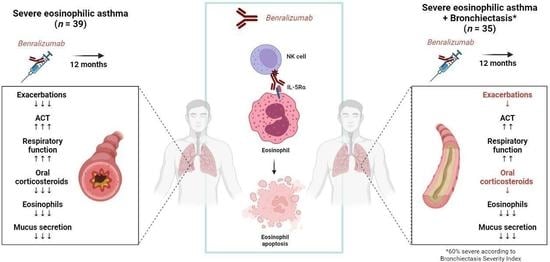
Benralizumab Effectiveness in Severe Eosinophilic Asthma with Co-Presence of Bronchiectasis: A Real-World Multicentre Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Inclusion Criteria
- Diagnosed with SEA as defined by the European Respiratory Society/American Thoracic Society (ERS/ATS) guidelines [29] and compliant to maintenance therapy;
- Treated with benralizumab (30 mg once every 4 weeks for the first 3 doses, then once every 8 weeks) for at least 12 months between February 2020 and September 2022, with adequate prescription adherence;
- Underwent high-resolution computed tomography (HRCT) at baseline <6 months before starting the anti-IL-5Rα biologic.
2.3. Data Collection and Assessment
2.4. Diagnosis and Evaluation of the Severity of Bronchiectasis
2.5. Statistical Analysis
3. Results
3.1. Baseline Patient Demographics and Clinical Characteristics
3.2. Benralizumab Effectiveness in the Entire Cohort
3.3. Effectiveness of Benralizumab in Severe Eosinophilic Asthma with or without Bronchiectasis
3.4. Remission According to the Presence of Bronchiectasis
3.5. Benralizumab Effectiveness According to Bronchiectasis Severity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, K.F. Diagnosis and Management of Severe Asthma. Semin. Respir. Crit. Care Med. 2018, 39, 091–099. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.R.; Bateman, E.D.; Boulet, L.-P.; Boushey, H.A.; Busse, W.W.; Casale, T.B.; Chanez, P.; Enright, P.L.; Gibson, P.G.; de Jongste, J.C.; et al. A new perspective on concepts of asthma severity and control. Eur. Respir. J. 2008, 32, 545–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porsbjerg, C.; Menzies-Gow, A. Co-morbidities in severe asthma: Clinical impact and management. Respirology 2017, 22, 651–661. [Google Scholar] [CrossRef] [Green Version]
- Denlinger, L.C.; Phillips, B.R.; Ramratnam, S.; Ross, K.; Bhakta, N.R.; Cardet, J.C.; Castro, M.; Peters, S.P.; Phipatanakul, W.; Aujla, S.; et al. Inflammatory and Comorbid Features of Patients with Severe Asthma and Frequent Exacerbations. Am. J. Respir. Crit. Care Med. 2017, 195, 302–313. [Google Scholar] [CrossRef] [Green Version]
- Crimi, C.; Campisi, R.; Cacopardo, G.; Intravaia, R.; Nolasco, S.; Porto, M.; Pelaia, C.; Crimi, N. Real-life effectiveness of mepolizumab in patients with severe refractory eosinophilic asthma and multiple comorbidities. World Allergy Organ. J. 2020, 13, 100462. [Google Scholar] [CrossRef] [PubMed]
- Bardin, P.G.; Rangaswamy, J.; Yo, S. Managing comorbid conditions in severe asthma. Med. J. Aust. 2018, 209, S11–S17. [Google Scholar] [CrossRef]
- Tay, T.R.; Radhakrishna, N.; Hore-Lacy, F.; Smith, C.; Hoy, R.; Dabscheck, E.; Hew, M. Comorbidities in difficult asthma are independent risk factors for frequent exacerbations, poor control and diminished quality of life. Respirology 2016, 21, 1384–1390. [Google Scholar] [CrossRef]
- Global Strategy for Asthma Management and Prevention. Ginasthma.org. Available online: https://ginasthma.org/wp-content/uploads/2023/05/GINA-2023-Full-Report-2023-WMS.pdf (accessed on 2 June 2023).
- Guan, W.-J.; Oscullo, G.; He, M.-Z.; Xu, D.-Y.; Gómez-Olivas, J.D.; Martinez-Garcia, M.A. Significance and Potential Role of Eosinophils in Non-Cystic Fibrosis Bronchiectasis. J. Allergy Clin. Immunol. Pract. 2022, 11, 1089–1099. [Google Scholar] [CrossRef]
- Martínez-García, M.Á. Bronchiectasis and Eosinophils. Arch. Bronconeumol. 2021, 57, 671–672. [Google Scholar] [CrossRef]
- Keir, H.R.; Chalmers, J.D. Bronchiectasis enters the inflammation era. Respirology 2022, 27, 488–489. [Google Scholar] [CrossRef]
- Crimi, C.; Ferri, S.; Crimi, N. Bronchiectasis and asthma: A dangerous liaison? Curr. Opin. Allergy Clin. Immunol. 2019, 19, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Ferri, S.; Crimi, C.; Campisi, R.; Cacopardo, G.; Paoletti, G.; Puggioni, F.; Crimi, N.; Heffler, E. Impact of asthma on bronchiectasis severity and risk of exacerbations. J. Asthma 2022, 59, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Muñoz, G.; López-De-Andrés, A.; Jiménez-García, R.; Hernández-Barrera, V.; Pedraza-Serrano, F.; Puente-Maestu, L.; de Miguel-Díez, J. Trend from 2001 to 2015 in the prevalence of bronchiectasis among patients hospitalized for asthma and effect of bronchiectasis on the in-hospital mortality. J. Asthma 2021, 58, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H. Bronchiectasis in severe asthma and asthmatic components in bronchiectasis. Respir. Investig. 2022, 60, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Crimi, C.; Ferri, S.; Campisi, R.; Crimi, N. The Link between Asthma and Bronchiectasis: State of the Art. Respiration 2020, 99, 463–476. [Google Scholar] [CrossRef]
- Crimi, C.; Campisi, R.; Nolasco, S.; Ferri, S.; Cacopardo, G.; Impellizzeri, P.; Pistorio, M.P.; Fagone, E.; Pelaia, C.; Heffler, E.; et al. Type 2-High Severe Asthma with and without Bronchiectasis: A Prospective Observational Multicentre Study. J. Asthma Allergy 2021, 14, 1441–1452. [Google Scholar] [CrossRef]
- Dagher, R.; Kumar, V.; Copenhaver, A.M.; Gallagher, S.; Ghaedi, M.; Boyd, J.; Newbold, P.; Humbles, A.A.; Kolbeck, R. Novel mechanisms of action contributing to benralizumab’s potent anti-eosinophilic activity. Eur. Respir. J. 2022, 59, 2004306. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkström, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- Nair, P.; Wenzel, S.; Rabe, K.F.; Bourdin, A.; Lugogo, N.L.; Kuna, P.; Barker, P.; Sproule, S.; Ponnarambil, S.; Goldman, M. Oral Glucocorticoid–Sparing Effect of Benralizumab in Severe Asthma. N. Engl. J. Med. 2017, 376, 2448–2458. [Google Scholar] [CrossRef]
- Bleecker, E.R.; Wechsler, M.E.; Fitzgerald, J.M.; Menzies-Gow, A.; Wu, Y.; Hirsch, I.; Goldman, M.; Newbold, P.; Zangrilli, J.G. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur. Respir. J. 2018, 52, 1800936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolasco, S.; Crimi, C.; Pelaia, C.; Benfante, A.; Caiaffa, M.F.; Calabrese, C.; Carpagnano, G.E.; Ciotta, D.; D’Amato, M.; Macchia, L.; et al. Benralizumab Effectiveness in Severe Eosinophilic Asthma with and without Chronic Rhinosinusitis with Nasal Polyps: A Real-World Multicenter Study. J. Allergy Clin. Immunol. Pract. 2021, 9, 4371–4380. [Google Scholar] [CrossRef]
- Pelaia, C.; Crimi, C.; Nolasco, S.; Carpagnano, G.E.; Brancaccio, R.; Buonamico, E.; Campisi, R.; Gagliani, C.; Patella, V.; Pelaia, G.; et al. Switch from Omalizumab to Benralizumab in Allergic Patients with Severe Eosinophilic Asthma: A Real-Life Experience from Southern Italy. Biomedicines 2021, 9, 1822. [Google Scholar] [CrossRef]
- Pelaia, C.; Crimi, C.; Benfante, A.; Caiaffa, M.F.; Calabrese, C.; Carpagnano, G.E.; Ciotta, D.; D’Amato, M.; Macchia, L.; Nolasco, S.; et al. Therapeutic Effects of Benralizumab Assessed in Patients with Severe Eosinophilic Asthma: Real-Life Evaluation Correlated with Allergic and Non-Allergic Phenotype Expression. J. Asthma Allergy 2021, 14, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Nolasco, S.; Campisi, R.; Intravaia, R.; Porto, M.; Pelaia, C.; Crimi, N.; Crimi, C. Case Report: Acute effect of benralizumab on asthma exacerbation without concomitant corticosteroid use. F1000Research 2020, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Busceti, M.T.; Crimi, C.; Carpagnano, G.E.; Lombardo, N.; Terracciano, R.; Vatrella, A.; Pelaia, G. Real-Life effects of benralizumab on exacerbation number and lung hyperinflation in atopic patients with severe eosinophilic asthma. Biomed. Pharmacother. 2020, 129, 110444. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Busceti, M.T.; Vatrella, A.; Ciriolo, M.; Garofalo, E.; Crimi, C.; Terracciano, R.; Lombardo, N.; Pelaia, G. Effects of the first three doses of benralizumab on symptom control, lung function, blood eosinophils, oral corticosteroid intake, and nasal polyps in a patient with severe allergic asthma. SAGE Open Med. Case Rep. 2020, 8, 2050313X20906963. [Google Scholar] [CrossRef]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef] [Green Version]
- Reddel, H.K.; Taylor, D.R.; Bateman, E.D.; Boulet, L.-P.; Boushey, H.A.; Busse, W.W.; Casale, T.B.; Chanez, P.; Enright, P.L.; Gibson, P.G.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Asthma Control and Exacerbations. Am. J. Respir. Crit. Care Med. 2009, 180, 59–99. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, C.M.; Pride, N.B. Definitions of emphysema, chronic bronchitis, asthma, and airflow obstruction: 25 years on from the Ciba symposium. Thorax 1984, 39, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathan, R.A.; Sorkness, C.A.; Kosinski, M.; Schatz, M.; Li, J.T.; Marcus, P.; Murray, J.J.; Pendergraft, T.B. Development of the asthma control test: A survey for assessing asthma control. J. Allergy Clin. Immunol. 2004, 113, 59–65. [Google Scholar] [CrossRef]
- Crimi, C.; Campisi, R.; Noto, A.; Genco, S.; Cacopardo, G.; Nolasco, S.; Crimi, N. Comparability of asthma control test scores between self and physician-administered test. Respir. Med. 2020, 170, 106015. [Google Scholar] [CrossRef] [PubMed]
- ATS/ERS—American Thoracic Society, European Respiratory Society. ATS/ERS Recommendations for Standardized Procedures for the Online and Offline Measurement of Exhaled Lower Respiratory Nitric Oxide and Nasal Nitric Oxide, 2005. Am. J. Respir. Crit. Care Med. 2005, 171, 912–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menzies-Gow, A.; Hoyte, F.L.; Price, D.B.; Cohen, D.; Barker, P.; Kreindler, J.; Jison, M.; Brooks, C.L.; Papeleu, P.; Katial, R. Clinical Remission in Severe Asthma: A Pooled Post Hoc Analysis of the Patient Journey with Benralizumab. Adv. Ther. 2022, 39, 2065–2084. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Bafadhel, M.; Busse, W.W.; Casale, T.B.; Kocks, J.W.; Pavord, I.D.; Szefler, S.J.; Woodruff, P.G.; de Giorgio-Miller, A.; Trudo, F.; et al. An expert consensus framework for asthma remission as a treatment goal. J. Allergy Clin. Immunol. 2020, 145, 757–765. [Google Scholar] [CrossRef] [Green Version]
- Aliberti, S.; Goeminne, P.C.; E O’Donnell, A.; Aksamit, T.R.; Al-Jahdali, H.; Barker, A.F.; Blasi, F.; Boersma, W.G.; Crichton, M.L.; De Soyza, A.; et al. Criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults for use in clinical trials: International consensus recommendations. Lancet Respir. Med. 2022, 10, 298–306. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Goeminne, P.; Aliberti, S.; McDonnell, M.J.; Lonni, S.; Davidson, J.; Poppelwell, L.; Salih, W.; Pesci, A.; Dupont, L.J.; et al. The Bronchiectasis Severity Index. An International Derivation and Validation Study. Am. J. Respir. Crit. Care Med. 2014, 189, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Pasteur, M.C.; Helliwell, S.M.; Houghton, S.J.; Webb, S.C.; Foweraker, J.E.; Coulden, R.A.; Flower, C.D.; Bilton, D.; Keogan, M.T. An Investigation into Causative Factors in Patients with Bronchiectasis. Am. J. Respir. Crit. Care Med. 2000, 162, 1277–1284. [Google Scholar] [CrossRef] [Green Version]
- Kwok, W.C.; Ho, J.C.M.; Tam, T.C.C.; Ip, M.S.M.; Lam, D.C.L. Risk factors for Pseudomonas aeruginosa colonization in non-cystic fibrosis bronchiectasis and clinical implications. Respir. Res. 2021, 22, 132. [Google Scholar] [CrossRef]
- Coman, I.; Pola-Bibián, B.; Barranco, P.; Vila-Nadal, G.; Dominguez-Ortega, J.; Romero, D.; Villasante, C.; Quirce, S. Bronchiectasis in severe asthma. Ann. Allergy, Asthma Immunol. 2018, 120, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Dimakou, K.; Gousiou, A.; Toumbis, M.; Kaponi, M.; Chrysikos, S.; Thanos, L.; Triantafillidou, C. Investigation of bronchiectasis in severe uncontrolled asthma. Clin. Respir. J. 2018, 12, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Polverino, E.; Dimakou, K.; Hurst, J.; Martinez-Garcia, M.-A.; Miravitlles, M.; Paggiaro, P.; Shteinberg, M.; Aliberti, S.; Chalmers, J.D. The overlap between bronchiectasis and chronic airway diseases: State of the art and future directions. Eur. Respir. J. 2018, 52, 1800328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lujan, M.; Gallardo, X.; Amengual, M.J.; Bosque, M.; Mirapeix, R.M.; Domingo, C. Prevalence of Bronchiectasis in Asthma according to Oral Steroid Requirement: Influence of Immunoglobulin Levels. BioMed. Res. Int. 2013, 2013, 109219. [Google Scholar] [CrossRef] [PubMed]
- Carpagnano, G.E.; Scioscia, G.; Lacedonia, D.; Curradi, G.; Barbaro, M.P.F. Severe uncontrolled asthma with bronchiectasis: A pilot study of an emerging phenotype that responds to mepolizumab. J. Asthma Allergy 2019, 12, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crimi, C.; Campisi, R.; Nolasco, S.; Cacopardo, G.; Intravaia, R.; Porto, M.; Impellizzeri, P.; Pelaia, C.; Crimi, N. Mepolizumab effectiveness in patients with severe eosinophilic asthma and co-presence of bronchiectasis: A real-world retrospective pilot study. Respir. Med. 2021, 185, 106491. [Google Scholar] [CrossRef]
- Rademacher, J.; Konwert, S.; Fuge, J.; Dettmer, S.; Welte, T.; Ringshausen, F.C. Anti-IL5 and anti-IL5Rα therapy for clinically significant bronchiectasis with eosinophilic endotype: A case series. Eur. Respir. J. 2020, 55, 1901333. [Google Scholar] [CrossRef]
- Bendien, S.A.; van Loon-Kooij, S.; Kramer, G.; Huijgen, W.; Altenburg, J.; Brinke, A.T.; der Zee, A.-H.M.-V. Bronchiectasis in Severe Asthma: Does It Make a Difference? Respiration 2020, 99, 1136–1144. [Google Scholar] [CrossRef]
- García-Clemente, M.; Enríquez-Rodríguez, A.I.; Iscar-Urrutia, M.; Escobar-Mallada, B.; Arias-Guillén, M.; López-González, F.J.; Madrid-Carbajal, C.; Pérez-Martínez, L.; Gonzalez-Budiño, T. Severe asthma and bronchiectasis. J. Asthma 2020, 57, 505–509. [Google Scholar] [CrossRef]
- Shoemark, A.; Shteinberg, M.; De Soyza, A.; Haworth, C.S.; Richardson, H.; Gao, Y.; Perea, L.; Dicker, A.J.; Goeminne, P.C.; Cant, E.; et al. Characterization of Eosinophilic Bronchiectasis: A European Multicohort Study. Am. J. Respir. Crit. Care Med. 2022, 205, 894–902. [Google Scholar] [CrossRef]
- Oriano, M.; Gramegna, A.; Amati, F.; D’adda, A.; Gaffuri, M.; Contoli, M.; Bindo, F.; Simonetta, E.; Di Francesco, C.; Santambrogio, M.; et al. T2-High Endotype and Response to Biological Treatments in Patients with Bronchiectasis. Biomedicines 2021, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Heffler, E.; Terranova, G.; Chessari, C.; Frazzetto, V.; Crimi, C.; Fichera, S.; Picardi, G.; Nicolosi, G.; Porto, M.; Intravaia, R.; et al. Point-of-care blood eosinophil count in a severe asthma clinic setting. Ann. Allergy Asthma Immunol. 2017, 119, 16–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferri, S.; Crimi, C.; Heffler, E.; Campisi, R.; Noto, A.; Crimi, N. Vitamin D and disease severity in bronchiectasis. Respir. Med. 2019, 148, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dunican, E.M.; Elicker, B.M.; Gierada, D.S.; Nagle, S.K.; Schiebler, M.L.; Newell, J.D.; Raymond, W.W.; Lachowicz-Scroggins, M.E.; Di Maio, S.; Hoffman, E.A.; et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J. Clin. Investig. 2018, 128, 997–1009. [Google Scholar] [CrossRef] [Green Version]
- McIntosh, M.J.; Kooner, H.K.; Eddy, R.L.; Jeimy, S.; Licskai, C.; Mackenzie, C.A.; Svenningsen, S.; Nair, P.; Yamashita, C.; Parraga, G. Asthma Control, Airway Mucus, and 129Xe MRI Ventilation After a Single Benralizumab Dose. Chest 2022, 162, 520–533. [Google Scholar] [CrossRef]
- Campisi, R.; Crimi, C.; Nolasco, S.; Beghè, B.; Antonicelli, L.; Guarnieri, G.; Scichilone, N.; Porto, M.; Macchia, L.; Scioscia, G.; et al. Real-World Experience with Dupilumab in Severe Asthma: One-Year Data from an Italian Named Patient Program. J. Asthma Allergy 2021, 14, 575–583. [Google Scholar] [CrossRef]
- Harb, H.; Chatila, T.A. Mechanisms of Dupilumab. Clin. Exp. Allergy 2020, 50, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Nolasco, S.; Pelaia, C.; Scioscia, G.; Campisi, R.; Crimi, C. Tezepelumab for asthma. Drugs Today 2022, 58, 591. [Google Scholar] [CrossRef]
- Kudlaty, E.; Patel, G.B.; Prickett, M.L.; Yeh, C.; Peters, A.T. Efficacy of type 2-targeted biologics in patients with asthma and bronchiectasis. Ann. Allergy Asthma Immunol. 2021, 126, 302–304. [Google Scholar] [CrossRef]




| All (n = 74) | SEA (n = 39) | SEA + BE (n = 35) | p-Value | |
|---|---|---|---|---|
| Age, years, mean (SD) | 54.8 (11.9) | 53.9 (12.2) | 55.8 (11.8) | 0.4980 |
| Female, n (%) | 43 (58.1) | 22 (56.4) | 21 (60) | 0.8161 |
| BMI, mean (SD) | 25.9 (3.8) | 25.6 (4.2) | 26.2 (3.3) | 0.4737 |
| Age at onset, years, mean (SD) | 33.8 (15.3) | 35.8 (16.2) | 31.7 (14.1) | 0.2520 |
| Patients with positive Skin Prick Tests, n (%) | 36 (48.6) | 19 (48.7) | 17 (48.6) | 0.9999 |
| Smoking status | ||||
| Smoking history, n (%) | 17 (23) | 6 (15.4) | 11 (31.4) | 0.1655 |
| Current smoker, n (%) | 6 (8.1) | 4 (10.3) | 2 (5.7) | 0.6771 |
| Comorbidities | ||||
| Patients with GERD, n (%) | 34 (45.9) | 16 (41) | 18 (51.4) | 0.4940 |
| Patients with CRSwNP, n (%) | 37 (50) | 15 (38.5) | 22 (62.9) | 0.0618 |
| Patients with BE, n (%) | 35 (47.3) | 0 (0) | 35 (100) | n/a |
| Bronchiectasis assessment | ||||
| BSI, median (IQR) | 9 (7–11) | n/a | 9 (7–11) | n/a |
| Mild BSI (≤4), n (%) | 3 (4) | n/a | 3 (8.6) | n/a |
| Moderate BSI (5–8), n (%) | 11 (14.9) | n/a | 11 (31.4) | n/a |
| Severe BSI (≥9), n (%) | 21 (28.4) | n/a | 21 (60) | n/a |
| Patients with chronic mucus hypersecretion | 49 (66.2) | 20 (51.3) | 29 (82.9) | 0.0064 |
| Patients with microbial colonization, n (%) | 16 (21.6) | 4 (10.3) | 12 (34.3) | 0.0219 |
| P. Aeruginosa, n (%) | 4 (5.4) | 0 (0) | 4 (11.4) | 0.0455 |
| A. Fumigatus, n (%) | 4 (5.4) | 0 (0) | 4 (11.4) | 0.0455 |
| S. Aureus, n (%) | 5 (6.8) | 3 (7.7) | 2 (5.7) | 0.9999 |
| Other, n (%) | 3 (4) | 1 (2.6) | 2 (5.7) | 0.5999 |
| Asthma outcomes | ||||
| Asthma exacerbations/year, median (IQR) | 6 (4–8) | 5 (3.5–7) | 7 (6–12) | 0.0012 |
| ACT, median (IQR) | 14 (9–17) | 15 (10–18) | 13 (8–16) | 0.0175 |
| FEV1, %, median (IQR) | 61 (46–77) | 67.5 (45.8–84.3) | 59 (46–71) | 0.2032 |
| FEV1, L, median (IQR) | 1.7 (1.2–2.3) | 2.0 (1.1–2.7) | 1.6 (1.2–2.0) | 0.1119 |
| FVC, %, median (IQR) | 78 (64–93) | 83 (60.8–99) | 76 (68–90) | 0.8298 |
| FEV1/FVC, %, median (IQR) | 60 (55–71) | 64 (55.9–76) | 57 (54–65) | 0.0202 |
| FEF25–75, %, median (IQR) | 30 (21–42) | 32 (22–52) | 30 (20–40) | 0.1501 |
| Pharmacologic therapies | ||||
| High dose ICS-LABA, n (%) | 74 (100) | 39 (100) | 35 (100) | 0.9999 |
| LAMA, n (%) | 64 (86.5) | 31 (79.5) | 33 (94.3) | 0.0905 |
| Previous anti-IgE/anti IL-5 mAbs, n (%) | 4 (5.4) | 1 (2.6) | 3 (8.6) | 0.3388 |
| Patients on OCS, n, (%) | 62 (73) | 27 (69.2) | 35 (100) | 0.0078 |
| OCS, mg/day, median (IQR) | 12.5 (5–18.3) | 5 (0–12.5) | 12.5 (10–25) | 0.0001 |
| Biomarkers | 54.8 (11.9) | 53.9 (12.2) | 55.8 (11.8) | 0.4980 |
| Eosinophil counts in peripheral blood, cells/μL median (IQR) | 630 (435–835) | 550 (450–777) | 680 (400–870) | 0.4800 |
| IgE, UI/mL, median (IQR) | 152 (66–465) | 158 (54–650) | 151 (72.3–387) | 0.8465 |
| FeNO, ppb, median (IQR) | 47 (35–63) | 43 (33–63) | 51 (38–66) | 0.0678 |
| Total (n = 74) | Baseline | 6 Months | p-Value | 12 Months | p-Value |
|---|---|---|---|---|---|
| Asthma outcomes | |||||
| Annual exacerbation rate, median (IQR) | 6 (4–8) | n/a | n/a | 1 (0–2) | <0.0001 |
| Exacerbation-free, n (%) | n/a | 35 (47.3) | n/a | 32 (43.2) | n/a |
| ACT, median (IQR) | 14 (9–17) | 20 (18–21) | <0.0001 | 22 (20–24) | <0.0001 |
| FEV1, %, median (IQR) | 61 (46–77) | 79 (63–95.5) | <0.0001 | 92.5 (67.3–107) | <0.0001 |
| FEV1, L, median (IQR) | 1.7 (1.2–2.3) | 2.1 (1.4–2.9) | <0.0001 | 2.5 (1.8–3.4) | <0.0001 |
| FVC, %, median (IQR) | 78 (64–93) | 93 (80–104) | <0.0001 | 98 (83–112) | <0.0001 |
| FEV1/FVC, %, median (IQR) | 60 (55–71) | 67 (60–76) | 0.0038 | 70 (62–78) | 0.0002 |
| FEF25–75, %, median (IQR) | 30 (21–42) | 41.5 (30.8–62) | <0.0001 | 57 (35.3–73) | <0.0001 |
| Pharmacologic therapies | |||||
| Patients on OCS, n, (%) | 62 (83.8) | 41 (55.4) | <0.0001 | 20 (27) | <0.0001 |
| OCS, mg/day, median (IQR) | 12.5 (5–18.3) | 2.5 (0–5) | <0.0001 | 0 (0–2.5) | <0.0001 |
| Biomarkers | |||||
| Eosinophil counts in peripheral blood, cells/μL median (IQR) | 630 (435–835) | 0 (0–50) | <0.0001 | 0 (0–0.0) | <0.0001 |
| Patients with chronic mucus hypersecretion, n (%) | 49 (66.2) | 28 (37.8) | <0.0001 | 19 (25.7) | <0.0001 |
| 6 Months | 12 Months | |||||
|---|---|---|---|---|---|---|
| Total (n = 74) | SEA (n = 39) | SEA + BE (n = 35) | p-Value | SEA (n = 39) | SEA + BE (n = 35) | p-Value |
| Asthma outcomes | ||||||
| Annual exacerbation rate, change from baseline, median (IQR) | n/a | n/a | n/a | −5 (−6 to −3) | −6 (−8 to −4) | 0.1308 |
| Exacerbation-free, n, (%) | 28 (71.8) | 7 (20) | <0.0001 | 25 (64.1) | 7 (20) | 0.0002 |
| ACT, change from baseline, median (IQR) | +5 (+2 to +7) | +6 (+3 to +10) | 0.3637 | +7 (+3 to +13) | +8 (+5 to +12) | 0.6022 |
| ACT MCID, n, (%) | 29 (74.4) | 31 (88.6) | 0.1458 | 31 (79.5) | 33 (94.3) | 0.0905 |
| FEV1, %, change from baseline, median (IQR) | +10 (+0.8 to +25) | +12 (+4 to +26) | 0.9672 | +20 (+3 to +32) | +25 (+12 to +44) | 0.2055 |
| FEV1, L, change from baseline, median (IQR) | +0.25 (+0.05 to +0.8) | +0.23 (+0.02 to +0.59) | 0.8972 | +0.65 (+0.24 to +0.98) | +0.61 (+0.25 to +1.16) | 0.6662 |
| FVC, %, change from baseline, median (IQR) | +7 (−0.3 to +17) | +10 (+2 to +20) | 0.4447 | +12 (+2 to +28) | +19 (+10 to +30) | 0.4023 |
| FEV1/FVC, %, change from baseline, median (IQR) | +6 (0 to +12) | +5.4 (−3.6 to +13.4) | 0.9822 | +6.8 (−1.9 to +15.6) | +6.8 (−1 to +18) | 0.5599 |
| FEF25–75, %, change from baseline, median (IQR) | +14.5 (+1.3 to +28.8) | +7 (0 to +13) | 0.0811 | +19.5 (+6 to +44) | +16 (+5 to +42) | 0.9010 |
| Pharmacologic therapies | ||||||
| Patients on OCS, n, (%) | −13 (−48.1) | −8 (−22.9) | 0.0577 | −25 (−92.6) | −17 (−48.6) | 0.0003 |
| OCS, mg/day, change from baseline, median (IQR) | −5 (0 to −10) | −10 (−5 to −18.8) | 0.0121 | −5 (0 to −12.5) | −12.5 (−7.5 to −20) | 0.0112 |
| Biomarkers | ||||||
| Eosinophil counts in peripheral blood, cells/μL median (IQR) | 0 (0–0.0) | 0 (0–50) | 0.9999 | 0 (0–0.0) | 0 (0–0.0) | 0.9999 |
| Patients with chronic mucus hypersecretion, n (%) | −10 (−50) | −11 (−37.9) | 0.0311 | −13 (−65) | −17 (−58.6) | 0.1204 |
| Remission Criteria | Overall (n = 74) | SEA (n = 39) | SEA + BE (n = 35) | OR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Zero exacerbations + zero OCS, n (%) | 31 (41.9) | 26 (66.7) | 5 (14.3) | 0.08 (0.03–0.27) | <0.0001 |
| Zero exacerbations + zero OCS + ACT ≥ 20, n (%) | 30 (40.5) | 25 (64.1) | 5 (14.3) | 0.09 (0.03–0.30) | <0.0001 |
| Zero exacerbations + zero OCS + ACT ≥ 20 + FEV1 ≥ 80%, n (%) | 27 (36.5) | 23 (59) | 4 (11.4) | 0.09 (0.03–0.31) | <0.0001 |
| Zero exacerbations + zero OCS + ACT ≥ 20 + FEV1 +100 mL, n (%) | 23 (31.1) | 19 (48.7) | 4 (11.4) | 0.13 (0.05–0.50) | 0.0009 |
| SEA + BE (n = 35) | Mild-to-Moderate BSI (n = 14) | Severe BSI (n = 21) | p-Value | |
|---|---|---|---|---|
| Age, years, mean (SD) | 55.8 (11.8) | 56 (13.2) | 55.6 (11) | 0.9269 |
| Female, n (%) | 21 (60) | 5 (35.7) | 16 (76.2) | 0.0332 |
| BMI, mean (SD) | 26.2 (3.3) | 26.9 (3.4) | 25.7 (3.2) | 0.2189 |
| Age at onset, years, mean (SD) | 31.7 (14.1) | 31.6 (14.3) | 31.6 (14.4) | 0.8746 |
| Patients with positive Skin Prick Tests, n (%) | 17 (48.6) | 5 (35.7) | 12 (57.4) | 0.3053 |
| Smoking status | ||||
| Smoking history, n (%) | 11 (31.4) | 7 (50) | 4 (19.1) | 0.0725 |
| Current smoker, n (%) | 2 (5.7) | 2 (14.3) | 0 (0) | 0.1529 |
| Comorbidities | ||||
| Patients with GERD, n (%) | 18 (51.4) | 7 (50) | 11 (52.4) | 0.4940 |
| Patients with CRSwNP, n (%) | 22 (62.9) | 11 (78.6) | 11 (52.4) | 0.1621 |
| Patients with BE, n (%) | 35 (100) | 14 (100) | 21 (100) | 0.9999 |
| Bronchiectasis assessment | ||||
| BSI, median (IQR) | 9 (7–11) | 7 (4.8–7) | 11 (9.5–12) | <0.0001 |
| Mild BSI (≤4), n (%) | 3 (8.6) | 3 (21.4) | n/a | n/a |
| Moderate BSI (5–8), n (%) | 11 (31.4) | 11 (78.6) | n/a | n/a |
| Severe BSI (≥9), n (%) | 21 (60) | n/a | 21 (60) | n/a |
| Patients with chronic mucus hypersecretion | 29 (82.9) | 13 (92.9) | 16 (76.2) | 0.3662 |
| Patients with microbial colonization, n (%) | 12 (89.7) | 3 (21.4) | 9 (42.9) | 0.2816 |
| P. Aeruginosa, n (%) | 4 (11.4) | 1 (7.1) | 3 (14.3) | 0.1243 |
| A. Fumigatus, n (%) | 4 (11.4) | 1 (7.1) | 3 (14.3) | 0.1243 |
| S. Aureus, n (%) | 2 (5.7) | 1 (7.1) | 1 (4.8) | 0.9999 |
| Other, n (%) | 2 (5.7) | 1 (7.1) | 1 (4.8) | 0.9999 |
| Pharmacologic therapies | ||||
| High dose ICS-LABA, n (%) | 35 (100) | 14 (100) | 21 (100) | 0.9999 |
| LAMA, n (%) | 33 (94.3) | 13 (92.9) | 20 (95.3) | 0.9999 |
| Previous anti-IgE/anti IL-5 mAbs, n (%) | 3 (8.6) | 1 (7.1) | 2 (9.5) | 0.9999 |
| Patients on OCS, n, (%) | 35 (100) | 14 (100) | 21 (100) | 0.9999 |
| OCS, mg/die, median (IQR) | 12.5 (10–25) | 13.8 (12.5–25) | 12.5 (7.5–25) | 0.5397 |
| Asthma outcomes | ||||
| Asthma exacerbations/year, median (IQR) | 7 (6–12) | 7 (4.7–12) | 7 (6–12) | 0.9999 |
| ACT, median (IQR) | 13 (8–16) | 13.5 (8–16) | 13 (8–14.5) | 0.3698 |
| FEV1, %, median (IQR) | 59 (46–71) | 70 (50–75.5) | 52 (38.8–66) | 0.0782 |
| FEV1, L, median (IQR) | 1.6 (1.2–2.0) | 1.8 (1.4–2.7) | 1.4 (1.1–1.7) | 0.0480 |
| FVC, %, median (IQR) | 76 (68–90) | 88 (77–102) | 72.5 (59.3–83.5) | 0.8298 |
| FEV1/FVC, %, median (IQR) | 57 (54–65) | 58 (51.5–75) | 56.7 (54.7–61.5) | 0.4725 |
| FEF25–75, %, median (IQR) | 30 (20–40) | 32 (22–37) | 26 (20–41.5) | 0.6299 |
| Biomarkers | ||||
| Eosinophil counts in peripheral blood, cells/μL median (IQR) | 680 (400–870) | 640 (389–1000) | 700 (450–835) | 0.9537 |
| IgE, UI/mL, median (IQR) | 151 (72.3–387) | 159 (54–467) | 148.5 (71–366) | 0.7555 |
| FeNO, ppb, median (IQR) | 51 (38–66) | 49 (33–63) | 52 (37–75) | 0.8674 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campisi, R.; Nolasco, S.; Pelaia, C.; Impellizzeri, P.; D’Amato, M.; Portacci, A.; Ricciardi, L.; Scioscia, G.; Crimi, N.; Scichilone, N.; et al. Benralizumab Effectiveness in Severe Eosinophilic Asthma with Co-Presence of Bronchiectasis: A Real-World Multicentre Observational Study. J. Clin. Med. 2023, 12, 3953. https://doi.org/10.3390/jcm12123953
Campisi R, Nolasco S, Pelaia C, Impellizzeri P, D’Amato M, Portacci A, Ricciardi L, Scioscia G, Crimi N, Scichilone N, et al. Benralizumab Effectiveness in Severe Eosinophilic Asthma with Co-Presence of Bronchiectasis: A Real-World Multicentre Observational Study. Journal of Clinical Medicine. 2023; 12(12):3953. https://doi.org/10.3390/jcm12123953
Chicago/Turabian StyleCampisi, Raffaele, Santi Nolasco, Corrado Pelaia, Pietro Impellizzeri, Maria D’Amato, Andrea Portacci, Luisa Ricciardi, Giulia Scioscia, Nunzio Crimi, Nicola Scichilone, and et al. 2023. "Benralizumab Effectiveness in Severe Eosinophilic Asthma with Co-Presence of Bronchiectasis: A Real-World Multicentre Observational Study" Journal of Clinical Medicine 12, no. 12: 3953. https://doi.org/10.3390/jcm12123953
APA StyleCampisi, R., Nolasco, S., Pelaia, C., Impellizzeri, P., D’Amato, M., Portacci, A., Ricciardi, L., Scioscia, G., Crimi, N., Scichilone, N., Foschino Barbaro, M. P., Pelaia, G., Carpagnano, G. E., Vatrella, A., & Crimi, C., on behalf of the Southern Italy Network on Severe Asthma Therapy. (2023). Benralizumab Effectiveness in Severe Eosinophilic Asthma with Co-Presence of Bronchiectasis: A Real-World Multicentre Observational Study. Journal of Clinical Medicine, 12(12), 3953. https://doi.org/10.3390/jcm12123953