Neck Pain Disability on Headache Impact and the Association between Sleep Disturbance and Neck Pain in Migraine
Abstract
1. Introduction
2. Materials and Methods
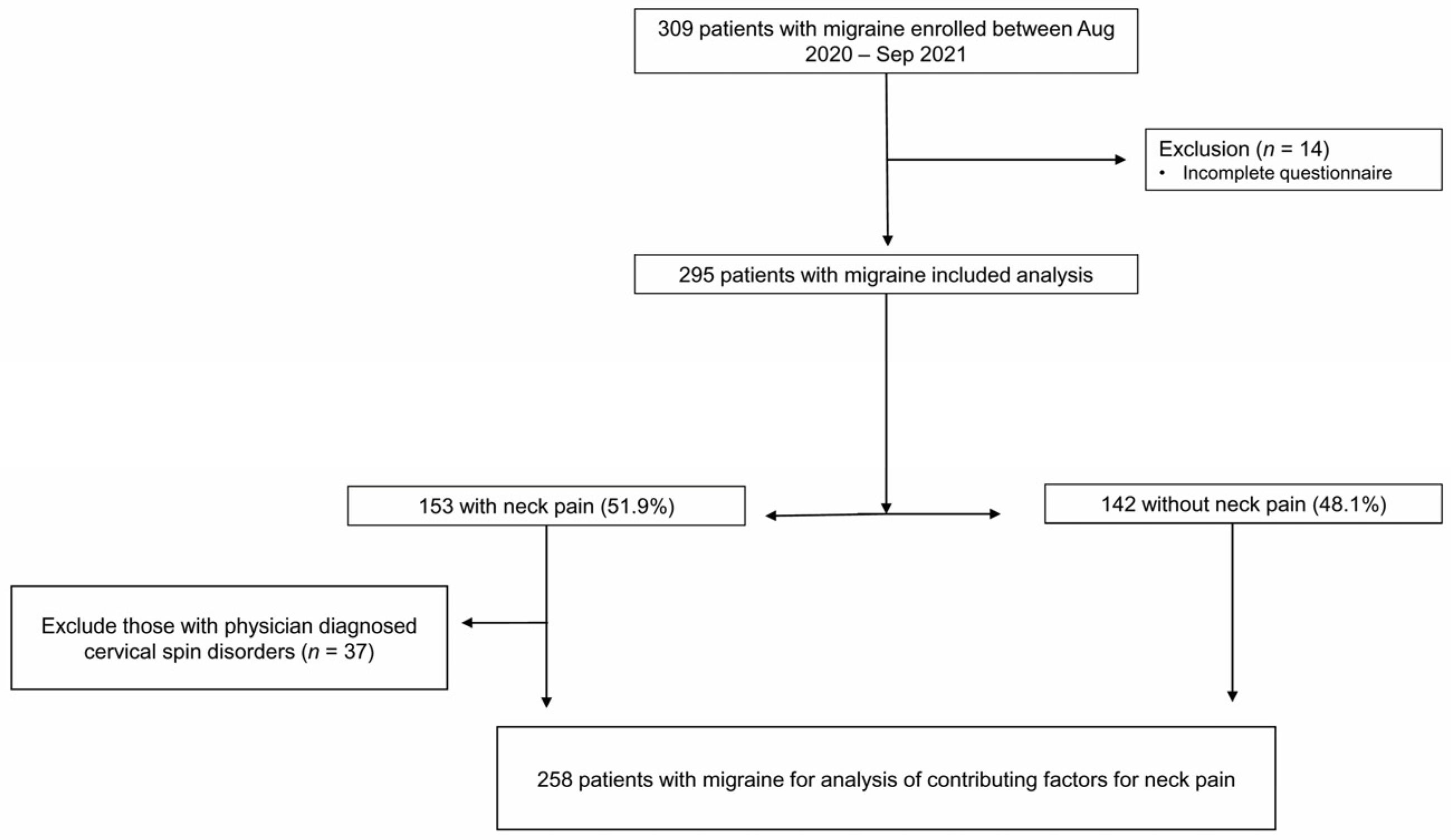
2.1. Participants
2.2. Assessment of Headache
2.3. Assessment of NP
2.4. Assessment of Sleep and Mood Parameters
2.5. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Disability of NP as the Predictor for Severe Impact of Headache in Migraineurs
3.3. Contributing Factors for the Presence of NP in 258 Migraine Patients after Excluding Those with Physician-Diagnosed Cervical Spine or Disc Disorders
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Safiri, S.; Kolahi, A.A.; Hoy, D.; Buchbinder, R.; Mansournia, M.A.; Bettampadi, D.; Ashrafi-Asgarabad, A.; Almasi-Hashiani, A.; Smith, E.; Sepidarkish, M.; et al. Global, regional, and national burden of neck pain in the general population, 1990–2017: Systematic analysis of the Global Burden of Disease Study 2017. BMJ 2020, 368, m791. [Google Scholar] [CrossRef]
- Shin, D.W.; Shin, J.I.; Koyanagi, A.; Jacob, L.; Smith, L.; Lee, H.; Chang, Y.; Song, T.J. Global, regional, and national neck pain burden in the general population, 1990–2019: An analysis of the global burden of disease study 2019. Front. Neurol. 2022, 13, 955367. [Google Scholar] [CrossRef]
- Evers, S. Comparison of cervicogenic headache with migraine. Cephalalgia 2008, 28 (Suppl. S1), 16–17. [Google Scholar] [CrossRef]
- Ashina, S.; Bendtsen, L.; Lyngberg, A.C.; Lipton, R.B.; Hajiyeva, N.; Jensen, R. Prevalence of neck pain in migraine and tension-type headache: A population study. Cephalalgia 2015, 35, 211–219. [Google Scholar] [CrossRef]
- Krøll, L.S.; Hammarlund, C.S.; Westergaard, M.L.; Nielsen, T.; Sloth, L.B.; Jensen, R.H.; Gard, G. Level of physical activity, well-being, stress and self-rated health in persons with migraine and co-existing tension-type headache and neck pain. J. Headache Pain 2017, 18, 46. [Google Scholar] [CrossRef]
- Al-Khazali, H.M.; Younis, S.; Al-Sayegh, Z.; Ashina, S.; Ashina, M.; Schytz, H.W. Prevalence of neck pain in migraine: A systematic review and meta-analysis. Cephalalgia 2022, 42, 663–673. [Google Scholar] [CrossRef]
- Penzien, D.; Rains, J.; Andrew, M.; Galovski, T.; Mohammad, Y.; Mosley, T. Relationships of daily stress, sleep, and headache: A time-series analysis. Cephalalgia Int. J. Headache 2001, 21, 262–263. [Google Scholar]
- Kim, S.H.; Lee, D.H.; Yoon, K.B.; An, J.R.; Yoon, D.M. Factors associated with increased risk for clinical insomnia in patients with chronic neck pain. Pain Physician 2015, 18, 593–598. [Google Scholar]
- Artner, J.; Cakir, B.; Spiekermann, J.-A.; Kurz, S.; Leucht, F.; Reichel, H.; Lattig, F. Prevalence of sleep deprivation in patients with chronic neck and back pain: A retrospective evaluation of 1016 patients. J. Pain Res. 2013, 6, 1–6. [Google Scholar] [CrossRef]
- Shimohata, K.; Hasegawa, K.; Onodera, O.; Nishizawa, M.; Shimohata, T. The Clinical Features, Risk Factors, and Surgical Treatment of Cervicogenic Headache in Patients with Cervical Spine Disorders Requiring Surgery. Headache 2017, 57, 1109–1117. [Google Scholar] [CrossRef]
- Bir, S.C.; Nanda, A.; Patra, D.P.; Maiti, T.K.; Liendo, C.; Minagar, A.; Chernyshev, O.Y. Atypical presentation and outcome of cervicogenic headache in patients with cervical degenerative disease: A single-center experience. Clin. Neurol. Neurosurg. 2017, 159, 62–69. [Google Scholar] [CrossRef]
- The International Headache Society’s Core Curriculum on Headache for Neurologists. Br. J. Pain 2012, 6, 103–105. [CrossRef]
- Yang, M.; Rendas-Baum, R.; Varon, S.F.; Kosinski, M. Validation of the Headache Impact Test (HIT-6™) across episodic and chronic migraine. Cephalalgia 2011, 31, 357–367. [Google Scholar] [CrossRef]
- Stewart, W.F.; Lipton, R.; Kolodner, K.; Liberman, J.; Sawyer, J. Reliability of the migraine disability assessment score in a population-based sample of headache sufferers. Cephalalgia 1999, 19, 107–114. [Google Scholar] [CrossRef]
- Guzman, J.; Hurwitz, E.L.; Carroll, L.J.; Haldeman, S.; Cote, P.; Carragee, E.J.; Peloso, P.M.; van der Velde, G.; Holm, L.W.; Hogg-Johnson, S.; et al. A new conceptual model of neck pain: Linking onset, course, and care: The Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. J. Manip. Physiol. Ther. 2009, 32, S17–S28. [Google Scholar] [CrossRef]
- Von Korff, M.; Ormel, J.; Keefe, F.J.; Dworkin, S.F. Grading the severity of chronic pain. Pain 1992, 50, 133–149. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Chung, F.; Abdullah, H.R.; Liao, P. STOP-Bang questionnaire: A practical approach to screen for obstructive sleep apnea. Chest 2016, 149, 631–638. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr. Ann. 2002, 32, 509–515. [Google Scholar] [CrossRef]
- Hoy, D.G.; Protani, M.; De, R.; Buchbinder, R. The epidemiology of neck pain. Best Pract. Res. Clin. Rheumatol. 2010, 24, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Hvedstrup, J.; Kolding, L.T.; Younis, S.; Ashina, M.; Schytz, H.W. Ictal neck pain investigated in the interictal state—A search for the origin of pain. Cephalalgia 2020, 40, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, A.H.; Ford, S.; Millen, C.; Finkel, A.G.; Truong, Y.; Nie, Y. The prevalence of neck pain in migraine. Headache J. Head Face Pain 2010, 50, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Thomas, L.; Jull, G.; Treleaven, J. The Neck Disability Index Reflects Allodynia and Headache Disability but Not Cervical Musculoskeletal Dysfunction in Migraine. Phys. Ther. 2022, 102, pzac027. [Google Scholar] [CrossRef]
- Castien, R.; De Hertogh, W. A Neuroscience Perspective of Physical Treatment of Headache and Neck Pain. Front. Neurol. 2019, 10, 276. [Google Scholar] [CrossRef]
- Ambalavanar, R.; Dessem, D.; Moutanni, A.; Yallampalli, C.; Yallampalli, U.; Gangula, P.; Bai, G. Muscle inflammation induces a rapid increase in calcitonin gene-related peptide (CGRP) mRNA that temporally relates to CGRP immunoreactivity and nociceptive behavior. Neuroscience 2006, 143, 875–884. [Google Scholar] [CrossRef]
- Olmos, S.R. Comorbidities of chronic facial pain and obstructive sleep apnea. Curr. Opin. Pulm. Med. 2016, 22, 570–575. [Google Scholar] [CrossRef]
- Charokopos, A.; Card, M.E.; Gunderson, C.; Steffens, C.; Bastian, L.A. The association of obstructive sleep apnea and pain outcomes in adults: A systematic review. Pain Med. 2018, 19, S69–S75. [Google Scholar] [CrossRef]
- Hatipoğlu, U.; Rubinstein, I. Inflammation and obstructive sleep apnea syndrome pathogenesis: A working hypothesis. Respiration 2003, 70, 665–671. [Google Scholar] [CrossRef]
- Stepanski, E.J. The effect of sleep fragmentation on daytime function. Sleep 2002, 25, 268–276. [Google Scholar] [CrossRef]
- Sutton, B.C.; Opp, M.R. Sleep fragmentation exacerbates mechanical hypersensitivity and alters subsequent sleep-wake behavior in a mouse model of musculoskeletal sensitization. Sleep 2014, 37, 515–524. [Google Scholar] [CrossRef]
- Waliszewska-Prosół, M.; Nowakowska-Kotas, M.; Chojdak-Łukasiewicz, J.; Budrewicz, S. Migraine and Sleep—An Unexplained Association? Int. J. Mol. Sci. 2021, 22, 5539. [Google Scholar] [CrossRef]
- Vgontzas, A.; Pavlović, J.M. Sleep Disorders and Migraine: Review of Literature and Potential Pathophysiology Mechanisms. Headache 2018, 58, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Sjaastad, O.; Fredriksen, T.A.; Pfaffenrath, V. Cervicogenic headache: Diagnostic criteria. The Cervicogenic Headache International Study Group. Headache 1998, 38, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Fredriksen, T.A.; Antonaci, F.; Sjaastad, O. Cervicogenic headache: Too important to be left un-diagnosed. J. Headache Pain 2015, 16, 6. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Presence of Neck Pain (n = 153) | Absence of Neck Pain (n = 142) | p-Value |
|---|---|---|---|
| Age (y) | 39.9 (32, 47) | 38.0 (31, 44) | 0.166 |
| Female sex | 123 (80.4) | 94 (66.2) | 0.009 |
| BMI (kg/m2) | 22.9 (20.8, 24.9) | 23.2 (20.9, 25.0) | 0.373 |
| Chronic migraine | 61 (39.9) | 40 (28.2) | 0.046 |
| Migraine with aura | 15 (9.8) | 22 (15.5) | 0.194 |
| Monthly headache days | 12.8 (5, 20) | 10.1 (4, 15) | 0.006 |
| Medication day per month | 7.6 (2, 10) | 6.4 (2, 10) | 0.261 |
| Medication overuse headache | 48 (31.4) | 28 (19.7) | 0.031 |
| MIDAS ≥ 21 | 70 (45.8) | 42 (29.6) | 0.006 |
| Severe impact of headache (HIT-6 ≥ 60) | 101 (66.0) | 71 (50.0) | 0.008 |
| Anxiety (GAD-7 ≥ 6) | 67 (43.8) | 56 (39.4) | 0.522 |
| Depressive mood (PHQ-9 ≥ 10) | 48 (31.4) | 30 (21.1) | 0.063 |
| Excessive daytime sleepiness (ESS ≥ 11) | 32 (20.9) | 26 (18.3) | 0.677 |
| Moderate-to-severe insomnia (ISI ≥ 15) | 40 (26.1) | 23 (16.2) | 0.052 |
| High risk of obstructive sleep apnea (STOP-bang ≥ 3) | 16 (10.5) | 6 (4.2) | 0.070 |
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Disability of neck pain | ||||
| Absence of neck pain | 1 | 1 | ||
| Low disability of neck pain | 1.55 (0.95–2.52) | 0.077 | 1.10 (0.56–2.16) | 0.785 |
| High disability of neck pain | 8.33 (2.41–28.85) | 0.008 | 6.14 (1.41–26.70) | 0.016 |
| Age | 0.98 (0.96–1.00) | 0.108 | 0.98 (0.95–1.01) | 0.213 |
| Female sex | 1.19 (0.71–2.01) | 0.507 | 1.03 (0.50–2.10) | 0.946 |
| BMI | 0.98 (0.92–1.06) | 0.663 | 0.97 (0.88–1.07) | 0.575 |
| Chronic migraine | 3.61 (2.09–6.21) | <0.001 | 0.97 (0.39–2.45) | 0.953 |
| Migraine with aura | 0.82 (0.41–1.64) | 0.575 | 1.38 (0.56–3.39) | 0.484 |
| MOH | 5.48 (2.80–10.72) | <0.001 | 2.40 (0.83–6.91) | 0.106 |
| Monthly headache days | 1.09 (1.05–1.13) | <0.001 | 0.99 (0.94–1.04) | 0.765 |
| Medication day per month | 1.15 (1.09–1.21) | <0.001 | 1.10 (1.03–1.18) | 0.008 |
| MIDAS ≥ 21 | 11.48 (5.99–21.97) | <0.001 | 7.19 (3.42–15.14) | <0.001 |
| Anxiety (GAD-7 ≥ 6) | 2.19 (1.34–3.55) | 0.002 | 1.07 (0.55–2.01) | 0.844 |
| Depressive mood (PHQ-9 ≥ 10) | 4.16 (2.23–7.76) | <0.001 | 2.82 (1.14–7.00) | 0.025 |
| Excessive daytime sleepiness (ESS ≥ 11) | 3.83 (1.89–7.74) | <0.001 | 2.57 (1.05–6.30) | 0.038 |
| Moderate to severe insomnia (ISI ≥ 15) | 2.51 (1.35–4.69) | 0.004 | 0.89 (0.35–2.24) | 0.802 |
| High risk of obstructive sleep apnea (STOP-bang ≥ 3) | 2.00 (0.76–5.27) | 0.161 | 1.53 (0.40–5.90) | 0.536 |
| Physician diagnosed cervical spine or disc disorders | 1.81 (0.86–3.83) | 0.118 | 1.45 (0.52–4.01) | 0.479 |
| Factors | OR (95% CI) | p-Value | |
|---|---|---|---|
| Demographic | Age | 1.00 (0.97–1.03) | 0.926 |
| Female sex | 1.92 (1.02–3.61) | 0.042 | |
| BMI | 0.96 (0.86–1.04) | 0.294 | |
| Headache related | Chronic migraine | 0.97 (0.51–1.86) | 0.370 |
| Migraine with aura | 0.71 (0.31–1.62) | 0.415 | |
| Monthly headache days | 1.04 (1.00–1.08) | 0.059 | |
| Monthly medication days | 0.93 (0.94–1.08) | 0.370 | |
| Severe impact of headache (HIT-6 ≥ 60) | 1.55 (0.81–2.59) | 0.215 | |
| Mood related | GAD-7 score | 1.00 (0.92–1.08) | 0.904 |
| PHQ-9 score | 0.99 (0.92–1.08) | 0.866 | |
| Sleep related | Moderate to severe insomnia (ISI ≥ 15) | 1.86 (0.87–4.00) | 0.112 |
| High risk of obstructive sleep apnea (STOP-bang ≥ 3) | 3.61 (1.19–11.00) | 0.024 | |
| Excessive daytime sleepiness (ESS ≥ 11) | 0.78 (0.38–1.63) | 0.510 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, H.-J.; Hong, Y.-H.; Cho, S.-J. Neck Pain Disability on Headache Impact and the Association between Sleep Disturbance and Neck Pain in Migraine. J. Clin. Med. 2023, 12, 3989. https://doi.org/10.3390/jcm12123989
Im H-J, Hong Y-H, Cho S-J. Neck Pain Disability on Headache Impact and the Association between Sleep Disturbance and Neck Pain in Migraine. Journal of Clinical Medicine. 2023; 12(12):3989. https://doi.org/10.3390/jcm12123989
Chicago/Turabian StyleIm, Hee-Jin, Yoo-Ha Hong, and Soo-Jin Cho. 2023. "Neck Pain Disability on Headache Impact and the Association between Sleep Disturbance and Neck Pain in Migraine" Journal of Clinical Medicine 12, no. 12: 3989. https://doi.org/10.3390/jcm12123989
APA StyleIm, H.-J., Hong, Y.-H., & Cho, S.-J. (2023). Neck Pain Disability on Headache Impact and the Association between Sleep Disturbance and Neck Pain in Migraine. Journal of Clinical Medicine, 12(12), 3989. https://doi.org/10.3390/jcm12123989





