Quantification of Microvascular Lesions in the Central Retinal Field: Could It Predict the Severity of Diabetic Retinopathy?
Abstract
1. Introduction
1.1. Diabetic Retinopathy
1.2. Diabetic Retinopathy Classification
2. Materials and Methods
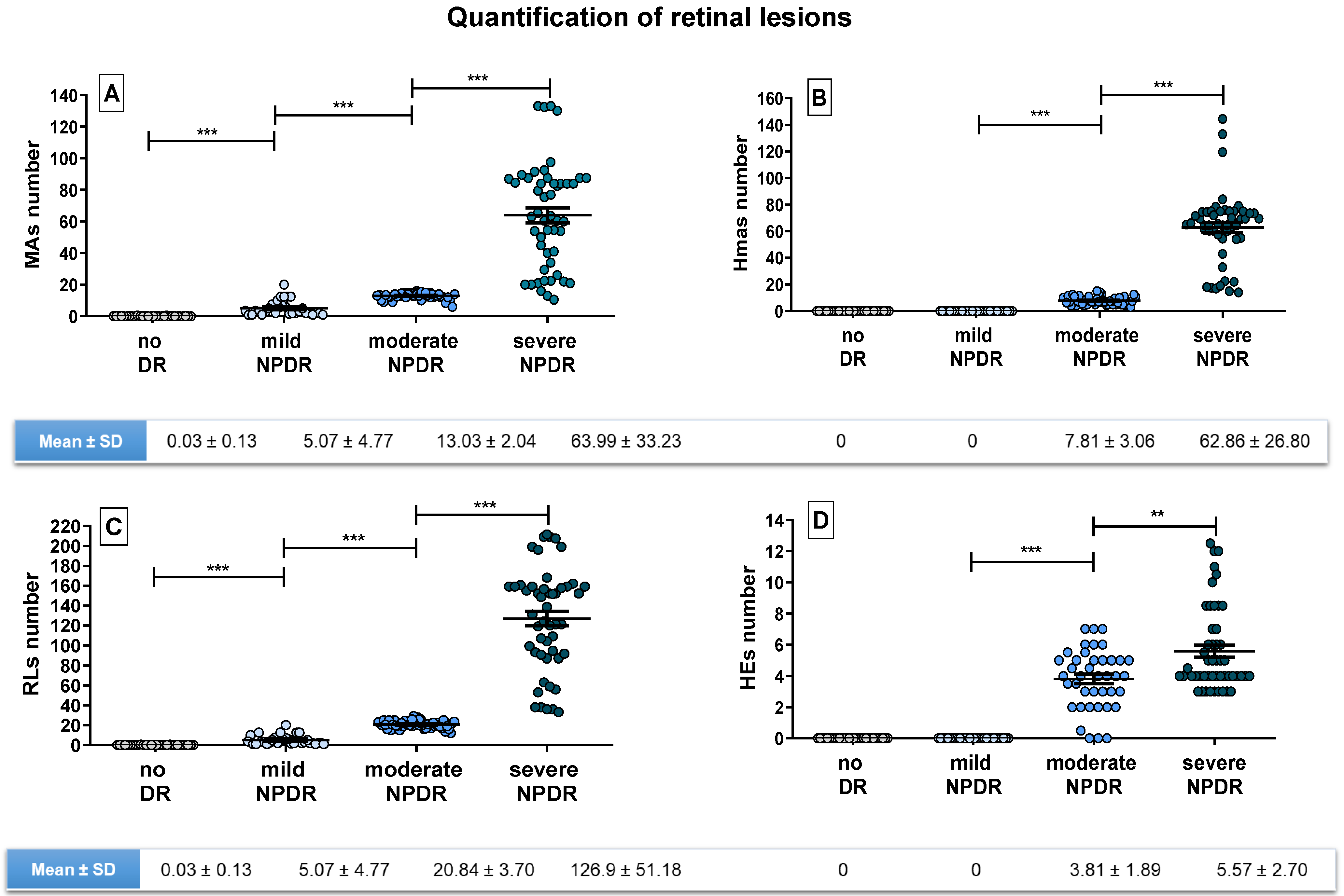
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://diabetesatlas.org/ (accessed on 28 May 2022).
- Martins, T.G.D.S. Diabetic Retinopathy: A Neuropathy. Einstein 2020, 19, eED6110. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Sabanayagam, C. Strategies to Tackle the Global Burden of Diabetic Retinopathy: From Epidemiology to Artificial Intelligence. Ophthalmologica 2020, 243, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef]
- Vujosevic, S.; Aldington, S.J.; Silva, P.; Hernández, C.; Scanlon, P.; Peto, T.; Simó, R. Screening for Diabetic Retinopathy: New Perspectives and Challenges. Lancet Diabetes Endocrinol. 2020, 8, 337–347. [Google Scholar]
- Whitehead, M.; Wickremasinghe, S.; Osborne, A.; Van Wijngaarden, P.; Martin, K.R. Diabetic Retinopathy: A Complex Pathophysiology Requiring Novel Therapeutic Strategies. Expert Opin. Biol. Ther. 2018, 18, 1257–1270. [Google Scholar] [CrossRef]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Seminar Diabetic Retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Tarr, J.M.; Kaul, K.; Chopra, M.; Kohner, E.M.; Chibber, R. Pathophysiology of Diabetic Retinopathy. ISRN Ophthalmol. 2013, 2013, 343560. [Google Scholar] [CrossRef]
- Rodríguez, M.L.; Pérez, S.; Mena-Mollá, S.; Desco, M.C.; Ortega, Á.L. Review Article Oxidative Stress and Microvascular Alterations in Diabetic Retinopathy: Future Therapies. Oxidative Med. Cell. Longev. 2019, 2019, 4940825. [Google Scholar] [CrossRef]
- Solomon, S.D.; Goldberg, M.F. ETDRS Grading of Diabetic Retinopathy: Still the Gold Standard? Ophthalmic Res. 2019, 62, 190–195. [Google Scholar] [CrossRef]
- Simó, R.; Simó-Servat, O.; Bogdanov, P.; Hernández, C. Neurovascular Unit: A New Target for Treating Early Stages of Diabetic Retinopathy. Pharmaceutics 2021, 13, 1320. [Google Scholar] [CrossRef]
- Soriano, M.E.T.; Aguirre, G.G.; Gordon, M. Ophthalmology: Current and Future Developments; Bentham Science Publishers: Mexico City, Mexico, 2016; ISBN 9781681083575. [Google Scholar]
- Lechner, J.; O’Leary, O.E.; Stitt, A.W. The Pathology Associated with Diabetic Retinopathy. Vision Res 2017, 139, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Madeira, M.H.; Marques, I.P.; Ferreira, S.; Tavares, D.; Santos, T.; Santos, A.R.; Figueira, J.; Lobo, C.; Cunha-Vaz, J. Retinal Neurodegeneration in Different Risk Phenotypes of Diabetic Retinal Disease. Front. Neurosci. 2021, 15, 800004. [Google Scholar] [CrossRef] [PubMed]
- Kohner, E.M.; Stratton, I.M.; Aldington, S.J.; Turner, R.C.; Matthews, D.R. Microaneurysms in the development of diabetic retinopathy (UKPDS 42). UK Prospective Diabetes Study Group. Diabetologia 1999, 42, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Parrulli, S.; Corvi, F.; Cozzi, M.; Monteduro, D.; Zicarelli, F.; Staurenghi, G. Microaneurysms Visualisation Using Five Different Optical Coherence Tomography Angiography Devices Compared to Fluorescein Angiography. Br. J. Ophthalmol. 2021, 105, 526–530. [Google Scholar] [CrossRef]
- Romila, A.; Potop, V.; Ciuluvica, R.; Monica, B.; Mehedinti Hincu, M.; Jurja, S. Correlation between Metabolic Status and Diabetic Retinopathy Evolution in Type 1 Diabetes. Exp. Ther. Med. 2021, 22, 1214. [Google Scholar] [CrossRef]
- Nunes, S.; Pires, I.; Rosa, A.; Duarte, L.; Bernardes, R.; Cunha-Vaz, J. Microaneurysm Turnover Is a Biomarker for Diabetic Retinopathy Progression to Clinically Significant Macular Edema: Findings for Type 2 Diabetics with Nonproliferative Retinopathy. Ophthalmologica 2009, 223, 292–297. [Google Scholar] [CrossRef]
- Santos, A.R.; Mendes, L.; Madeira, M.H.; Marques, I.P.; Tavares, D.; Figueira, J.; Lobo, C.; Cunha-Vaz, J. Microaneurysm Turnover in Mild Non-Proliferative Diabetic Retinopathy Is Associated with Progression and Development of Vision-Threatening Complications: A 5-Year Longitudinal Study. J. Clin. Med. 2021, 10, 2142. [Google Scholar] [CrossRef]
- Klein, R.; Moss, S.E.; Klein, B.E.K.; Dams, M.D.; DeMets, D.L. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XI. The Incidence of Macular Edema. Ophthalmology 1989, 96, 1501–1510. [Google Scholar] [CrossRef]
- Klein, R.; Meuer, S.M.; Moss, S.E.; Barbara, M.; Klein, E.K. The Relationship of Retinal Microaneurysm Counts to the 4-Year Progression of Diabetic Retinopathy. Arch. Ophthalmol. 1989, 107, 1780–1785. [Google Scholar] [CrossRef]
- Wilson, C.; Horton, M.; Cavallerano, J.; Aiello, L.M. Addition of Primary Care-Based Retinal Imaging Technology to an Existing Eye Care Professional Referral Program Increased the Rate of Surveillance and Treatment of Diabetic Retinopathy. Diabetes Care 2005, 28, 318–322. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Grading Diabetic Retinopathy from Stereoscopic Color Fundus Photographs—An Extension of the Modified Airlie House Classification: ETDRS Report Number 10. Ophthalmology 1991, 98, 786–806. [Google Scholar] [CrossRef]
- Wilkinson, C.P.; Ferris, F.L.; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T.; et al. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Nguyen, L.; Hynan, L.S.; Blomquist, P.H. Comparison of 1-Field, 2-Fields, and 3-Fields Fundus Photography for Detection and Grading of Diabetic Retinopathy. J. Diabetes Complicat. 2019, 33, 107441. [Google Scholar] [CrossRef] [PubMed]
- Bursell, S.-E.; Cavallerano, J.D.; Cavallerano, A.A.; Clermont, A.C.; Birkmire-Peters, D.; Aiello, L.P.; Aiello, L.M.; Botti, R.; Bursell, D.K.; Calderon, R.M.; et al. Stereo Nonmydriatic Digital-Video Color Retinal Imaging Compared with Early Treatment Diabetic Retinopathy Study Seven Standard Field 35-Mm Stereo Color Photos for Determining Level of Diabetic Retinopathy. Ophthalmology 2001, 108, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Abràmoff, M.D.; Lavin, P.T.; Birch, M.; Shah, N.; Folk, J.C. Pivotal Trial of an Autonomous AI-Based Diagnostic System for Detection of Diabetic Retinopathy in Primary Care Offices. NPJ Digit. Med. 2018, 1, 39. [Google Scholar] [CrossRef]
- González-Gonzalo, C.; Thee, E.F.; Klaver, C.C.W.; Lee, A.Y.; Schlingemann, R.O.; Tufail, A.; Verbraak, F.; Sánchez, C.I. Trustworthy AI: Closing the Gap between Development and Integration of AI Systems in Ophthalmic Practice. Prog. Retin. Eye Res. 2021, 90, 101034. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.G. Pathophysiology of Diabetic Retinopathy. Br. J. Ophthalmol. 1978, 62, 351–355. [Google Scholar] [CrossRef]
- Munuera-Gifre, E.; Saez, M.; Juvinyà-Canals, D.; Rodríguez-Poncelas, A.; Barrot-de-la–Puente, J.F.; Franch-Nadal, J.; Romero-Aroca, P.; Barceló, M.A.; Coll-de-Tuero, G. Analysis of the Location of Retinal Lesions in Central Retinographies of Patients with Type 2 Diabetes. Acta Ophthalmol. 2020, 98, e13–e21. [Google Scholar] [CrossRef]
- Sánchez, C.I.; García, M.; Mayo, A.; López, M.I.; Hornero, R. Retinal Image Analysis Based on Mixture Models to Detect Hard Exudates. Med. Image Anal. 2009, 13, 650–658. [Google Scholar] [CrossRef]
- Marupally, A.G.; Vupparaboina, K.K.; Peguda, H.K.; Richhariya, A.; Jana, S.; Chhablani, J. Semi-Automated Quantification of Hard Exudates in Colour Fundus Photographs Diagnosed with Diabetic Retinopathy. BMC Ophthalmol. 2017, 17, 172. [Google Scholar] [CrossRef]
- Chalakkal, R.J.; Abdulla, W.H.; Hong, S.C. Fundus Retinal Image Analyses for Screening and Diagnosing Diabetic Retinopathy, Macular Edema, and Glaucoma Disorders. In Diabetes and Fundus OCT; Elsevier: Amsterdam, The Netherlands, 2020; pp. 59–111. [Google Scholar]
- Durzhinskaya, M.K. Mikroanevrizmy Kak Marker Diabeticheskoi Retinopatii [Microaneurysms as a Biomarker of Diabetic Retinopathy]. Vestn. Oftalmol. 2021, 137, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Gatti, V.; Muraca, A.; Brambilla, M.; Villani, E.; Nucci, P.; Rossetti, L.; De Cilla’, S. Optical coherence tomography angiography changes after subthreshold micropulse yellow laser in diabetic macular edema. Retina 2020, 40, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Jiang, A.C.; Boss, J.D.; Hu, M.; Figueiredo, N.; Babiuch, A.; Talcott, K.; Sharma, S.; Hach, J.; Le, T.; et al. Quantitative Ultra-Widefield Angiography and Diabetic Retinopathy Severity: An Assessment of Panretinal Leakage Index, Ischemic Index and Microaneurysm Count. Ophthalmology 2019, 126, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Cavallerano, J.D.; Sun, J.K.; Silva, P.S.; Aiello, L.P. Ultrawide Field Imaging in Diabetic Retinopathy: Exploring the Role of Quantitative Metrics. J. Clin. Med. 2021, 10, 3300. [Google Scholar] [CrossRef]
- Wan, C.; Chen, Y.; Li, H.; Zheng, B.; Chen, N.; Yang, W.; Wang, C.; Li, Y. EAD-Net: A Novel Lesion Segmentation Method in Diabetic Retinopathy Using Neural Networks. Dis. Markers 2021, 2021, 6482665. [Google Scholar] [CrossRef]
- Hervella, Á.S.; Rouco, J.; Novo, J.; Ortega, M. Retinal Microaneurysms Detection Using Adversarial Pre-Training with Unlabeled Multimodal Images. Inf. Fusion 2022, 79, 146–161. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Liu, B.; Tang, L.; Lv, L.; Ke, X.; Ling, S.; Lu, L.; Zou, H. The Diagnostic Accuracy of an Intelligent and Automated Fundus Disease Image Assessment System with Lesion Quantitative Function (SmartEye) in Diabetic Patients. BMC Ophthalmol. 2019, 19, 184. [Google Scholar] [CrossRef]
- Karasu, B.; Akbas, Y.B.; Aykut, A.; Çelebi, A.R.C. Subthreshold Photocoagulation, Laser Endpoint Management Based on Optical Coherence Tomography Angiography in Cases of Diabetic Macular Edema Refractory to Anti-VEGF. Klin. Monbl. Augenheilkd. 2022. [Google Scholar] [CrossRef]
- AttaAllah, H.R.; Mohamed, A.A.M.; Ali, M.A. Macular Vessels Density in Diabetic Retinopathy: Quantitative Assessment Using Optical Coherence Tomography Angiography. Int. Ophthalmol. 2019, 39, 1845–1859. [Google Scholar] [CrossRef]
- Li, X.; Xie, J.; Zhang, L.; Cui, Y.; Zhang, G.; Wang, J.; Zhang, A.; Chen, X.; Huang, T.; Meng, Q. Differential Distribution of Manifest Lesions in Diabetic Retinopathy by Fundus Fluorescein Angiography and Fundus Photography. BMC Ophthalmol. 2020, 20, 470. [Google Scholar] [CrossRef]
- Silva, P.S.; Cavallerano, J.D.; Sun, J.K.; Soliman, A.Z.; Aiello, L.M.; Aiello, L.P. Peripheral Lesions Identified by Mydriatic Ultrawide Field Imaging: Distribution and Potential Impact on Diabetic Retinopathy Severity. Ophthalmology 2013, 120, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- Sears, C.M.; Nittala, M.G.; Jayadev, C.; Verhoek, M.; Fleming, A.; van Hemert, J.; Tsui, I.; Sadda, S.V.R. Comparison of Subjective Assessment and Precise Quantitative Assessment of Lesion Distribution in Diabetic Retinopathy. JAMA Ophthalmol. 2018, 136, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Sadda, S.R.; Nittala, M.G.; Taweebanjongsin, W.; Verma, A.; Velaga, S.B.; Alagorie, A.R.; Sears, C.M.; Silva, P.S.; Aiello, L.P. Quantitative Assessment of the Severity of Diabetic Retinopathy. Am. J. Ophthalmol. 2020, 218, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Bellemo, V.; Xie, Y.; Lee, X.Q.; Yip, M.Y.T.; Ting, D.S.W. Different Fundus Imaging Modalities and Technical Factors in AI Screening for Diabetic Retinopathy: A Review. Eye Vis. 2020, 7, 21. [Google Scholar] [CrossRef]
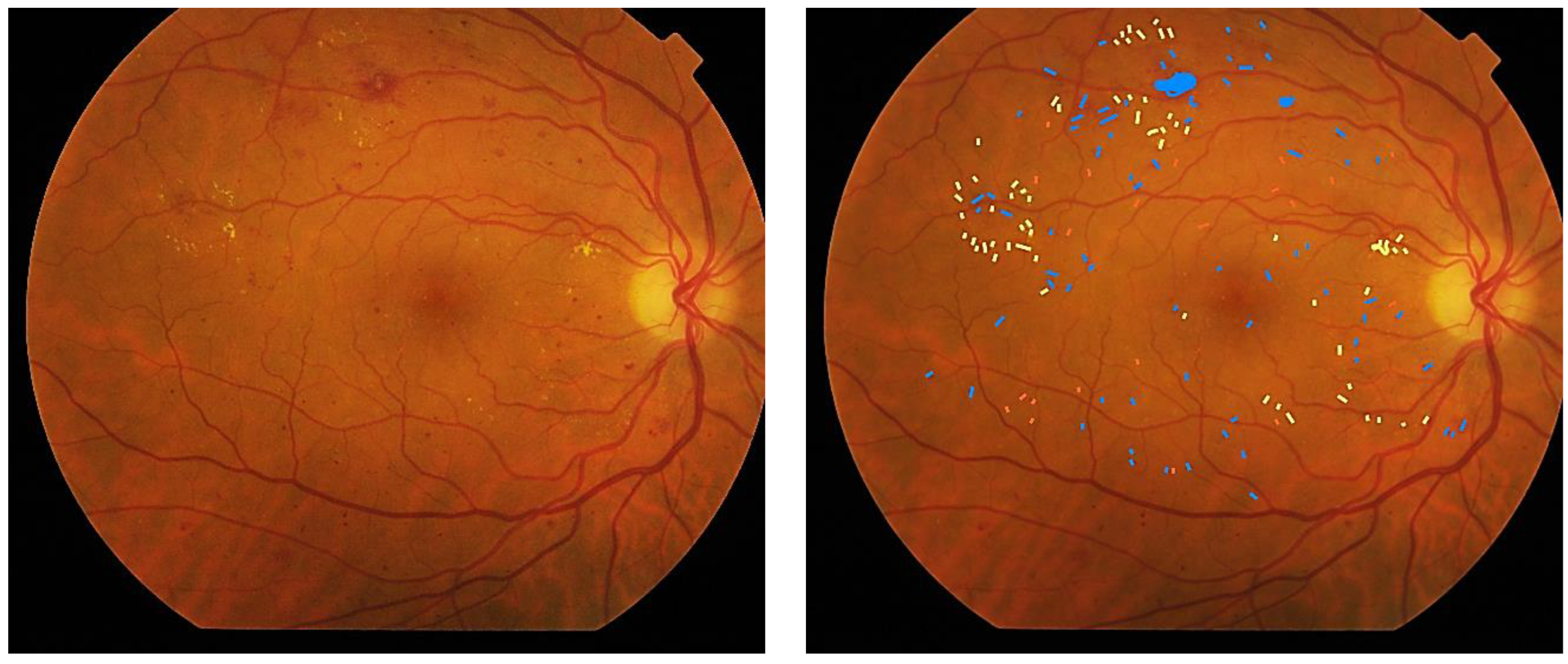

| ICDR Disease Severity Level | Findings Observable upon Dilated Ophthalmoscopy | ETDRS Severity Scale Level |
|---|---|---|
| No apparent DR | No abnormalities | 10—DR absent |
| Mild NPDR | Microaneurysms only | 20—Microaneurysms only |
| Moderate NPDR | More than just microaneurysms but less severe NPDR | 35—Mild NPDR 43—Moderate NPDR [The risk of subsequent development of PDR or CSME * is appreciable from this level] 47—Moderately severe NPDR |
| Severe NPDR | Any of the following and no signs of PDR
| 53—Severe NPDR |
| PDR | One of both of the following:
| 61—Mild PDR 65—Moderate PDR 75—High-risk PDR 81, 85—Advanced PDR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Carneado, J.; Almazán-Moga, A.; Ramírez-Lamelas, D.T.; Cuscó, C.; Alonso de la Fuente, J.I.; Pastor, J.C.; López Gálvez, M.I.; Ponsati, B. Quantification of Microvascular Lesions in the Central Retinal Field: Could It Predict the Severity of Diabetic Retinopathy? J. Clin. Med. 2023, 12, 3948. https://doi.org/10.3390/jcm12123948
Fernández-Carneado J, Almazán-Moga A, Ramírez-Lamelas DT, Cuscó C, Alonso de la Fuente JI, Pastor JC, López Gálvez MI, Ponsati B. Quantification of Microvascular Lesions in the Central Retinal Field: Could It Predict the Severity of Diabetic Retinopathy? Journal of Clinical Medicine. 2023; 12(12):3948. https://doi.org/10.3390/jcm12123948
Chicago/Turabian StyleFernández-Carneado, Jimena, Ana Almazán-Moga, Dolores T. Ramírez-Lamelas, Cristina Cuscó, José Ignacio Alonso de la Fuente, J. Carlos Pastor, María Isabel López Gálvez, and Berta Ponsati. 2023. "Quantification of Microvascular Lesions in the Central Retinal Field: Could It Predict the Severity of Diabetic Retinopathy?" Journal of Clinical Medicine 12, no. 12: 3948. https://doi.org/10.3390/jcm12123948
APA StyleFernández-Carneado, J., Almazán-Moga, A., Ramírez-Lamelas, D. T., Cuscó, C., Alonso de la Fuente, J. I., Pastor, J. C., López Gálvez, M. I., & Ponsati, B. (2023). Quantification of Microvascular Lesions in the Central Retinal Field: Could It Predict the Severity of Diabetic Retinopathy? Journal of Clinical Medicine, 12(12), 3948. https://doi.org/10.3390/jcm12123948





