Use of Automated Machine Learning for Classifying Hemoperitoneum on Ultrasonographic Images of Morrison’s Pouch: A Multicenter Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
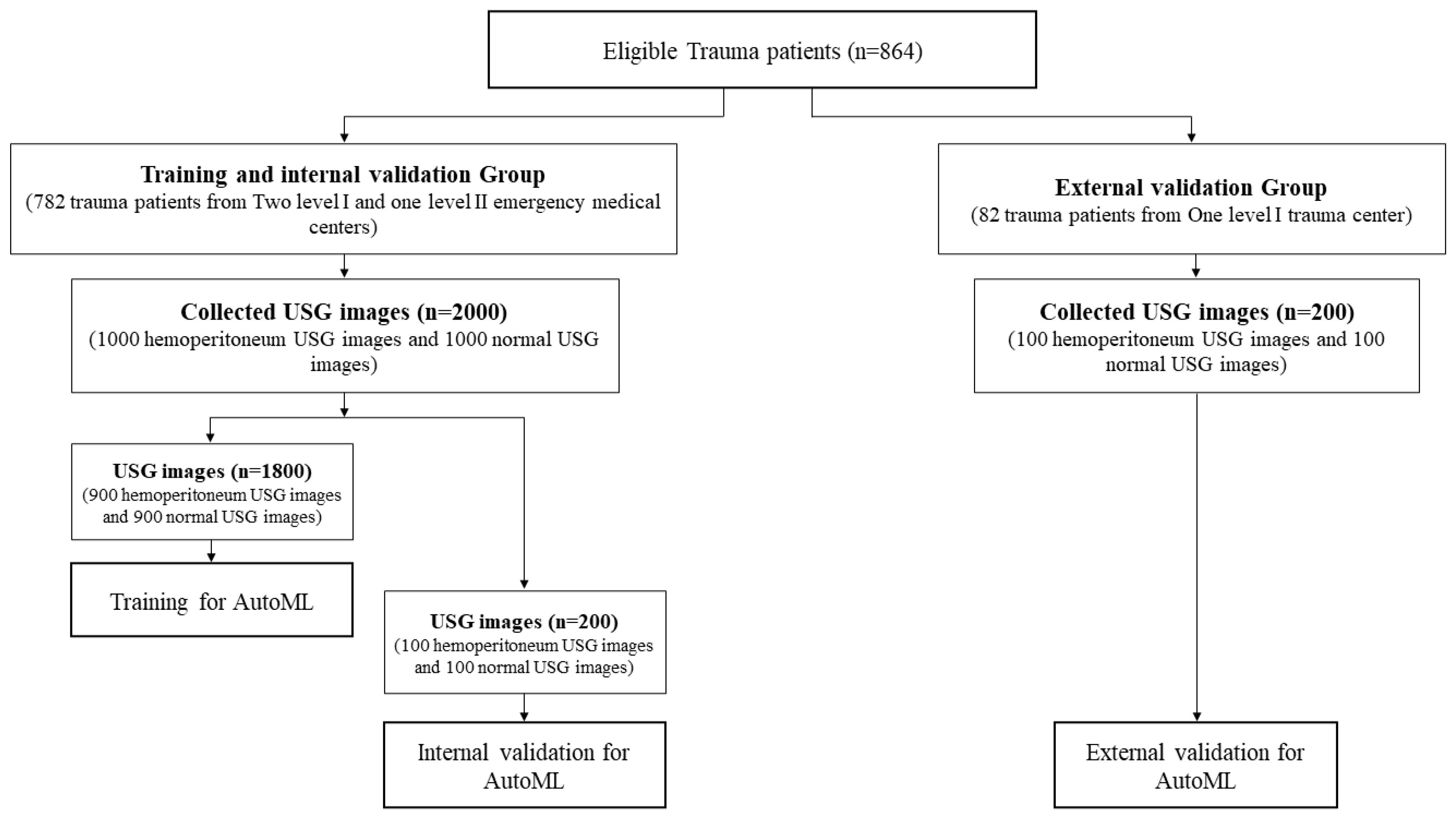
2.2. Study Population
2.3. Data Collection
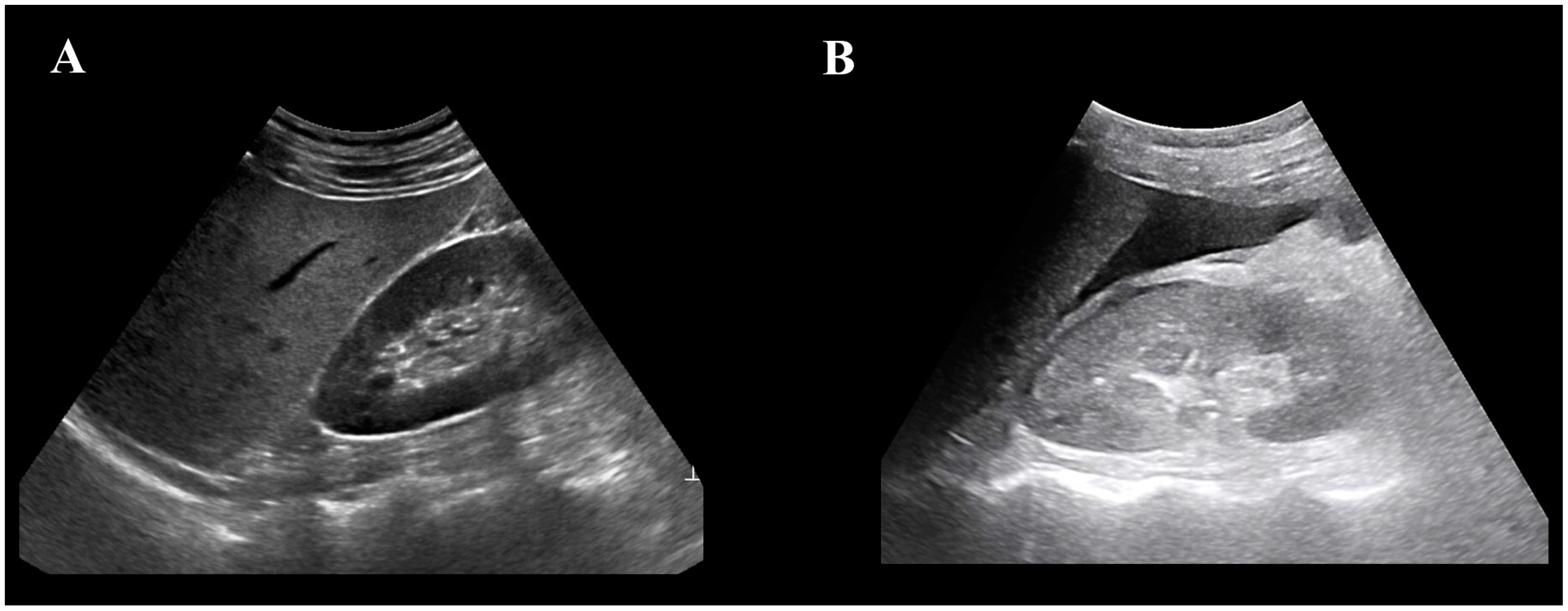
2.4. USG Image Distribution
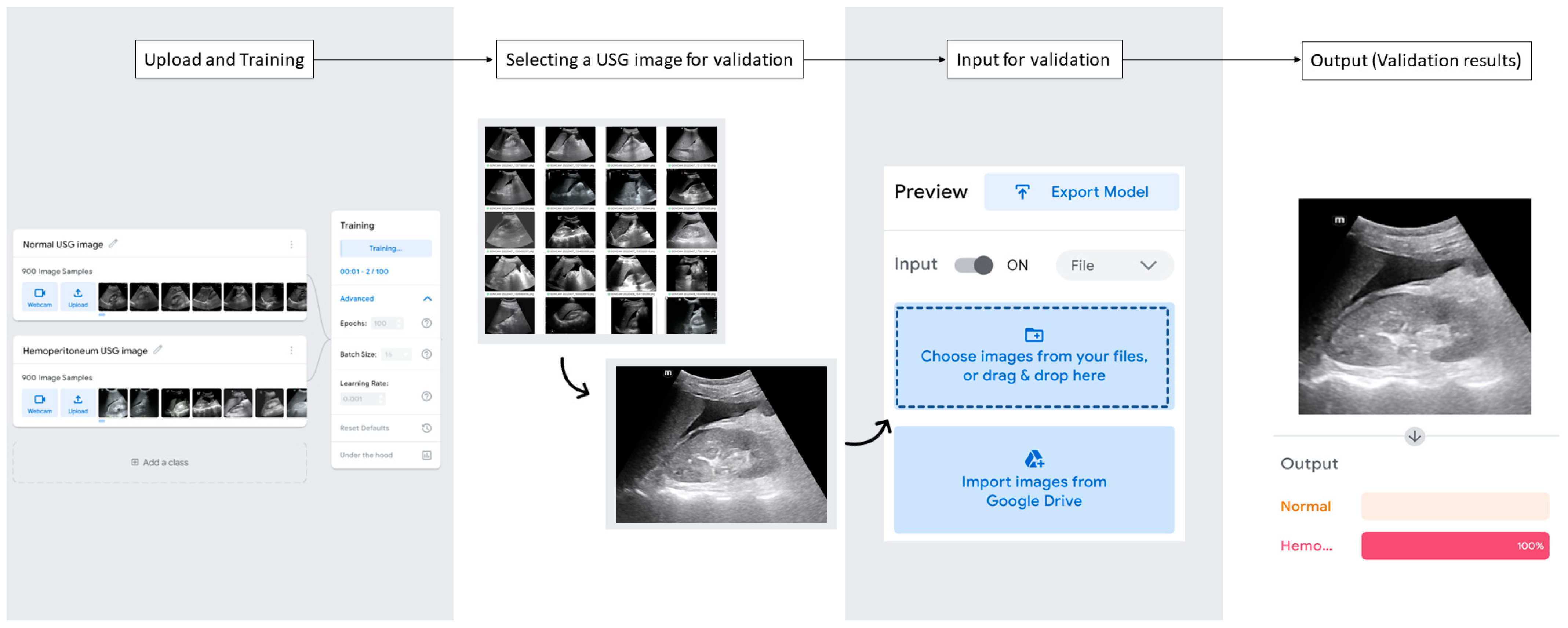
2.5. USG Image Analysis with AutoML
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Enrolled Trauma Patients
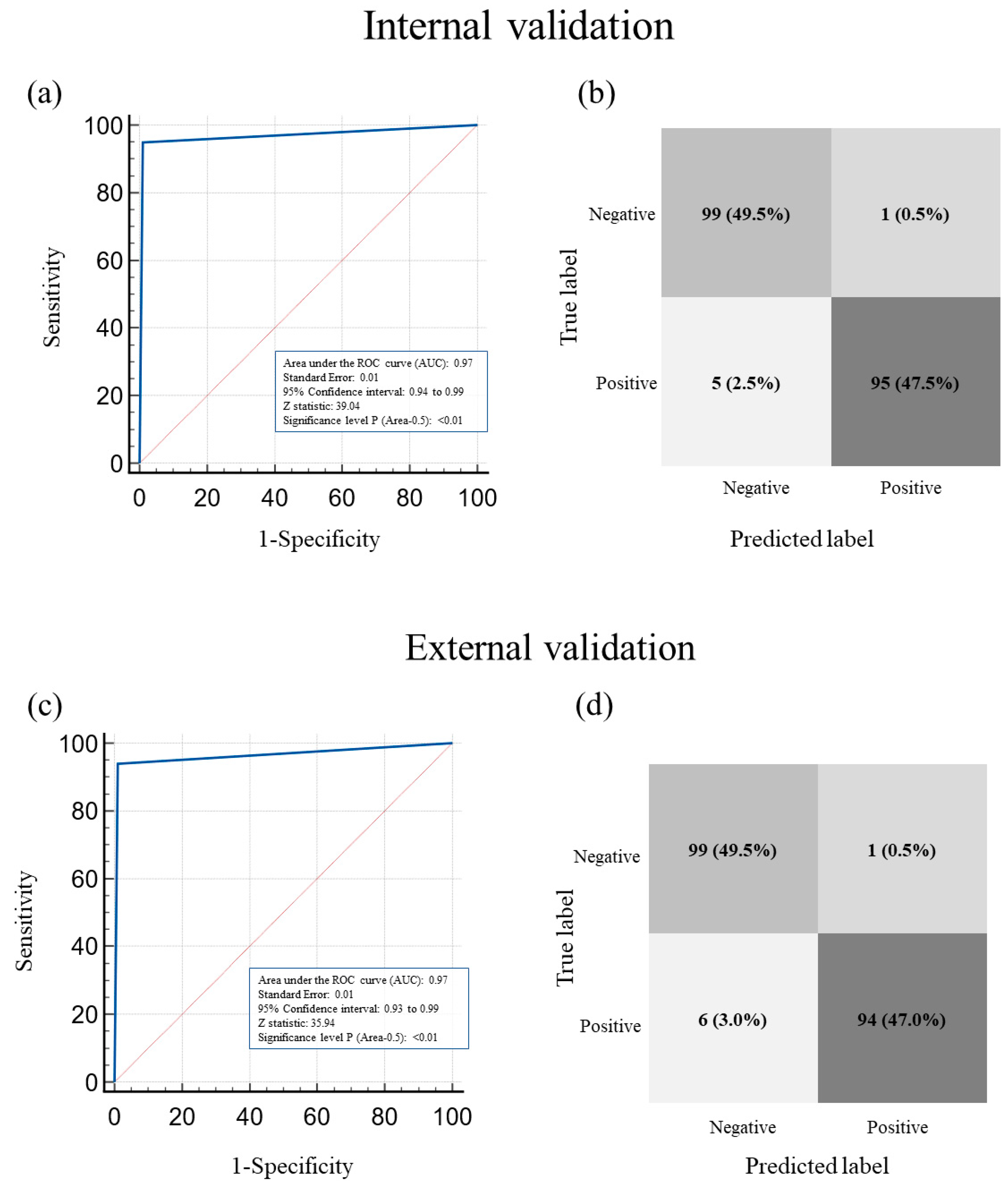
3.2. Performance of AutoML in Classifying Hemoperitoneum in Morrison’s Pouch USG Images: The Internal Validation Group
3.3. Performance of AutoML in Classifying Hemoperitoneum in Morrison’s Pouch USG Images: The External Validation Group
3.4. Comparison of Internal and External Validation of the Performance of AutoML in Classifying the Presence or Absence of Hemoperitoneum on USG Images of Morrison’s Pouch
3.5. ROC Curve of AutoML in Classifying the Presence or Absence of Hemoperitoneum in Morrison’s Pouch USG Images
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harvin, J.A.; Maxim, T.; Inaba, K.; Martinez-Aguilar, M.A.; King, D.R.; Choudhry, A.J.; Zielinski, M.D.; Akinyeye, S.; Todd, S.R.; Griffin, R.L. Mortality following emergent trauma laparotomy: A multicenter, retrospective study: Mortality after emergent trauma laparotomy. J. Trauma Acute Care Surg. 2017, 83, 464. [Google Scholar] [CrossRef]
- Eastridge, B.J.; Holcomb, J.B.; Shackelford, S. Outcomes of traumatic hemorrhagic shock and the epidemiology of preventable death from injury. Transfusion 2019, 59, 1423–1428. [Google Scholar] [CrossRef] [Green Version]
- Holcomb, J.B.; Pati, S. Optimal trauma resuscitation with plasma as the primary resuscitative fluid: The surgeon’s perspective. Hematol. Am. Soc. Hematol. Educ. Program 2013, 2013, 656–659. [Google Scholar] [CrossRef] [Green Version]
- Murao, S.; Yamakawa, K.; Kabata, D.; Kinoshita, T.; Umemura, Y.; Shintani, A.; Fujimi, S. Effect of earlier door-to-CT and door-to-bleeding control in severe blunt trauma: A retrospective cohort study. J. Clin. Med. 2021, 10, 1522. [Google Scholar] [CrossRef]
- Lateef, A.U.; Khan, A.A.; Rana, M.M. Comparison of efficacy of FAST and CT scan in patients with blunt abdominal trauma. Ann. Punjab Med. Coll. (APMC) 2019, 13, 10–13. [Google Scholar]
- Kumar, S.; Bansal, V.K.; Muduly, D.K.; Sharma, P.; Misra, M.C.; Chumber, S.; Singh, S.; Bhardwaj, D. Accuracy of focused assessment with sonography for trauma (fast) in blunt trauma abdomen—A prospective study. Indian J. Surg. 2015, 77, 393–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingeman, J.E.; Plewa, M.C.; Okasinski, R.E.; King, R.W.; Knotts, F.B. Emergency physician use of ultrasonography in blunt abdominal trauma. Acad. Emerg. Med. 1996, 3, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Basnet, S.; Shrestha, S.K.; Pradhan, A.; Shrestha, R.; Shrestha, A.P.; Sharma, G.; Bade, S.; Giri, L. Diagnostic performance of the extended focused assessment with sonography for trauma (EFAST) patients in a tertiary care hospital of Nepal. Trauma Surg. Acute Care Open 2020, 5, e000438. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, K.; Mirvis, S.E.; Sherbourne, C.D.; Chiu, W.C.; Rodriguez, A. Hemoperitoneum as the sole indicator of abdominal visceral injuries: A potential limitation of screening abdominal US for trauma. Radiology 1999, 212, 423–430. [Google Scholar] [CrossRef]
- Emery, K.H.; McAneney, C.M.; Racadio, J.M.; Johnson, N.D.; Evora, D.K.; Garcia, V.F. Absent peritoneal fluid on screening trauma ultrasonography in children: A prospective comparison with computed tomography. J. Pediatr. Surg. 2001, 36, 565–569. [Google Scholar] [CrossRef]
- Körner, M.; Krötz, M.M.; Degenhart, C.; Pfeifer, K.J.; Reiser, M.F.; Linsenmaier, U. Current role of emergency US in patients with major trauma. RadioGraphics 2008, 28, 225–242. [Google Scholar] [CrossRef]
- Boulanger, B.R.; McLellan, B.A.; Brenneman, F.D.; Wherrett, L.; Rizoli, S.B.; Culhane, J.; Hamilton, P. Emergent abdominal sonography as a screening test in a new diagnostic algorithm for blunt trauma. J. Trauma Acute Care Surg. 1996, 40, 867–874. [Google Scholar] [CrossRef]
- Netherton, S.; Milenkovic, V.; Taylor, M.; Davis, P.J. Diagnostic accuracy of eFAST in the trauma patient: A systematic review and meta-analysis. Can. J. Emerg. Med. 2019, 21, 727–738. [Google Scholar] [CrossRef]
- Currie, G.; Hawk, K.E.; Rohren, E.; Vial, A.; Klein, R. Machine learning and deep learning in medical imaging: Intelligent imaging. J. Med. Imaging Radiat. Sci. 2019, 50, 477–487. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.K.; Greenspan, H.; Davatzikos, C.; Duncan, J.S.; Van Ginneken, B.; Madabhushi, A.; Prince, J.L.; Rueckert, D.; Summers, R.M. A review of deep learning in medical imaging: Imaging traits, technology trends, case studies with progress highlights, and future promises. Proc. IEEE 2021, 109, 820–838. [Google Scholar] [CrossRef]
- Stewart, J.; Sprivulis, P.; Dwivedi, G. Artificial intelligence and machine learning in emergency medicine. Emerg. Med. Australas. 2018, 30, 870–874. [Google Scholar] [CrossRef]
- Tang, K.J.W.; Ang, C.K.E.; Constantinides, T.; Rajinikanth, V.; Acharya, U.R.; Cheong, K.H. Artificial intelligence and machine learning in emergency medicine. Biocybern. Biomed. Eng. 2021, 41, 156–172. [Google Scholar] [CrossRef]
- McBee, M.P.; Awan, O.A.; Colucci, A.T.; Ghobadi, C.W.; Kadom, N.; Kansagra, A.P.; Tridandapani, S.; Auffermann, W.F. Deep learning in radiology. Acad. Radiol. 2018, 25, 1472–1480. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.B.; Lee, S.H.; Lee, D.E.; Park, S.-Y.; Kim, J.K.; Cho, J.W.; Cho, J.; Kim, K.B.; Park, B.; Park, J. Deep-learning algorithms for the interpretation of chest radiographs to aid in the triage of COVID-19 patients: A multicenter retrospective study. PLoS ONE 2020, 15, e0242759. [Google Scholar] [CrossRef]
- Meng, X.H.; Wu, D.J.; Wang, Z.; Ma, X.L.; Dong, X.M.; Liu, A.E.; Chen, L. A fully automated rib fracture detection system on chest CT images and its impact on radiologist performance. Skelet. Radiol. 2021, 50, 1821–1828. [Google Scholar] [CrossRef]
- Alom, M.Z.; Taha, T.M.; Yakopcic, C.; Westberg, S.; Sidike, P.; Nasrin, M.S.; Hasan, M.; Van Essen, B.C.; Awwal, A.A.S.; Asari, V.K. A state-of-the-art survey on deep learning theory and architectures. Electronics 2019, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Misra, R.; O’Byrne, C.; Keane, P. Code-Free Deep Learning: A Step into the Future of Ophthalmology. 2022. Available online: https://www.eyenews.uk.com/features/ophthalmology/post/code-free-deep-learning-a-step-into-the-future-of-ophthalmology (accessed on 1 April 2023).
- Soares, T.R.; de Oliveira, R.D.; Liu, Y.E.; da Silva Santos, A.; dos Santos, P.C.P.; Monte, L.R.S.; de Oliveira, L.M.; Park, C.M.; Hwang, E.J.; Andrews, J.R.; et al. Evaluation of chest X-ray with automated interpretation algorithms for mass tuberculosis screening in prisons: A cross-sectional study. Lancet Reg. Health Am. 2023, 17, 100388. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Lee, S.H.; Arru, C.D.; Doda Khera, R.; Singh, R.; Siebert, S.; Kim, D.; Lee, Y.; Park, J.H.; Eom, H.J.; et al. AI-based improvement in lung cancer detection on chest radiographs: Results of a multi-reader study in NLST dataset. Eur. Radiol. 2021, 31, 9664–9674. [Google Scholar] [CrossRef] [PubMed]
- van Beek, E.; Ahn, J.; Kim, M.; Murchison, J. Validation study of machine-learning chest radiograph software in primary and emergency medicine. Clin. Radiol. 2023, 78, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kalra, M.K.; Nitiwarangkul, C.; Patti, J.A.; Homayounieh, F.; Padole, A.; Rao, P.; Putha, P.; Muse, V.V.; Sharma, A.; et al. Deep learning in chest radiography: Detection of findings and presence of change. PLoS ONE 2018, 13, e0204155. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.J.; Gandomkar, Z.; Wu, W.J.; Zhang, G.; Gao, W.; He, X.; Wang, Y.; Reed, W. Artificial intelligence and convolution neural networks assessing mammographic images: A narrative literature review. J. Med. Radiat. Sci. 2020, 67, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Jin, K.N.; Kim, E.Y.; Kim, Y.J.; Lee, G.P.; Kim, H.; Oh, S.; Kim, Y.S.; Han, J.H.; Cho, Y.J. Diagnostic effect of artificial intelligence solution for referable thoracic abnormalities on chest radiography: A multicenter respiratory outpatient diagnostic cohort study. Eur. Radiol. 2022, 32, 3469–3479. [Google Scholar] [CrossRef]
- Raimondo, D.; Raffone, A.; Aru, A.C.; Giorgi, M.; Giaquinto, I.; Spagnolo, E.; Travaglino, A.; Galatolo, F.A.; Cimino, M.G.C.A.; Lenzi, J. Application of deep learning model in the sonographic diagnosis of uterine adenomyosis. Int. J. Environ. Health Res. 2023, 20, 1724. [Google Scholar] [CrossRef]
- Li, J.; Bu, Y.; Lu, S.; Pang, H.; Luo, C.; Liu, Y.; Qian, L. Development of a deep learning–based model for diagnosing breast nodules with ultrasound. J. Ultrasound Med. 2021, 40, 513–520. [Google Scholar] [CrossRef]
- Sjogren, A.R.; Leo, M.M.; Feldman, J.; Gwin, J.T. Image segmentation and machine learning for detection of abdominal free fluid in focused assessment with sonography for trauma examinations: A pilot study. J. Ultrasound Med. 2016, 35, 2501–2509. [Google Scholar] [CrossRef]
- Kornblith, A.E.; Addo, N.; Dong, R.; Rogers, R.; Grupp-Phelan, J.; Butte, A.; Gupta, P.; Callcut, R.A.; Arnaout, R. Development and validation of a deep learning model for automated view classification of pediatric focused assessment with sonography for trauma (FAST). MedRxiv 2020. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Chiu, I.M.; Hsu, M.Y.; Pan, H.Y.; Tsai, C.M.; Lin, C.-H.R. Deep learning assisted detection of abdominal free fluid in Morison’s pouch during focused assessment with sonography in trauma. Front. Med. 2021, 8, 707437. [Google Scholar] [CrossRef]
- Shokoohi, H.; LeSaux, M.A.; Roohani, Y.H.; Liteplo, A.; Huang, C.; Blaivas, M. Enhanced point-of-care ultrasound applications by integrating automated feature-learning systems using deep learning. J. Ultrasound Med. 2019, 38, 1887–1897. [Google Scholar] [CrossRef]
- Rozycki, G.S.; Ochsner, M.G.; Feliciano, D.V.; Thomas, B.; Boulanger, B.R.; Davis, F.E.; Falcone, R.E.; Schmidt, J.A. Early detection of hemoperitoneum by USG examination of the right upper quadrant: A multicenter study. J. Trauma. 1998, 45, 878–883. [Google Scholar] [CrossRef]
- Ma, O.J.; Kefer, M.P.; Mateer, J.R.; Thoma, B. Evaluation of hemoperitoneum using a single-vs multiple-view ultrasonographic examination. Acad. Emerg. Med. 1995, 2, 581–586. [Google Scholar] [CrossRef]
| Entire Sample (n = 864) | Training and Internal Validation Group (n = 782) | External Validation Group (n = 82) | p | |
|---|---|---|---|---|
| Sex (n, %) | 0.17 | |||
| Male | 596 (68.98) | 534 (68.27) | 62 (75.61) | |
| Female | 268 (31.02) | 248 (31.71) | 20 (24.39) | |
| Median age (yr) (95% CI *) | 58.0 (56.0–60.0) | 58 (56.0–60.0) | 57 (51.7–62.3) | 0.81 |
| Hemoperitoneum (n, %) | 429 (49.65) | 388 (49.62) | 41 (50.00) | 0.95 |
| Internal Validation Group | Difference (95% CI) with Standard Reference * | p | |
|---|---|---|---|
| Sensitivity | 95.00% (88.72–98.36%) | 2.00% (−0.38–4.38%) | 0.22 |
| Specificity | 99.00% (94.55–99.97%) | ||
| PPV | 98.96% (93.11–99.85%) | ||
| NPV | 95.19% (89.39–97.90%) | ||
| Accuracy | 97.00% (93.58–98.89%) |
| External Validation Group | Difference (95% CI) with Standard Reference * | p | |
|---|---|---|---|
| Sensitivity | 94.00% (87.40–97.77%) | 2.50% (−0.07–5.07%) | 0.13 |
| Specificity | 99.00% (94.55–99.97%) | ||
| PPV | 98.95% (93.04–99.85%) | ||
| NPV | 94.29% (88.36–97.29%) | ||
| Accuracy | 96.50% (92.92–98.58%) |
| Difference (95% CI) between Internal and External Validation | p | |
|---|---|---|
| Sensitivity | 1% (−3.70–5.76%) | 0.66 |
| Specificity | 0% (−2.67–2.67%) | 1.0 |
| PPV | 0.01% (−2.69–2.71%) | 0.99 |
| NPV | 0.90% (−3.72–5.58%) | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, D.; Jeong, W.; Lee, J.H.; Park, S.-Y., on behalf of the Society of Emergency & Critical Care Imaging (SECCI). Use of Automated Machine Learning for Classifying Hemoperitoneum on Ultrasonographic Images of Morrison’s Pouch: A Multicenter Retrospective Study. J. Clin. Med. 2023, 12, 4043. https://doi.org/10.3390/jcm12124043
Jeong D, Jeong W, Lee JH, Park S-Y on behalf of the Society of Emergency & Critical Care Imaging (SECCI). Use of Automated Machine Learning for Classifying Hemoperitoneum on Ultrasonographic Images of Morrison’s Pouch: A Multicenter Retrospective Study. Journal of Clinical Medicine. 2023; 12(12):4043. https://doi.org/10.3390/jcm12124043
Chicago/Turabian StyleJeong, Dongkil, Wonjoon Jeong, Ji Han Lee, and Sin-Youl Park on behalf of the Society of Emergency & Critical Care Imaging (SECCI). 2023. "Use of Automated Machine Learning for Classifying Hemoperitoneum on Ultrasonographic Images of Morrison’s Pouch: A Multicenter Retrospective Study" Journal of Clinical Medicine 12, no. 12: 4043. https://doi.org/10.3390/jcm12124043
APA StyleJeong, D., Jeong, W., Lee, J. H., & Park, S.-Y., on behalf of the Society of Emergency & Critical Care Imaging (SECCI). (2023). Use of Automated Machine Learning for Classifying Hemoperitoneum on Ultrasonographic Images of Morrison’s Pouch: A Multicenter Retrospective Study. Journal of Clinical Medicine, 12(12), 4043. https://doi.org/10.3390/jcm12124043








