae of COVID-19" Journal of Clinical Medicine 12, no. 12: 4158. https://doi.org/10.3390/jcm12124158
APA StyleBadenes Bonet, D., Caguana Vélez, O. A., Duran Jordà, X., Comas Serrano, M., Posso Rivera, M., Admetlló, M., Herranz Blasco, A., Cuadrado Godia, E., Marco Navarro, E., Martin Ezquerra, G., Pineiro Aguin, Z., Cumpli Gargallo, M. C., Gonzalez Garcia, J. G., Balcells Vilarnau, E., Rodriguez Chiaradia, D., Castells, X., Gea, J., Horcajada, J. P., & Villar-García, J., on behalf of MAR Post-COVID-19 Unit. (2023). Treatment of COVID-19 during the Acute Phase in Hospitalized Patients Decreases Post-Acute Sequelae of COVID-19. Journal of Clinical Medicine, 12(12), 4158. https://doi.org/10.3390/jcm12124158









