Association of Temporalis Muscle Mass with Early Cognitive Impairment in Older Patients with Acute Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. TMT Measurement Using Brain MRI (T2-Weighted Image)
2.3. Cognitive Evaluation
2.4. Clinical Examinations
2.5. Statistical Analysis
3. Results
3.1. Participants and Characteristics
3.2. Parameters According to the MoCA-Defined Subgroups
3.3. Univariate and Multivariate Linear Regression Analyses of Predictors Associated with Early Post-Stroke Cognitive Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaillard, A.; Naegele, B.; Trabucco-Miguel, S.; LeBas, J.F.; Hommel, M. Hidden dysfunctioning in subacute stroke. Stroke 2009, 40, 2473–2479. [Google Scholar] [CrossRef]
- Pendlebury, S.T.; Rothwell, P.M. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009, 8, 1006–1018. [Google Scholar] [CrossRef]
- Suda, S.; Nishimura, T.; Ishiwata, A.; Muraga, K.; Aoki, J.; Kanamaru, T.; Suzuki, K.; Sakamoto, Y.; Katano, T.; Nishiyama, Y.; et al. Early cognitive impairment after minor stroke: Associated factors and functional outcome. J. Stroke Cerebrovasc. Dis. 2020, 29, 104749. [Google Scholar] [CrossRef]
- Kiyohara, T.; Kumai, Y.; Yubi, T.; Ishikawa, E.; Wakisaka, Y.; Ago, T.; Kitazono, T. Association between Early Cognitive Impairment and Short-Term Functional Outcome in Acute Ischemic Stroke. Cerebrovasc. Dis. 2023, 52, 61–67. [Google Scholar] [CrossRef]
- Bertschi, D.; Kiss, C.M.; Beerli, N.; Kressig, R.W. Sarcopenia in hospitalized geriatric patients: Insights into prevalence and associated parameters using new EWGSOP2 guidelines. Eur. J. Clin. Nutr. 2021, 75, 653–660. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Nagano, A.; Shimizu, A.; Maeda, K.; Ueshima, J.; Inoue, T.; Murotani, K.; Ishida, Y.; Mori, N. Predictive Value of Temporal Muscle Thickness for Sarcopenia after Acute Stroke in Older Patients. Nutrients 2022, 14, 5048. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Yoshida, M.; Sato, A.; Fujimoto, Y.; Minematsu, T.; Sugama, J.; Sanada, H. Temporal muscle thickness as a new indicator of nutritional status in older individuals. Geriatr. Gerontol. Int. 2019, 19, 135–140. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Yoshida, M.; Sato, A.; Fujimoto, Y.; Minematsu, T.; Sugama, J.; Sanada, H. A change in temporal muscle thickness is correlated with past energy adequacy in bedridden older adults: A prospective cohort study. BMC Geriatr. 2021, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Abzhandadze, T.; Rafsten, L.; Lundgren Nilsson, Å.; Palstam, A.; Sunnerhagen, K.S. Very early MoCA can predict functional dependence at 3 months after stroke: A longitudinal, cohort study. Front. Neurol. 2019, 10, 1051. [Google Scholar] [CrossRef]
- Li, W.; Yue, T.; Liu, Y. New understanding of the pathogenesis and treatment of stroke-related sarcopenia. Biomed. Pharmacother. 2020, 131, 110721. [Google Scholar] [CrossRef]
- Heruti, R.J.; Lusky, A.; Dankner, R.; Ring, H.; Dolgopiat, M.; Barell, V.; Levenkrohn, S.; Adunsky, A. Rehabilitation outcome of elderly patients after a first stroke: Effect of cognitive status at admission on the functional outcome. Arch. Phys. Med. Rehabil. 2002, 83, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Coshall, C.; Rudd, A.G.; Wolfe, C.D. Natural history of cognitive impairment after stroke and factors associated with its recovery. Clin. Rehabil. 2003, 17, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, M.; Kakizawa, Y.; Nishikawa, A.; Yamamoto, Y.; Uchiyama, T.; Agata, M.; Wada, N.; Kawamura, S.; Koh, A. Temporal Muscle and Stroke-A Narrative Review on Current Meaning and Clinical Applications of Temporal Muscle Thickness, Area, and Volume. Nutrients 2022, 14, 687. [Google Scholar] [CrossRef]
- Furtner, J.; Berghoff, A.S.; Albtoush, O.M.; Woitek, R.; Asenbaum, U.; Prayer, D.; Widhalm, G.; Gatterbauer, B.; Dieckmann, K.; Birner, P.; et al. Survival prediction using temporal muscle thickness measurements on cranial magnetic resonance images in patients with newly diagnosed brain metastases. Eur. Radiol. 2017, 27, 3167–3173. [Google Scholar] [CrossRef] [PubMed]
- Furtner, J.; Berghoff, A.S.; Schöpf, V.; Reumann, R.; Pascher, B.; Woitek, R.; Asenbaum, U.; Pelster, S.; Leitner, J.; Widhalm, G.; et al. Temporal muscle thickness is an independent prognostic marker in melanoma patients with newly diagnosed brain metastases. J. Neurooncol. 2018, 140, 173–178. [Google Scholar] [CrossRef]
- Furtner, J.; Genbrugge, E.; Gorlia, T.; Bendszus, M.; Nowosielski, M.; Golfinopoulos, V.; Weller, M.; Van Den Bent, M.J.; Wick, W.; Preusser, M. Temporal muscle thickness is an independent prognostic marker in patients with progressive glioblastoma: Translational imaging analysis of the EORTC 26101 trial. Neuro Oncol. 2019, 21, 1587–1594. [Google Scholar] [CrossRef]
- Chiti, G.; Pantoni, L. Use of Montreal Cognitive Assessment in patients with stroke. Stroke 2014, 45, 3135–3140. [Google Scholar] [CrossRef]
- Jaywant, A.; Toglia, J.; Gunning, F.M.; O’Dell, M.W. Subgroups defined by the Montreal Cognitive Assessment differ in functional gain during acute inpatient stroke rehabilitation. Arch. Phys. Med. Rehabil. 2020, 101, 220–226. [Google Scholar] [CrossRef]
- Nasreddine, Z.; Lee, J. Korean Version of the Montreal Cognitive Assessment. 2006. [Google Scholar]
- Terwee, C.B.; Bot, S.D.; de Boer, M.R.; van der Windt, D.A.; Knol, D.L.; Dekker, J.; Bouter, L.M.; de Vet, H.C. Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiol. 2007, 60, 34–42. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, R.F.; Hillis, A.E. Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet Neurol. 2010, 9, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Snowdon, D.A.; Greiner, L.H.; Mortimer, J.A.; Riley, K.P.; Greiner, P.A.; Markesbery, W.R. Brain infarction and the clinical expression of Alzheimer disease: The Nun Study. JAMA 1997, 277, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.X.; Williams, L.J.; Holloway-Kew, K.L.; Hyde, N.K.; Anderson, K.B.; Tembo, M.C.; Addinsall, A.B.; Leach, S.; Pasco, J.A. Skeletal muscle density and cognitive function: A cross-sectional study in men. Calcif. Tissue Int. 2021, 108, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Akagi, J. Cognitive impairment is independently associated with definitive and possible sarcopenia in hospitalized older adults: The prevalence and impact of comorbidities. Geriatr. Gerontol. Int. 2017, 17, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Wirth, R.; Smoliner, C.; Sieber, C.; Volkert, D. Cognitive function is associated with body composition and nutritional risk cognitive function is associated with body composition and nutritional risk of geriatric patients. J. Nutr. Health Aging 2011, 15, 706–710. [Google Scholar] [CrossRef]
- Sui, S.X.; Williams, L.J.; Holloway-Kew, K.L.; Hyde, N.K.; Pasco, J.A. Skeletal muscle health and cognitive function: A narrative review. Int. J. Mol. Sci. 2020, 22, 255. [Google Scholar] [CrossRef]
- Sui, S.X.; Hordacre, B.; Pasco, J.A. Are sarcopenia and cognitive dysfunction comorbid after stroke in the context of brain–muscle crosstalk? Biomedicines 2021, 9, 223. [Google Scholar] [CrossRef]
- Oudbier, S.J.; Goh, J.; Looijaard, S.M.L.M.; Reijnierse, E.M.; Meskers, C.G.M.; Maier, A.B. Pathophysiological mechanisms explaining the association between low skeletal muscle mass and cognitive function. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1959–1968. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, X. Post-stroke cognitive impairment: A review focusing on molecular biomarkers. J. Mol. Neurosci. 2020, 70, 1244–1254. [Google Scholar] [CrossRef]
- Silakarma, D.; Sudewi, A.A.R. The role of brain-derived neurotrophic factor (BDNF) in cognitive functions. Bali Med. J. 2019, 8, 518–525. [Google Scholar] [CrossRef]
- Roh, E.; Hwang, S.Y.; Song, E.; Park, M.J.; Yoo, H.J.; Baik, S.H.; Kim, M.; Won, C.W.; Choi, K.M. Association of plasma brain-derived neurotrophic factor levels and frailty in community-dwelling older adults. Sci. Rep. 2022, 12, 18605. [Google Scholar] [CrossRef]
- Halloway, S.; Jung, M.; Yeh, A.-Y.; Liu, J.; McAdams, E.; Barley, M.; Dorsey, S.G.; Pressler, S.J. An integrative review of brain-derived neurotrophic factor and serious cardiovascular conditions. Nurs. Res. 2020, 69, 376–390. [Google Scholar] [CrossRef]
- Laurin, D.; Verreault, R.; Lindsay, J.; MacPherson, K.; Rockwood, K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch. Neurol. 2001, 58, 498–504. [Google Scholar] [CrossRef]
- Guimarães, F.C.; dos Santos Amorim, P.R.; Dos Reis, F.F.; Bonoto, R.T.; De Oliveira, W.C.; da Silva Moura, T.A.; De Assis, C.L.; Palotás, A.; Lima, L.M. Physical activity and better medication compliance improve mini-mental state examination scores in the elderly. Dement. Geriatr. Cogn. Disord. 2015, 39, 25–31. [Google Scholar] [CrossRef]
- Bowden Davies, K.A.; Pickles, S.; Sprung, V.S.; Kemp, G.J.; Alam, U.; Moore, D.R.; Tahrani, A.A.; Cuthbertson, D.J. Reduced physical activity in young and older adults: Metabolic and musculoskeletal implications. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819888824. [Google Scholar] [CrossRef] [PubMed]
- Lytle, M.E.; Vander Bilt, J.; Pandav, R.S.; Dodge, H.H.; Ganguli, M. Exercise level and cognitive decline: The MoVIES project. Alzheimer Dis. Assoc. Disord. 2004, 18, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Andel, R.; Crowe, M.; Pedersen, N.L.; Fratiglioni, L.; Johansson, B.; Gatz, M. Physical exercise at midlife and risk of dementia three decades later: A population-based study of Swedish twins. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Doorduijn, A.S.; de van der Schueren, M.A.; van de Rest, O.; de Leeuw, F.A.; Hendriksen, H.M.; Teunissen, C.E.; Scheltens, P.; van der Flier, W.M.; Visser, M. Nutritional Status Is Associated With Clinical Progression in Alzheimer ’ s Disease: The NUDAD Project. J. Am. Med. Dir. Assoc. 2023, 24, 638–644.e1. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Yu, W.; Liu, X.; Wan, T.; Chen, C.; Xiong, L.; Zhang, W.; Lü, Y. Associations between malnutrition and cognitive impairment in an elderly Chinese population: An analysis based on a 7-year database. Psychogeriatrics 2021, 21, 80–88. [Google Scholar] [CrossRef]
- Onodera, H.; Mogamiya, T.; Matsushima, S.; Sase, T.; Kawaguchi, K.; Nakamura, H.; Sakakibara, Y. High protein intake after subarachnoid hemorrhage improves oral intake and temporal muscle volume. Clin. Nutr. 2021, 40, 4187–4191. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Mathuranath, P. Issues in evaluation of cognition in the elderly in developing countries. Ann. Indian Acad. Neurol. 2008, 11, 82–88. [Google Scholar] [PubMed]
- Porrselvi, A.; Shankar, V. Status of cognitive testing of adults in India. Ann. Indian Acad. Neurol. 2017, 20, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Park, M.; Moon, W.J.; Han, S.H.; Moon, Y. Sarcopenia in patients with dementia: Correlation of temporalis muscle thickness with appendicular muscle mass. Neurol. Sci. 2022, 43, 3089–3095. [Google Scholar] [CrossRef]
- Ten Cate, C.; Huijs, S.M.H.; Willemsen, A.C.H.; Pasmans, R.C.O.S.; Eekers, D.B.P.; Zegers, C.M.L.; Ackermans, L.; Beckervordersandforth, J.; van Raak, E.P.M.; Anten, M.H.M.E.; et al. Correlation of reduced temporal muscle thickness and systemic muscle loss in newly diagnosed glioblastoma patients. J. Neurooncol. 2022, 160, 611–618. [Google Scholar] [CrossRef]
- Jorm, A.; Jacomb, P. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Socio-demographic correlates, reliability, validity and some norms. Psychol. Med. 1989, 19, 1015–1022. [Google Scholar] [CrossRef]
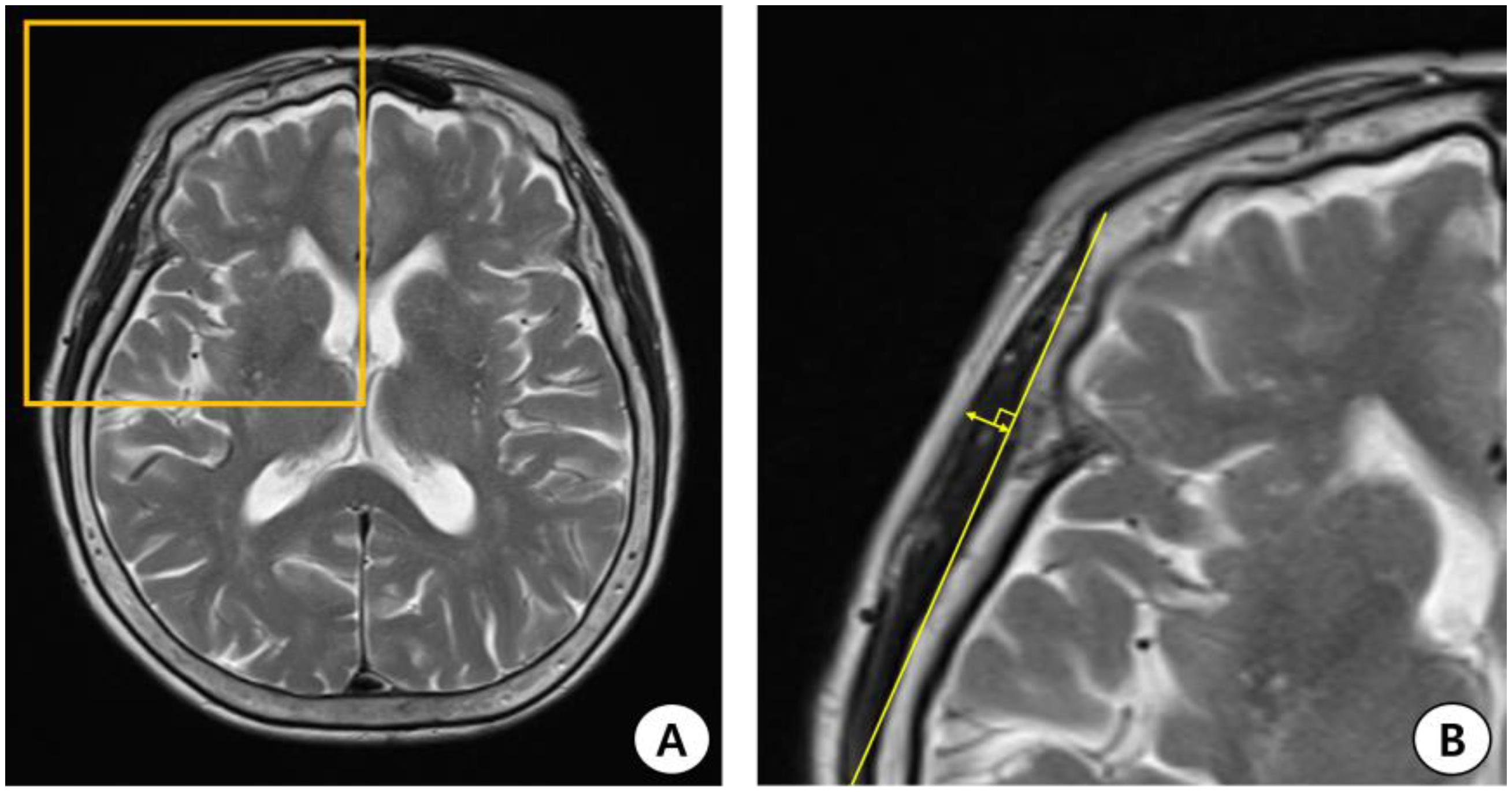
| MoCA Group | Normal to Mildly Impaired | Moderately Impaired | Severely Impaired | Total | p |
|---|---|---|---|---|---|
| (n = 18) | (n = 49) | (n = 59) | (n = 126) | ||
| Age, median [IQR] | 71.5 [66.0–74.0] | 77.0 [72.0–82.0] * | 80.0 [76.5–84.5] *† | 79.0 [72.0–82.0] | <0.001 |
| Sex, n (%) | 0.041 | ||||
| Male | 11 (61.1%) | 23 (46.9%) | 18 (30.5%) * | 52 (41.3%) | |
| Female | 7 (38.9%) | 26 (53.1%) | 41 (69.5%) | 74 (58.7%) | |
| Body mass index, kg/m2 | 24.9 (24.0–27.7) | 23.7 (21.4–26.0) | 22.9 (20.3–25.6) * | 23.6 (20.8–26.0) | 0.039 |
| Premorbid mRS score | 0.423 | ||||
| 0 | 13 (72.2%) | 41 (83.7%) | 43 (72.9%) | 97 (77.0%) | |
| 1 | 2 (11.1%) | 5 (10.2%) | 5 (8.5%) | 12 (9.5%) | |
| 2 | 3 (16.7%) | 3 (6.1%) | 11 (18.6%) | 17 (13.5%) | |
| Lesion side, n (%) | 0.321 | ||||
| Right | 9 (50.0%) | 28 (57.1%) | 25 (42.3%) | 62 (49.2%) | |
| Left | 9 (50.0%) | 19 (38.8%) | 28 (47.5%) | 56 (44.4%) | |
| Bilateral | 0 (0.0%) | 2 (4.1%) | 6 (10.2%) | 8 (6.4%) | |
| Lesion site, n (%) | 0.668 | ||||
| Supratentorial | 13 (72.2%) | 34 (69.4%) | 44 (74.6%) | 91 (72.2%) | |
| Infratentorial | 5 (27.8%) | 14 (28.6%) | 12 (20.3%) | 31 (24.6%) | |
| Both | 0 (0.0%) | 1 (2.0%) | 3 (5.1%) | 4 (3.2%) | |
| Stroke severity (NIHSS) | 4.0 (2.0–5.0) | 3.0 (1.0–6.0) | 5.0 (3.0–9.5) *† | 4.0 (2.0–7.0) | 0.001 |
| Cerebral atrophy, n (%) | 7 (38.9%) | 24 (49.0%) | 35 (59.3%) | 66 (52.4%) | 0.262 |
| Fazekas grade of PVH, grade | 0.138 | ||||
| Grade 0 | 1 (5.6%) | 1 (2.0%) | 0 (0.0%) | 2 (1.6%) | |
| Grade 1 | 8 (44.4%) | 15 (30.6%) | 12 (20.3%) | 35 (27.8%) | |
| Grade 2 | 7 (38.9%) | 23 (47.0%) | 27 (45.8%) | 57 (45.2%) | |
| Grade 3 | 2 (11.1%) | 10 (20.4%) | 20 (33.9%) | 32 (25.4%) | |
| Recurrent stroke, n (%) | 5 (27.8%) | 10 (20.4%) | 20 (33.9%) | 35 (27.8%) | 0.297 |
| CCI, point | 3.5 (3.0–4.0) | 4.0 (3.0–5.0) | 5.0 (4.0–5.0) * | 4.0 (3.0–5.0) | 0.003 |
| Hypertension, n (%) | 11 (61.1%) | 33 (67.3%) | 38 (64.4%) | 82 (65.1%) | 0.884 |
| Diabetes mellitus, n (%) | 6 (33.3%) | 19 (38.8%) | 26 (44.1%) | 51 (40.5%) | 0.685 |
| Afib, n (%) | 4 (22.2%) | 8 (16.3%) | 6 (10.2%) | 18 (14.3%) | 0.385 |
| Year of education, year | 12.0 (9.0–12.0) | 6.0 (4.0–9.0) * | 6.0 (1.5–8.5) * | 6.0 (3.0–9.0) | <0.001 |
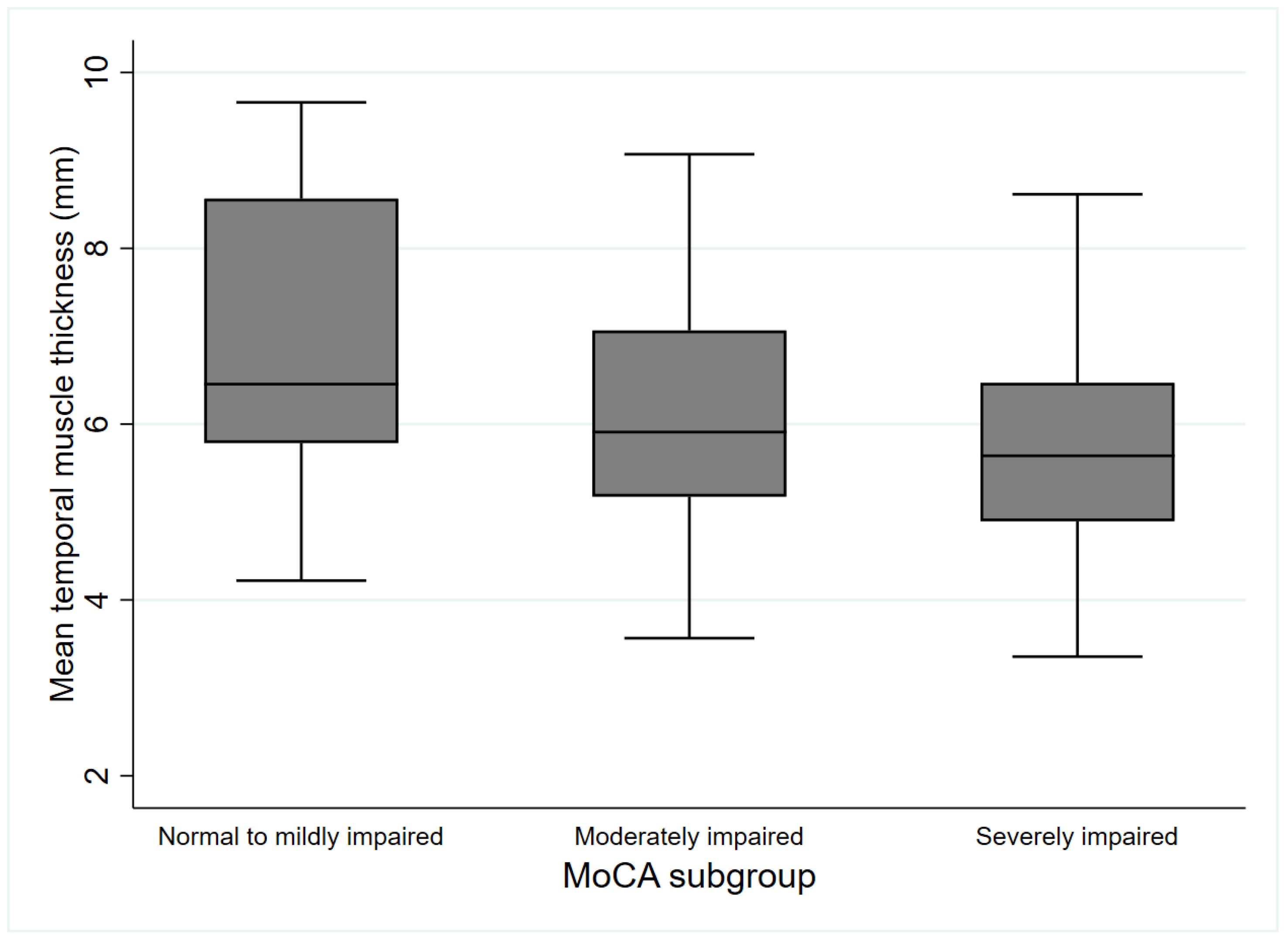
| MoCA scores, point | 22.5 (21.0–24.0) | 14.0 (11.0–17.0) * | 3.0 (1.0–6.0) *† | 10.0 (3.0–17.0) | <0.001 |
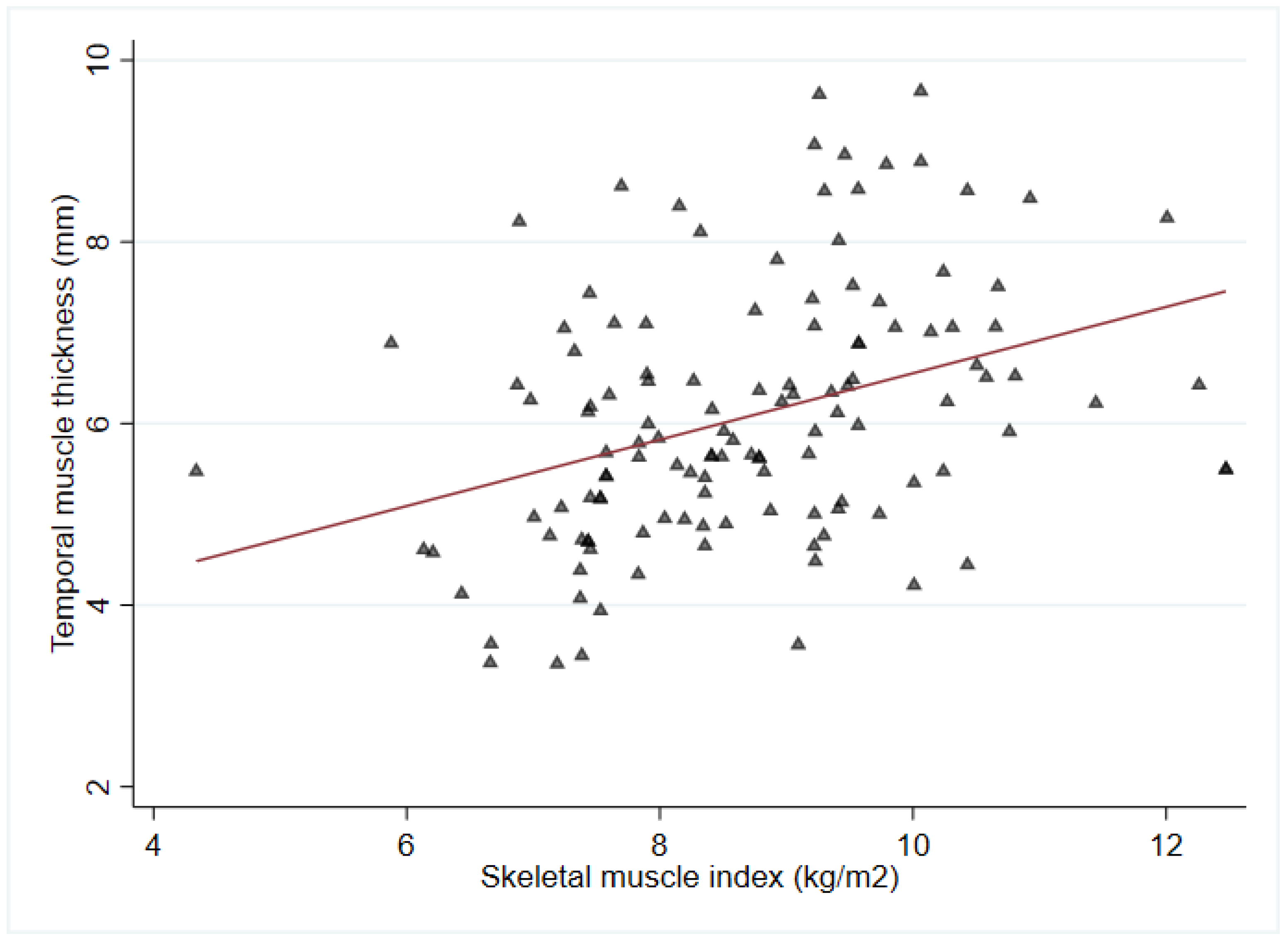
| SMI, kg/m2 | 9.7 (9.3–10.3) | 9.0 (7.9–9.6) * | 8.0 (7.4–9.1) * | 8.7 (7.6–9.5) | <0.001 |
| Mean TMT (mm) | 6.8 ± 1.7 | 6.2 ± 1.3 | 5.7 ± 1.3 * | 6.1 ± 1.4 | 0.01 |
| CRP, median [IQR] (mg/L) | 2.2 [0.8–7.9] | 1.7 [0.7–3.2] | 1.9 [0.6–13.4] | 1.8 [0.7–6.6] | 0.631 |
| Independent Variable | Unstandardized Coefficients | Standardized Beta Coefficients (β) | |||||
|---|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | VIF | |
| Age, year | −0.510 | 0.090 | <0.001 * | −0.27 | 0.097 | 0.006 * | 1.487 |
| Sex [male] | −3.523 | 1.322 | 0.009 * | 0.154 | 1.234 | 0.901 | 1.329 |
| BMI, kg/m2 | 0.371 | 0.152 | 0.016 * | 0.189 | 0.135 | 0.164 | 1.227 |
| CCI, point | −1.746 | 0.416 | <0.001 * | −0.512 | 0.416 | 0.221 | 1.415 |
| NIHSS, point | −0.520 | 0.116 | <0.001 * | −0.298 | 0.109 | 0.007 * | 1.223 |
| History of stroke | −0.912 | 1.492 | <0.001 * | −1.927 | 1.253 | 0.127 | 1.134 |
| Education year, year | 0.701 | 0.138 | <0.001 * | 0.38 | 0.140 | 0.008 * | 1.375 |
| CRP, (mg/L) | −0.034 | 0.027 | 0.211 | −0.034 | 0.024 | 0.162 | 1.208 |
| TMT, mm | −0.910 | 0.456 | <0.001 * | 1.040 | 0.430 | 0.017 * | 1.296 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namgung, H.-g.; Hong, S.; Choi, Y.-A. Association of Temporalis Muscle Mass with Early Cognitive Impairment in Older Patients with Acute Ischemic Stroke. J. Clin. Med. 2023, 12, 4071. https://doi.org/10.3390/jcm12124071
Namgung H-g, Hong S, Choi Y-A. Association of Temporalis Muscle Mass with Early Cognitive Impairment in Older Patients with Acute Ischemic Stroke. Journal of Clinical Medicine. 2023; 12(12):4071. https://doi.org/10.3390/jcm12124071
Chicago/Turabian StyleNamgung, Ho-geon, Seungho Hong, and Young-Ah Choi. 2023. "Association of Temporalis Muscle Mass with Early Cognitive Impairment in Older Patients with Acute Ischemic Stroke" Journal of Clinical Medicine 12, no. 12: 4071. https://doi.org/10.3390/jcm12124071
APA StyleNamgung, H.-g., Hong, S., & Choi, Y.-A. (2023). Association of Temporalis Muscle Mass with Early Cognitive Impairment in Older Patients with Acute Ischemic Stroke. Journal of Clinical Medicine, 12(12), 4071. https://doi.org/10.3390/jcm12124071






