Variations and Predictors of Post-COVID Syndrome Severity in Patients Attending a Post-COVID Outpatient Clinic
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analyses
3. Results
3.1. Patient Demographics
3.1.1. First Presentation (FP)
3.1.2. First Re-Presentation (1st RP)
3.1.3. Second Re-Presentation (2nd RP)
3.2. Post-COVID Score
3.3. Post-COVID Severity at the First Presentation: Associated Variables
Acute Treatment Setting, Post-COVID Symptoms and Time since Infection
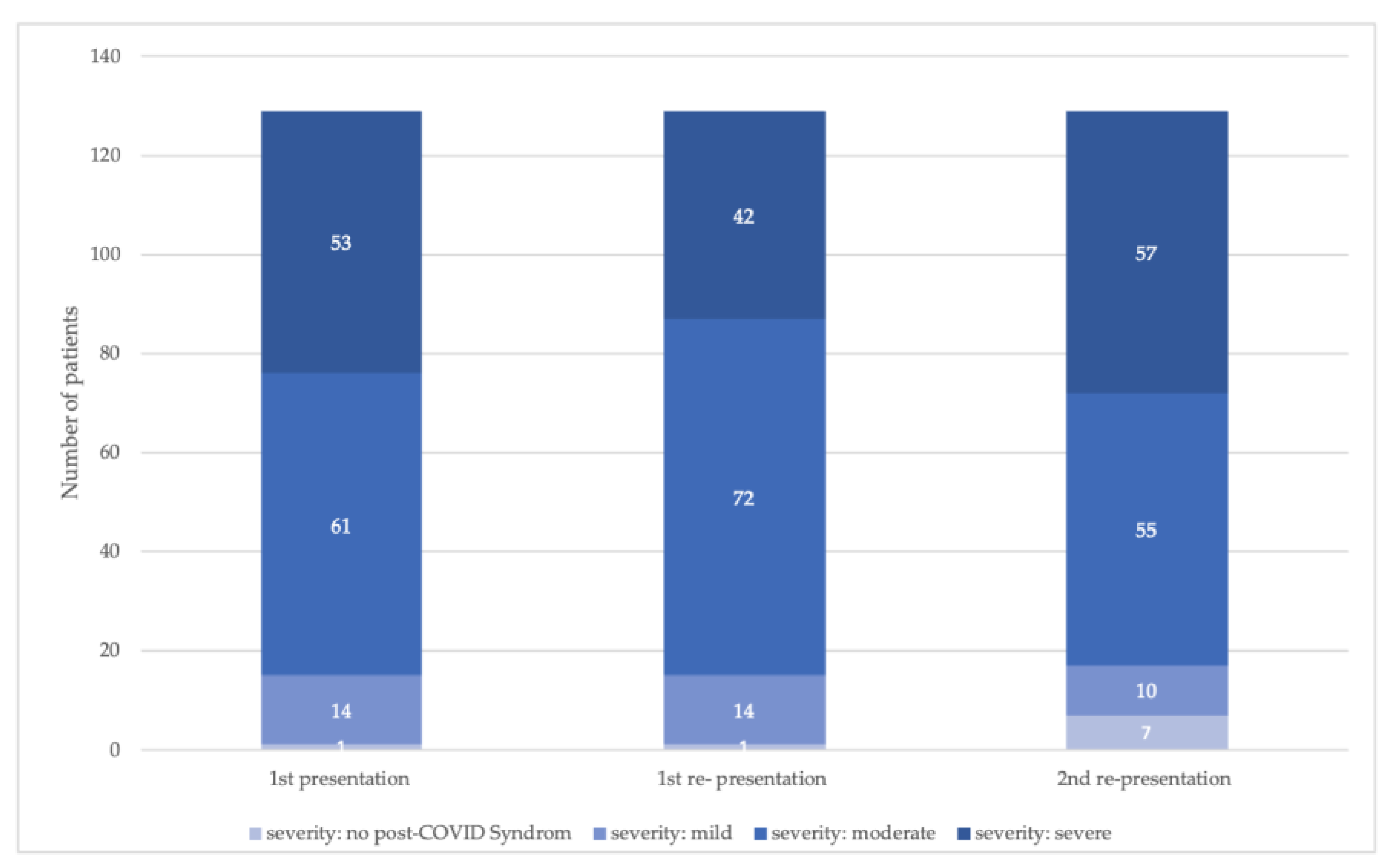
3.4. Frequencies of Re-Presentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- elisaperego78. Twitter 2020, May 20. Available online: https://twitter.com/elisaperego78/status/263172084055838721 (accessed on 15 December 2022).
- Giszas, B.; Trommer, S.; Schussler, N.; Rodewald, A.; Besteher, B.; Bleidorn, J.; Dickmann, P.; Finke, K.; Katzer, K.; Lehmann-Pohl, K.; et al. Post-COVID-19 condition is not only a question of persistent symptoms: Structured screening including health-related quality of life reveals two separate clusters of post-COVID. Infection 2022, 51, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Nehme, M.; Vetter, P.; Chappuis, F.; Kaiser, L.; Guessous, I.; CoviCare Study, T. Prevalence of Post-Coronavirus Disease Condition 12 Weeks After Omicron Infection Compared with Negative Controls and Association with Vaccination Status. Clin. Infect. Dis. 2023, 76, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 Long-term effects of COVID-19: A systematic review and meta-analysis. medRxiv 2021. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Jassat, W.; Mudara, C.; Vika, C.; Welch, R.; Arendse, T.; Dryden, M.; Blumberg, L.; Mayet, N.; Tempia, S.; Parker, A.; et al. A cohort study of post-COVID-19 condition across the Beta, Delta, and Omicron waves in South Africa: 6-month follow-up of hospitalized and nonhospitalized participants. Int. J. Infect. Dis. 2023, 128, 102–111. [Google Scholar] [CrossRef]
- Stallmach, A.; Kesselmeier, M.; Bauer, M.; Gramlich, J.; Finke, K.; Fischer, A.; Fleischmann-Struzek, C.; Heutelbeck, A.; Katzer, K.; Mutschke, S.; et al. Comparison of fatigue, cognitive dysfunction and psychological disorders in post-COVID patients and patients after sepsis: Is there a specific constellation? Infection 2022, 50, 661–669. [Google Scholar] [CrossRef]
- Peter, R.S.; Nieters, A.; Krausslich, H.G.; Brockmann, S.O.; Gopel, S.; Kindle, G.; Merle, U.; Steinacker, J.M.; Rothenbacher, D.; Kern, W.V.; et al. Post-acute sequelae of COVID-19 six to 12 months after infection: Population based study. BMJ Clin. Res. Ed. 2022, 379, e071050. [Google Scholar] [CrossRef]
- Kubota, T.; Kuroda, N.; Sone, D. Neuropsychiatric aspects of long COVID: A comprehensive review. Psychiatry Clin. Neurosci. 2023, 77, 84–93. [Google Scholar] [CrossRef]
- Lemhofer, C.; Sturm, C.; Loudovici-Krug, D.; Guntenbrunner, C.; Bulow, M.; Reuken, P.; Quickert, S.; Best, N. Quality of life and ability to work of patients with Post-COVID syndrome in relation to the number of existing symptoms and the duration since infection up to 12 months: A cross-sectional study. Qual. Life Res. 2023, 32, 1991–2002. [Google Scholar] [CrossRef]
- Lemhöfer, C.; Best, N.; Gutenbrunner, C.; Loudovici-Krug, D.; Teixido, L.; Sturm, C. Gefühlte und reale Arbeitsfähigkeit von Patient*innen mit Post-COVID Symptomatik nach mildem Akutverlauf: Eine Analyse des Rehabilitation Needs Questionnaire (RehabNeQ). Phys. Med. Rehabil. Kurortmed. 2021, 32, 151–158. [Google Scholar] [CrossRef]
- Bahmer, T.; Borzikowsky, C.; Lieb, W.; Horn, A.; Krist, L.; Fricke, J.; Scheibenbogen, C.; Rabe, K.F.; Maetzler, W.; Maetzler, C.; et al. Severity, predictors and clinical correlates of Post-COVID syndrome (PCS) in Germany: A prospective, multi-centre, population-based cohort study. eClinicalMedicine 2022, 51, 101549. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- De Vries, J.; Michielsen, H.; Van Heck, G.L.; Drent, M. Measuring fatigue in sarcoidosis: The Fatigue Assessment Scale (FAS). Br. J. Health Psychol. 2004, 9, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Lemhofer, C.; Gutenbrunner, C.; Schiller, J.; Loudovici-Krug, D.; Best, N.; Bokel, A.; Sturm, C. Assessment of rehabilitation needs in patients after COVID-19: Development of the COVID-19-rehabilitation needs survey. J. Rehabil. Med. 2021, 53, jrm00183. [Google Scholar] [CrossRef]
- Robert Koch Institut. SARS CoV-2 Virus Variants of Concern (VOC). Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Virusvariante.html?nn=2386228 (accessed on 15 December 2022).
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef]
- Sivan, M.; Preston, N.; Parkin, A.; Makower, S.; Gee, J.; Ross, D.; Tarrant, R.; Davison, J.; Halpin, S.; O’Connor, R.J.; et al. The modified COVID-19 Yorkshire Rehabilitation Scale (C19-YRSm) patient-reported outcome measure for Long Covid or Post-COVID-19 syndrome. J. Med. Virol. 2022, 94, 4253–4264. [Google Scholar] [CrossRef]
- Klok, F.A.; Boon, G.; Barco, S.; Endres, M.; Geelhoed, J.J.M.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The Post-COVID-19 Functional Status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020, 56, 2001494. [Google Scholar] [CrossRef]
- Núñez-Cortés, R.; Rivera-Lillo, G.; Arias-Campoverde, M.; Soto-García, D.; García-Palomera, R.; Torres-Castro, R. Use of sit-to-stand test to assess the physical capacity and exertional desaturation in patients post COVID-19. Chronic Respir. Dis. 2021, 18, 1479973121999205. [Google Scholar] [CrossRef] [PubMed]
- Torres-Castro, R.; Solis-Navarro, L.; Sitja-Rabert, M.; Vilaro, J. Functional Limitations Post-COVID-19: A Comprehensive Assessment Strategy. Arch. Bronconeumol. 2021, 57, 7–8. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Post COVID-19 Condition (Long COVID). Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (accessed on 9 December 2022).
- Lemhofer, C.; Appel, K.S.; Hauser, W.; Hettich, N.; Kohls, M.; Polidori, M.C. Post COVID—Just a matter of definition? Dtsch. Med. Wochenschr. 2022, 147, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Long-COVIDGermany, L.-C. Guidance for projects on research and care for ME/CFS and post-COVID syndrome. Available online: https://longcoviddeutschland.org/leitfaden/ (accessed on 15 March 2022).
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.; Sawant, H.B.; Flannery, T.; Tarrant, R.; Shardha, J.; Bannister, R.; Ross, D.; Halpin, S.; Greenwood, D.C.; Sivan, M. Effect of using a structured pacing protocol on post-exertional symptom exacerbation and health status in a longitudinal cohort with the post-COVID-19 syndrome. J. Med. Virol. 2023, 95, e28373. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, I.; Rodriguez-Mañas, L.; Laosa, O. Long COVID-19: The Need for an Interdisciplinary Approach. Clin. Geriatr. Med. 2022, 38, 533–544. [Google Scholar] [CrossRef]
- Kupferschmitt, A.; Etzrodt, F.; Kleinschmidt, J.; Kollner, V. Not Only Multimodal, but also Interdisciplinary: A Concept for Interdisciplinary Cooperation in the Rehabilitation of Post-COVID Syndrome. Psychother. Psychosom. Med. Psychol. 2023, 73, 34–41. [Google Scholar] [CrossRef]
- Hallek, M.; Adorjan, K.; Behrends, U.; Ertl, G.; Suttorp, N.; Lehmann, C. Post-COVID Syndrome. Dtsch. Arztebl. Int. 2023, 120, 48–55. [Google Scholar] [CrossRef]
- Decary, S.; De Groote, W.; Arienti, C.; Kiekens, C.; Boldrini, P.; Lazzarini, S.G.; Dugas, M.; Stefan, T.; Langlois, L.; Daigle, F.; et al. Scoping review of rehabilitation care models for post COVID-19 condition. Bull. World Health Organ. 2022, 100, 676–688. [Google Scholar] [CrossRef]
- Stallmach, A.; Katzer, K.; Besteher, B.; Finke, K.; Giszas, B.; Gremme, Y.; Abou Hamdan, R.; Lehmann-Pohl, K.; Legen, M.; Lewejohann, J.C.; et al. Mobile primary healthcare for post-COVID patients in rural areas: A proof-of-concept study. Infection 2023, 51, 337–345. [Google Scholar] [CrossRef]
- Lemhöfer, C.; Best, N.; Bökel, A.; Brugger, S.; Gutenbrunner, C.; Loudovici-Krug, D.; Sturm, C. Zufriedenheit COVID-19-Erkrankter mit den Akteuren des Gesundheitssystems und der rehabilitativen Therapieversorgung unter Verwendung des COVID-19-Rehabilitation Needs Questionnaire (C19-RehabNeQ) in Bayern. Phys. Med. Rehabil. Kurortmed. 2021, 32, 11–18. [Google Scholar] [CrossRef]
- Brown, S.M.; Bose, S.; Banner-Goodspeed, V.; Beesley, S.J.; Dinglas, V.D.; Hopkins, R.O.; Jackson, J.C.; Mir-Kasimov, M.; Needham, D.M.; Sevin, C.M.; et al. Approaches to Addressing Post-Intensive Care Syndrome among Intensive Care Unit Survivors. A Narrative Review. Ann. Am. Thorac. Soc. 2019, 16, 947–956. [Google Scholar] [CrossRef]
- Valverde-Martínez, M.; López-Liria, R.; Martínez-Cal, J.; Benzo-Iglesias, M.J.; Torres-Álamo, L.; Rocamora-Pérez, P. Telerehabilitation, A Viable Option in Patients with Persistent Post-COVID Syndrome: A Systematic Review. Healthcare 2023, 11, 187. [Google Scholar] [CrossRef]
- Reinert, G.; Müller, D.; Wagner, P.; Martínez-Pozas, O.; Cuenca-Záldivar, J.N.; Fernández-Carnero, J.; Sánchez Romero, E.A.; Corbellini, C. Pulmonary Rehabilitation in SARS-CoV-2: A Systematic Review and Meta-Analysis of Post-Acute Patients. Diagnostics 2022, 12, 3032. [Google Scholar] [CrossRef]

| N | % | ||
|---|---|---|---|
| Pre-Existing Conditions | |||
| Chronic lung disease (n = 919) | existing | 132 | 14.4% |
| Mental disorders (n = 914) | existing | 125 | 13.7% |
| Chronic pain (n = 912) | existing | 46 | 5.0% |
| Coronary artery disease (n = 912) | existing | 49 | 5.1% |
| Chronic heart failure (n = 913) | existing | 40 | 4.2% |
| Coagulation disorder (n = 913) | existing | 27 | 3.0% |
| Acute medical treatment | |||
| Inpatient treatment (n = 952) | no | 711 | 75.8% |
| yes | 148 | 15.5% | |
| yes, with ICU | 82 | 8.6% | |
| Duration since infection at the time points of presentation | |||
| Duration since infection (1st presentation) | 3–6 Mon | 249 | 26.2% |
| 6–9 Mon | 250 | 26.3% | |
| 9–12 Mon | 178 | 18.7% | |
| 12–15 Mon | 149 | 15.7% | |
| 15–18 Mon | 81 | 8.5% | |
| 18–24 Mon | 42 | 4.4% | |
| >24 Mon | 3 | 0.3% | |
| Duration since infection (1st re-presentation) | 3–6 Mon | 1 | 0.3% |
| 6–9 Mon | 67 | 17.7% | |
| 9–12 Mon | 97 | 25.6% | |
| 12–15 Mon | 99 | 26.1% | |
| 15–18 Mon | 66 | 17.4% | |
| 18–24 Mon | 46 | 12.1% | |
| >24 Mon | 3 | 0.8% | |
| Duration since infection (2nd re-presentation) | 3–6 Mon | 0 | 0.0% |
| 6–9 Mon | 0 | 0.0% | |
| 9–12 Mon | 8 | 6.2% | |
| 12–15 Mon | 39 | 30.2% | |
| 15–18 Mon | 40 | 31.0% | |
| 18–24 Mon | 33 | 25.6% | |
| >24 Mon | 9 | 7.0% | |
| 1st Presentation (n = 952) | 1st Re-Presentation (n = 379) | 2nd Re-Presentation (n = 129) | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Symptom complexes | |||||||
| Fatigue | existing | 766 | 80.5% | 304 | 80.2% | 106 | 82.2% |
| Neurological ailments | existing | 724 | 76.1% | 290 | 76.5% | 104 | 80.6% |
| Exercise intolerance | existing | 512 | 53.8% | 176 | 46.4% | 69 | 53.5% |
| Joint and muscle pain | existing | 400 | 42.0% | 206 | 54.4% | 72 | 55.8% |
| Sleeping disturbance | existing | 364 | 38.2% | 178 | 47.0% | 63 | 48.8% |
| Chemosensory deficits | existing | 281 | 29.5% | 104 | 27.4% | 26 | 20.2% |
| Gastrointestinal ailments | existing | 141 | 14.8% | 75 | 19.8% | 35 | 27.1% |
| Cough, wheezing | existing | 127 | 13.3% | 63 | 16.6% | 24 | 18.6% |
| Chest pain | existing | 75 | 7.9% | 22 | 5.8% | 6 | 4.7% |
| ENT ailments | existing | 67 | 7.0% | 40 | 10.6% | 15 | 11.6% |
| Dermatological ailments | existing | 66 | 6.9% | 25 | 6.6% | 5 | 3,9% |
| Infection signs | existing | 14 | 1.5% | 3 | 0.8% | 2 | 1.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemhöfer, C.; Bahmer, T.; Baumbach, P.; Besteher, B.; Boekel, A.; Finke, K.; Katzer, K.; Lehmann-Pohl, K.; Lewejohann, J.-C.; Loudovici-Krug, D.; et al. Variations and Predictors of Post-COVID Syndrome Severity in Patients Attending a Post-COVID Outpatient Clinic. J. Clin. Med. 2023, 12, 4013. https://doi.org/10.3390/jcm12124013
Lemhöfer C, Bahmer T, Baumbach P, Besteher B, Boekel A, Finke K, Katzer K, Lehmann-Pohl K, Lewejohann J-C, Loudovici-Krug D, et al. Variations and Predictors of Post-COVID Syndrome Severity in Patients Attending a Post-COVID Outpatient Clinic. Journal of Clinical Medicine. 2023; 12(12):4013. https://doi.org/10.3390/jcm12124013
Chicago/Turabian StyleLemhöfer, Christina, Thomas Bahmer, Philipp Baumbach, Bianca Besteher, Andrea Boekel, Kathrin Finke, Katrin Katzer, Katja Lehmann-Pohl, Jan-Christoph Lewejohann, Dana Loudovici-Krug, and et al. 2023. "Variations and Predictors of Post-COVID Syndrome Severity in Patients Attending a Post-COVID Outpatient Clinic" Journal of Clinical Medicine 12, no. 12: 4013. https://doi.org/10.3390/jcm12124013





