Bone Morphogenic Protein and Mesenchymal Stem Cells to Regenerate Bone in Calvarial Defects: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Study Selection and Data Collection Process
2.4. Risk Bias Assessment
3. Results
3.1. Study Selection and Characteristics
3.2. Area of Bone Regeneration and Bone Quality among the Included Studies
3.2.1. Mice Models
3.2.2. Rat Models
4. Discussion
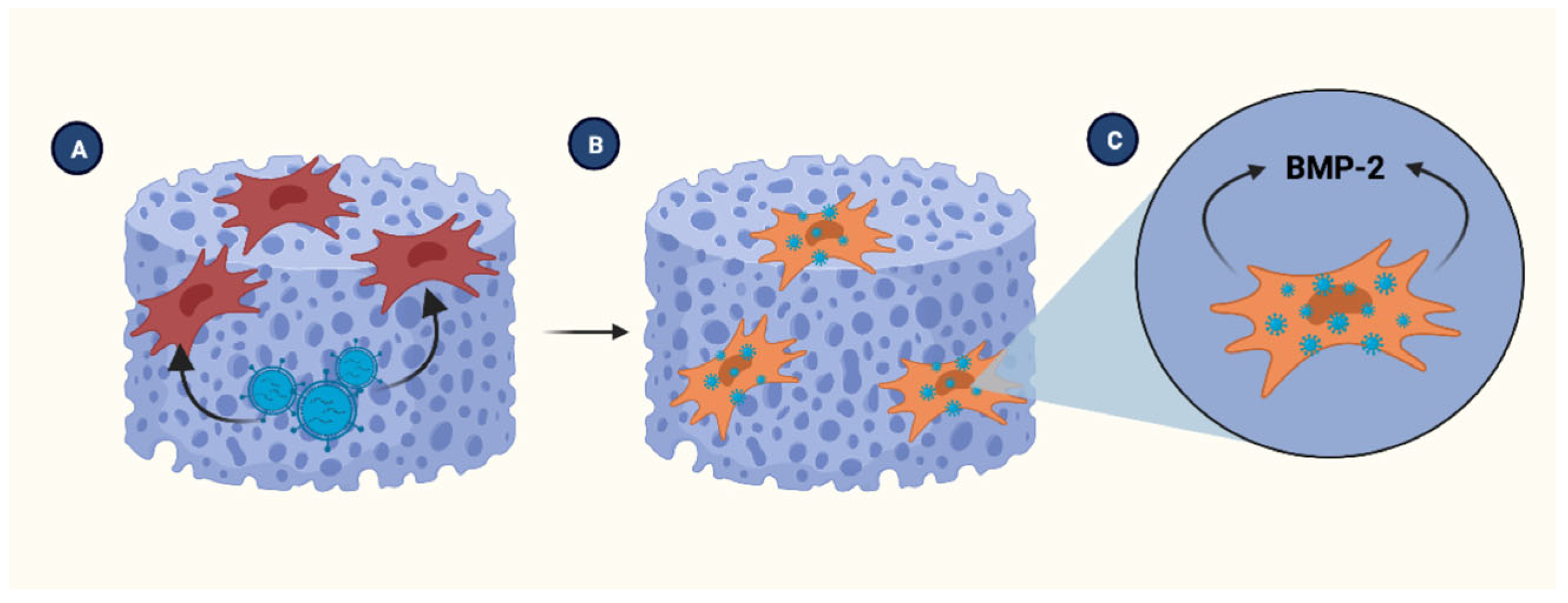
4.1. Clinical Implications of BMP-2 and Bone Regeneration
4.2. Scaffolds in Tissue Engineering
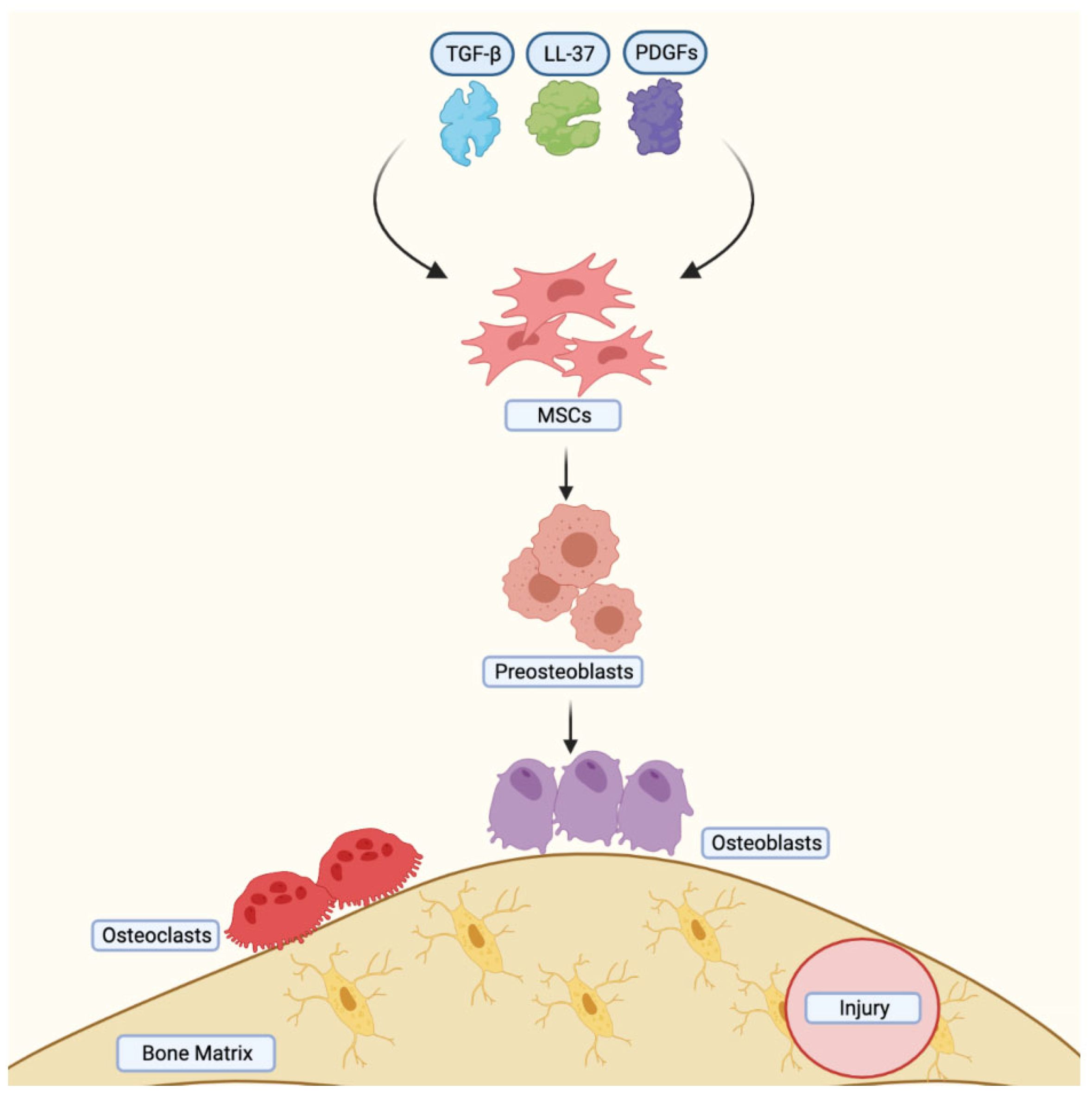
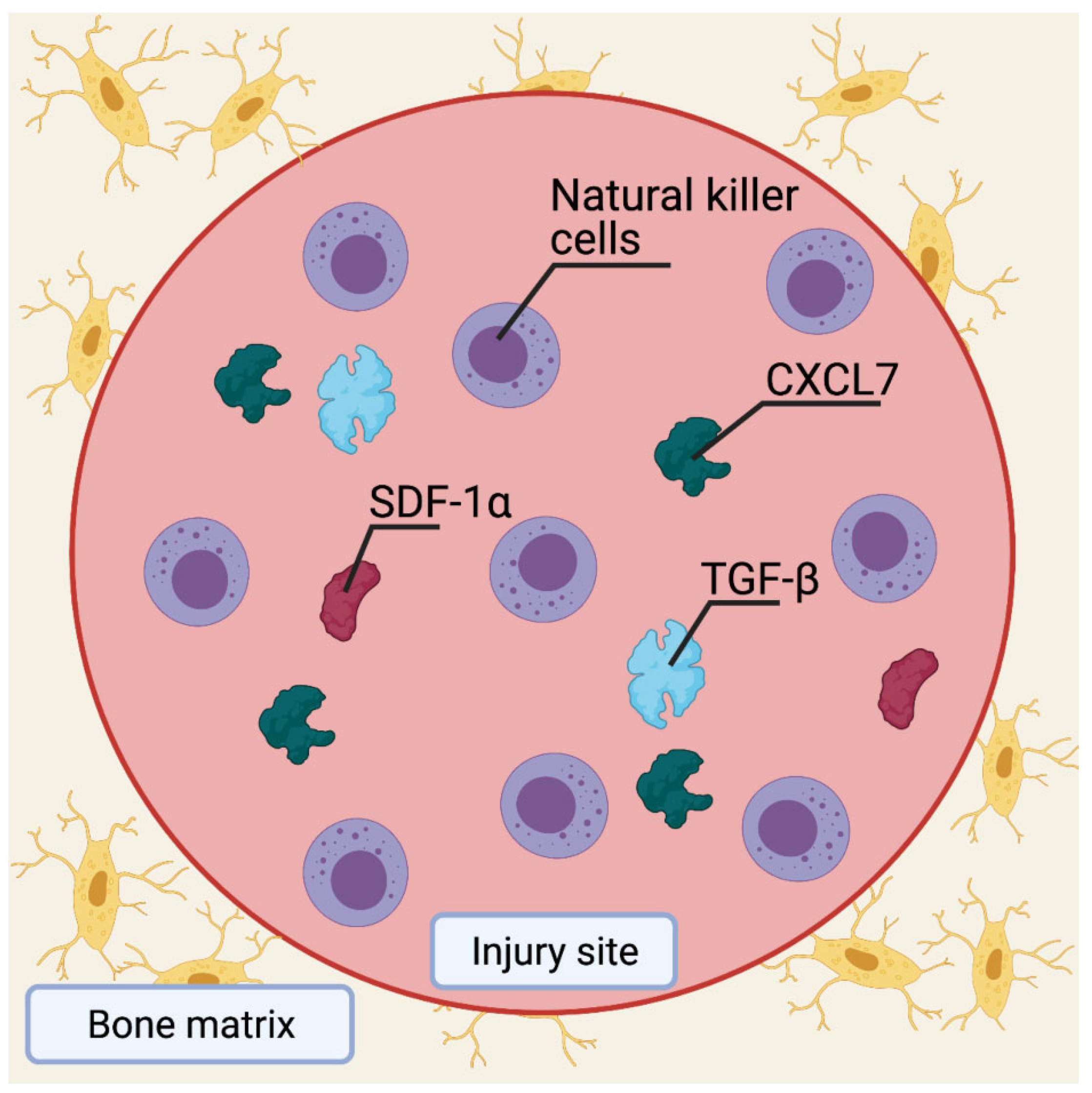
4.3. Role of MSCs in Bone Injury Healing
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, A.; Azad, T.D.; Veeravagu, A.; Bhatti, I.; Long, C.; Ratliff, J.K.; Li, G. Cranioplasty Complications and Costs: A National Population-Level Analysis Using the MarketScan Longitudinal Database. World Neurosurg. 2017, 102, 209–220. [Google Scholar] [CrossRef]
- Liao, H.T.; Chen, C.T. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J. Stem Cells 2014, 6, 288–295. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Aquino-Martinez, R.; Rodriguez-Carballo, E.; Gamez, B.; Artigas, N.; Carvalho-Lobato, P.; Manzanares-Cespedes, M.C.; Rosa, J.L.; Ventura, F. Mesenchymal Stem Cells Within Gelatin/CaSO4 Scaffolds Treated Ex Vivo with Low Doses of BMP-2 and Wnt3a Increase Bone Regeneration. Tissue Eng. Part A 2016, 22, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.K.; Lin, K.J.; Lin, C.Y.; Chang, Y.H.; Yen, T.C.; Hwang, S.M.; Sung, L.Y.; Chen, H.C.; Hu, Y.C. Xenotransplantation of human mesenchymal stem cells into immunocompetent rats for calvarial bone repair. Tissue Eng. Part A 2010, 16, 479–488. [Google Scholar] [CrossRef]
- Du, M.; Zhu, T.; Duan, X.; Ge, S.; Li, N.; Sun, Q.; Yang, P. Acellular dermal matrix loading with bFGF achieves similar acceleration of bone regeneration to BMP-2 via differential effects on recruitment, proliferation and sustained osteodifferentiation of mesenchymal stem cells. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017, 70 Pt 1, 62–70. [Google Scholar] [CrossRef]
- Gao, X.; Usas, A.; Tang, Y.; Lu, A.; Tan, J.; Schneppendahl, J.; Kozemchak, A.M.; Wang, B.; Cummins, J.H.; Tuan, R.S.; et al. A comparison of bone regeneration with human mesenchymal stem cells and muscle-derived stem cells and the critical role of BMP. Biomaterials 2014, 35, 6859–6870. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Hwang, M.P.; Wright, N.; Lu, A.; Ruzbarsky, J.J.; Huard, M.; Cheng, H.; Mullen, M.; Ravuri, S.; Wang, B.; et al. The use of heparin/polycation coacervate sustain release system to compare the bone regenerative potentials of 5 BMPs using a critical sized calvarial bone defect model. Biomaterials 2022, 288, 121708. [Google Scholar] [CrossRef]
- Gohil, S.V.; Kuo, C.L.; Adams, D.J.; Maye, P.; Rowe, D.W.; Nair, L.S. Evaluation of the donor cell contribution in rhBMP-2 mediated bone formation with chitosan thermogels using fluorescent protein reporter mice. J. Biomed. Mater. Res. A 2016, 104, 928–941. [Google Scholar] [CrossRef]
- He, X.; Liu, Y.; Yuan, X.; Lu, L. Enhanced healing of rat calvarial defects with MSCs loaded on BMP-2 releasing chitosan/alginate/hydroxyapatite scaffolds. PLoS ONE 2014, 9, e104061. [Google Scholar] [CrossRef]
- Hsieh, M.K.; Wu, C.J.; Chen, C.C.; Tsai, T.T.; Niu, C.C.; Wu, S.C.; Lai, P.L. BMP-2 gene transfection of bone marrow stromal cells to induce osteoblastic differentiation in a rat calvarial defect model. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 806–816. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, K.; Qiao, C.; Yuan, A.; Li, D.; Zhao, L.; Shi, C.; Xu, X.; Ni, S.; Zheng, C.; et al. Efficiently engineered cell sheet using a complex of polyethylenimine-alginate nanocomposites plus bone morphogenetic protein 2 gene to promote new bone formation. Int. J. Nanomed. 2014, 9, 2179–2190. [Google Scholar]
- Kong, Y.; Zhao, Y.; Li, D.; Shen, H.; Yan, M. Dual delivery of encapsulated BM-MSCs and BMP-2 improves osteogenic differentiation and new bone formation. J. Biomed. Mater. Res. A 2019, 107, 2282–2295. [Google Scholar] [CrossRef] [PubMed]
- Kuttappan, S.; Mathew, D.; Jo, J.I.; Tanaka, R.; Menon, D.; Ishimoto, T.; Nakano, T.; Nair, S.V.; Nair, M.B.; Tabata, Y. Dual release of growth factor from nanocomposite fibrous scaffold promotes vascularisation and bone regeneration in rat critical sized calvarial defect. Acta Biomater. 2018, 78, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jang, S.J.; Baek, H.R.; Lee, K.M.; Chang, B.S.; Lee, C.K. Synergistic induction of early stage of bone formation by combination of recombinant human bone morphogenetic protein-2 and epidermal growth factor. J. Tissue Eng. Regen. Med. 2015, 9, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, G.; Wang, Y.; Yang, G.; Ding, S.; Zhou, S. Controlled dual delivery of BMP-2 and dexamethasone by nanoparticle-embedded electrospun nanofibers for the efficient repair of critical-sized rat calvarial defect. Biomaterials 2015, 37, 218–229. [Google Scholar] [CrossRef]
- Park, S.H.; Doh, J.; Park, S.I.; Lim, J.Y.; Kim, S.M.; Youn, J.I.; Jin, H.T.; Seo, S.H.; Song, M.Y.; Sung, S.Y.; et al. Branched oligomerization of cell-permeable peptides markedly enhances the transduction efficiency of adenovirus into mesenchymal stem cells. Gene Ther. 2010, 17, 1052–1061. [Google Scholar] [CrossRef]
- Shao, N.; Guo, J.; Guan, Y.; Zhang, H.; Li, X.; Chen, X.; Zhou, D.; Huang, Y. Development of Organic/Inorganic Compatible and Sustainably Bioactive Composites for Effective Bone Regeneration. Biomacromolecules 2018, 19, 3637–3648. [Google Scholar] [CrossRef]
- Stephan, S.J.; Tholpady, S.S.; Gross, B.; Petrie-Aronin, C.E.; Botchway, E.A.; Nair, L.S.; Ogle, R.C.; Park, S.S. Injectable tissue-engineered bone repair of a rat calvarial defect. Laryngoscope 2010, 120, 895–901. [Google Scholar] [CrossRef]
- Strecker, S.E.; Unterman, S.; Charles, L.F.; Pivovarchick, D.; Maye, P.F.; Edelman, E.R.; Artzi, N. Osterix-mCherry Expression Allows for Early Bone Detection in a Calvarial Defect Model. Adv. Biosyst. 2019, 3, e1900184. [Google Scholar] [CrossRef]
- Subbiah, R.; Hwang, M.P.; Van, S.Y.; Do, S.H.; Park, H.; Lee, K.; Kim, S.H.; Yun, K.; Park, K. Osteogenic/angiogenic dual growth factor delivery microcapsules for regeneration of vascularized bone tissue. Adv. Healthc. Mater. 2015, 4, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Lin, H.; Tang, Y.; Xiang, S.; Xue, J.; Yin, W.; Tan, J.; Peng, H.; Alexander, P.G.; Tuan, R.S.; et al. Injectable BMP-2 gene-activated scaffold for the repair of cranial bone defect in mice. Stem Cells Transl. Med. 2020, 9, 1631–1642. [Google Scholar] [CrossRef]
- Terella, A.; Mariner, P.; Brown, N.; Anseth, K.; Streubel, S.O. Repair of a calvarial defect with biofactor and stem cell-embedded polyethylene glycol scaffold. Arch. Facial Plast. Surg. 2010, 12, 166–171. [Google Scholar] [CrossRef]
- Vila, O.F.; Martino, M.M.; Nebuloni, L.; Kuhn, G.; Perez-Amodio, S.; Muller, R.; Hubbell, J.A.; Rubio, N.; Blanco, J. Bioluminescent and micro-computed tomography imaging of bone repair induced by fibrin-binding growth factors. Acta Biomater. 2014, 10, 4377–4389. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, W.; Nothdurft, L.; Wu, C.; Zhou, Y.; Crawford, R.; Xiao, Y. In vitro and in vivo evaluation of adenovirus combined silk fibroin scaffolds for bone morphogenetic protein-7 gene delivery. Tissue Eng. Part C Methods 2011, 17, 789–797. [Google Scholar] [CrossRef]
- Zhou, C.; Ye, C.; Zhao, C.; Liao, J.; Li, Y.; Chen, H.; Huang, W. A Composite Tissue Engineered Bone Material Consisting of Bone Mesenchymal Stem Cells, Bone Morphogenetic Protein 9 (BMP9) Gene Lentiviral Vector, and P3HB4HB Thermogel (BMSCs-LV-BMP9-P3HB4HB) Repairs Calvarial Skull Defects in Rats by Expression of Osteogenic Factors. Med. Sci. Monit. 2020, 26, e924666. [Google Scholar]
- Marupanthorn, K.; Tantrawatpan, C.; Kheolamai, P.; Tantikanlayaporn, D.; Manochantr, S. Bone morphogenetic protein-2 enhances the osteogenic differentiation capacity of mesenchymal stromal cells derived from human bone marrow and umbilical cord. Int. J. Mol. Med. 2017, 39, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Dumic-Cule, I.; Peric, M.; Kucko, L.; Grgurevic, L.; Pecina, M.; Vukicevic, S. Bone morphogenetic proteins in fracture repair. Int. Orthop. 2018, 42, 2619–2626. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Q.; Wang, Z.; Zhou, W.; Zhang, L.; Liu, Y.; Xu, Z.; Li, Z.; Zhu, C.; Zhang, X. Bone regeneration materials and their application over 20 years: A bibliometric study and systematic review. Front. Bioeng. Biotechnol. 2022, 10, 921092. [Google Scholar] [CrossRef]
- Bagi, C.M.; Berryman, E.; Moalli, M.R. Comparative bone anatomy of commonly used laboratory animals: Implications for drug discovery. Comp. Med. 2011, 61, 76–85. [Google Scholar] [PubMed]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef]
- Su, P.; Tian, Y.; Yang, C.; Ma, X.; Wang, X.; Pei, J.; Qian, A. Mesenchymal Stem Cell Migration during Bone Formation and Bone Diseases Therapy. Int. J. Mol. Sci. 2018, 19, 2343. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.W.; Sim, K.B.; Kim, S.D. Development and Growth of the Normal Cranial Vault: An Embryologic Review. J. Korean Neurosurg. Soc. 2016, 59, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Kobayashi, T.; Selig, M.K.; Torrekens, S.; Roth, S.I.; Mackem, S.; Carmeliet, G.; Kronenberg, H.M. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell. 2010, 19, 329–344. [Google Scholar] [CrossRef]
- Cheng, H.; Jiang, W.; Phillips, F.M.; Haydon, R.C.; Peng, Y.; Zhou, L.; Luu, H.H.; An, N.; Breyer, B.; Vanichakarn, P.; et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J. Bone Joint Surg. Am. 2003, 85, 1544–1552. [Google Scholar] [CrossRef]
- Cho, T.J.; Gerstenfeld, L.C.; Einhorn, T.A. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J. Bone Miner. Res. 2002, 17, 513–520. [Google Scholar] [CrossRef]
- Edgar, C.M.; Chakravarthy, V.; Barnes, G.; Kakar, S.; Gerstenfeld, L.C.; Einhorn, T.A. Autogenous regulation of a network of bone morphogenetic proteins (BMPs) mediates the osteogenic differentiation in murine marrow stromal cells. Bone 2007, 40, 1389–1398. [Google Scholar] [CrossRef]
- Hyun, S.J.; Han, D.K.; Choi, S.H.; Chai, J.K.; Cho, K.S.; Kim, C.K.; Kim, C.S. Effect of recombinant human bone morphogenetic protein-2, -4, and -7 on bone formation in rat calvarial defects. J. Periodontol. 2005, 76, 1667–1674. [Google Scholar] [CrossRef] [PubMed]

| Authors and Year | Animal Model and Age | MSCs Type | Transduction/Transfection Method | BMP Therapy Delivery Method | Diameter Size of the Defect | Approximate Number of Cells Seeded | BMP Therapy | BMP Dose | Type of Scaffold Used to Seed MSCs | Endpoints |
|---|---|---|---|---|---|---|---|---|---|---|
| Aquino-Martinez, R. et al., 2016 [6] | Six to eight-week-old GFP transgenic BALB/c mice | BMSCs | Embedded in scaffold | Embedded in scaffold | 5 mm | 3.5 × 105 | BMP-2 | 2 nM | Gelatin/CaSO4 scaffold | 5 Weeks |
| Chuang, C.K. et al., 2010 [7] | Female immunocompetent Sprague-Dawley rats. 10 weeks | BMSCs | Baculovirus | Transduction | 8 mm | 5 × 106 | BMP-2 | Not reported | PLGA | 4 and 12 weeks |
| Du, M. et al., 2017 [8] | Seven to eight-week-old, adult female Wistar rats | BMSCs | Embedded in scaffold | Embedded in scaffold | 8 mm | 4 × 104 | BMP-2 | 800 ng/mL | Acellular dermal matrix membrane | 1 and 2 weeks |
| Gao, X. et al., 2014 [9]. | Eight-week-old Male CD-1 nude mice | BMSCs | Lentivirus | Transduction | 5 mm | 1.5 × 106 | BMP-2 | Not reported | Fibrin sealant | 1, 14, 28 and 42 days |
| Gao, X. et al., 2022 [10] | Seven-weeks-old ICRSCID mice at | hMDSCs | Embedded in scaffold | Embedded in scaffold | 5 mm | 2 × 104 | BMP-2, 4, 6, 7, 9 | 50 ng/mL | Fibrin sealant | 1, 14, 28 and 42 days |
| Gohil, S. V. et al., 2016 [11] | Col3.6Cyan (ECFP) mice | BMSCs | Embedded in scaffold | Embedded in scaffold | 3.5 mm | 3 × 106 cells/cm2 | rhBMP-2 | 2 ug | Chitosan thermogel | 4 and 8 weeks |
| He, X. et al., 2014 [12] | Male SD rats at 8 weeks of age | BMSCs | Embedded in scaffold | Embedded in scaffold | 8 mm | 1 × 106 | BMP-2 | 200 ng/mL | Chitosan/alginate/hydroxyapatite | 12 weeks |
| Hsieh, M. K. et al., 2018 [13] | Eight-week-old Sprague-Dawley male rats | BMSCs | E. coli/TransIT-2020 | Transfection | 8 mm | 1 × 106/mL | BMP-2 | Not reported | Corning Matrigel basement membrane Matrix High Concentration | 12 weeks |
| Jin, H. et al., 2014 [14] | Male Wistar rats | BMSCs | Cells in transfection media | Transfection | 5 mm | 2 × 105 | BMP-2 | Not reported | polyethylenimine–alginate (PEI–al) nanocomposite | 4 and 8 weeks |
| Kong, Y. et al., 2019 [15] | Eight-week-old Sprague-Dawley rats | BMSCs | Embedded in scaffold | Embedded in scaffold | 5 mm | 5 × 105 | BMP-2 | 0.5 ± 0.02 μg/mL | Sodium alginate microcapsules and polylactic acid (PLLA) microspheres | 4 and 8 weeks |
| Kuttappan, S. et al., 2018 [16] | Four to five-month-old male Wistar rats | ADSCs | Embedded in scaffold | Embedded in scaffold | 8 mm | 5 × 104 | BMP-2 | Not stated | Nanocomposite fibrous | 4 and 12 weeks |
| Lee, J.H. et al., 2015 [17] | Male Sprague-Dawley rats | hADSCs | E. coli | Transfection | 8 mm | 2 × 103–2 × 104 | rhBMP-2 | Not reported | Collagen sponge | 2 and 6 weeks |
| Li, L. et al., 2015 [18] | Female Sprague-Dawley rats | BMSCs | Embedded in scaffold | Embedded in scaffold | 8 mm | 2 × 104 | BMP-2 | 80 mg | Dexamethasone embedded PCE polymer | 4 and 12 weeks |
| Park, S.H. et al., 2010 [19] | Male immunocompetent Sprague-Dawley rats. 6 weeks | BMSCs | rAd | Transduction | 8 mm | 5 × 105 | BMP-2 | Not reported | Matrigel matrix | 4 weeks |
| Shao, N. et al., 2018 [20] | Six-week-old Sprague-Dawley (SD) male rats | BMSCs | Embedded in scaffold | Embedded in scaffold | 5 mm | 5 × 104 | BMP-2 | 340–400 μg | Inorganic hydroxyapatite gel | 12 weeks |
| Stephan, S.J. et al., 2010 [21] | Six to eight-month-old Sprague-Dawley rats | BMSCs | Embedded in scaffold | Embedded in scaffold | 8 mm | 0.3 × 106 | BMP-2 | 2 μg | Chitosan gel | 4 and 8 weeks |
| Strecker, S. E. et al., 2019 [22] | Osterix-mCherry mice | BMSCs | Embedded in scaffold | Embedded in scaffold | 4 mm | 1.2 × 106 cells/cm2 | rhBMP-2 | 0.2 μg | Dextran-Dendrimer Hydrogel Nanocomposite | 4 and 8 weeks |
| Subbiah, R. et al., 2015 [23] | Seven-week-old SD rats | UCMSCs | Embedded in scaffold | Embedded in scaffold | 9 mm | 2.5 × 106 | BMP-2 | 392 ± 18 ng | PLGA NP and alginate microcapsules | 4 and 8 weeks |
| Sun, K. et al., 2020 [24] | SCID mice. Age not specified | BMSCs | rAAV | Transduction | 5 mm | 1 × 107 | BMP-2 | Not reported | mGL hydrogel scaffold | 6 weeks |
| Terella, A. et al., 2010 [25] | Albino male Sprague Dawley rats aged 10–11 weeks | Not specified | Embedded in scaffold | Embedded in scaffold | 8 mm | 1 × 107 | BMP-2 | 5–15 ng/80 uL | PEG-DA, and PEG-MMP | 1, 4 and 8 weeks |
| Vila, O.F. et al., 2014 [26] | 10-week-old SCID mice | hADSCs | Lentivirus | Transduction | 3 mm | 0.2 × 106 | BMP-2 | Not reported | Fibrin matrix | 6 weeks |
| Zhang, Y. et al., 2011 [27] | SCID mice. Age not specified | BMSCs | Adenovirus | Transduction | 3 mm | 1 × 106 | BMP-7 | Not reported | Silk fibroin | 4 weeks |
| Zhou, C. et al., 2020 [28] | Two-week-old Sprague-Dawley rats | BMSCs | Lentivirus | Transduction | 5 mm | 2 × 106 cell/mL | BMP-9 | Not reported | P3HB4HB thermogel | 4 weeks |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Guzman, R.A.; Avila, F.R.; Maita, K.C.; Garcia, J.P.; De Sario, G.D.; Borna, S.; Eldaly, A.S.; Quinones-Hinojosa, A.; Zubair, A.C.; Ho, O.A.; et al. Bone Morphogenic Protein and Mesenchymal Stem Cells to Regenerate Bone in Calvarial Defects: A Systematic Review. J. Clin. Med. 2023, 12, 4064. https://doi.org/10.3390/jcm12124064
Torres-Guzman RA, Avila FR, Maita KC, Garcia JP, De Sario GD, Borna S, Eldaly AS, Quinones-Hinojosa A, Zubair AC, Ho OA, et al. Bone Morphogenic Protein and Mesenchymal Stem Cells to Regenerate Bone in Calvarial Defects: A Systematic Review. Journal of Clinical Medicine. 2023; 12(12):4064. https://doi.org/10.3390/jcm12124064
Chicago/Turabian StyleTorres-Guzman, Ricardo A., Francisco R. Avila, Karla C. Maita, John P. Garcia, Gioacchino D. De Sario, Sahar Borna, Abdullah S. Eldaly, Alfredo Quinones-Hinojosa, Abba C. Zubair, Olivia A. Ho, and et al. 2023. "Bone Morphogenic Protein and Mesenchymal Stem Cells to Regenerate Bone in Calvarial Defects: A Systematic Review" Journal of Clinical Medicine 12, no. 12: 4064. https://doi.org/10.3390/jcm12124064
APA StyleTorres-Guzman, R. A., Avila, F. R., Maita, K. C., Garcia, J. P., De Sario, G. D., Borna, S., Eldaly, A. S., Quinones-Hinojosa, A., Zubair, A. C., Ho, O. A., & Forte, A. J. (2023). Bone Morphogenic Protein and Mesenchymal Stem Cells to Regenerate Bone in Calvarial Defects: A Systematic Review. Journal of Clinical Medicine, 12(12), 4064. https://doi.org/10.3390/jcm12124064







