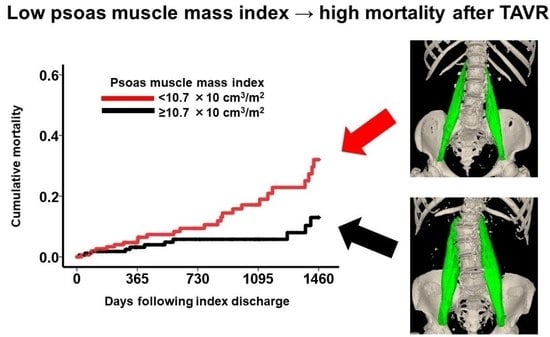
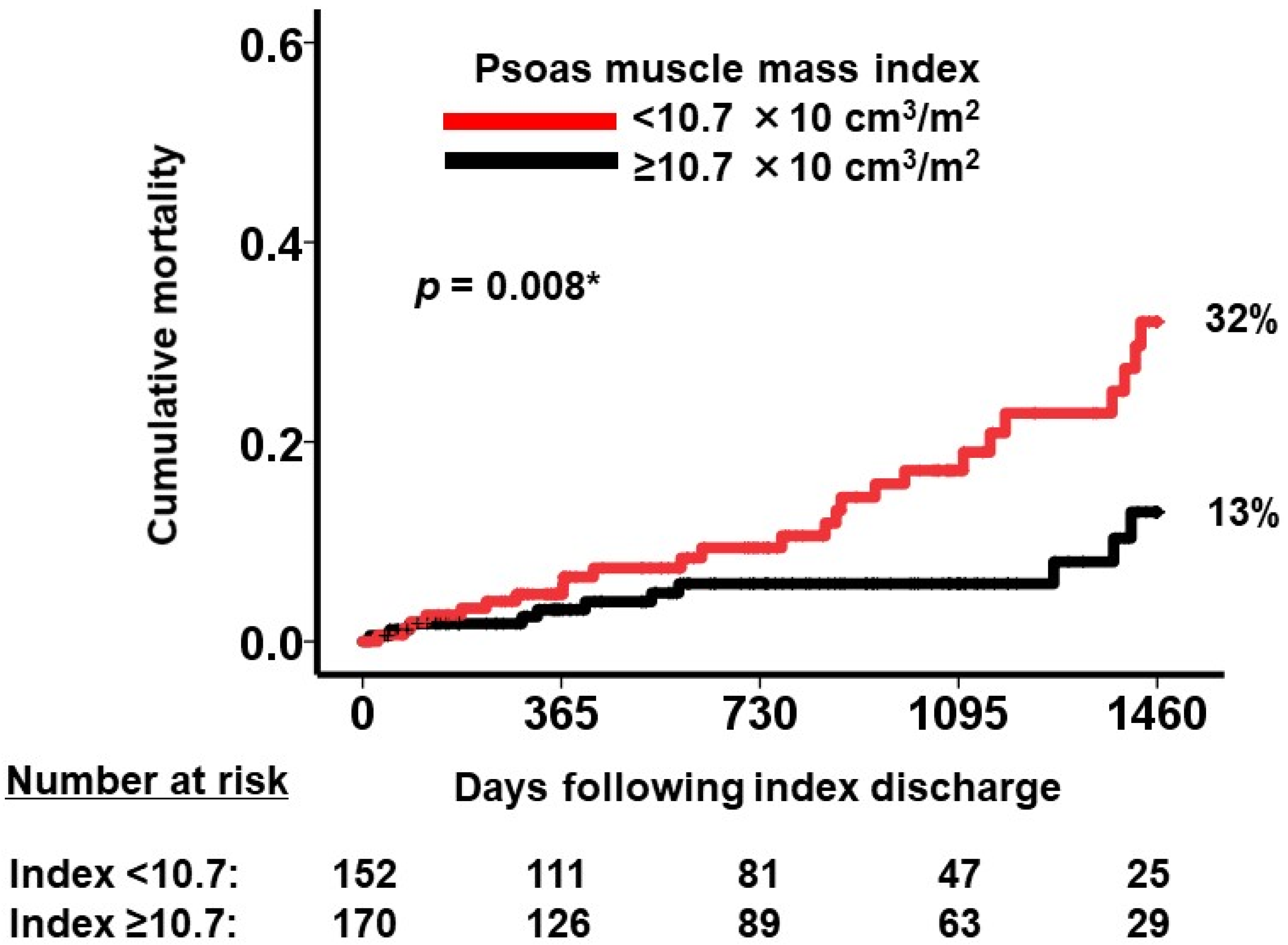
Prognostic Impact of Psoas Muscle Mass Index following Trans-Catheter Aortic Valve Replacement
Abstract
1. Background
2. Methods
2.1. Patient Selection
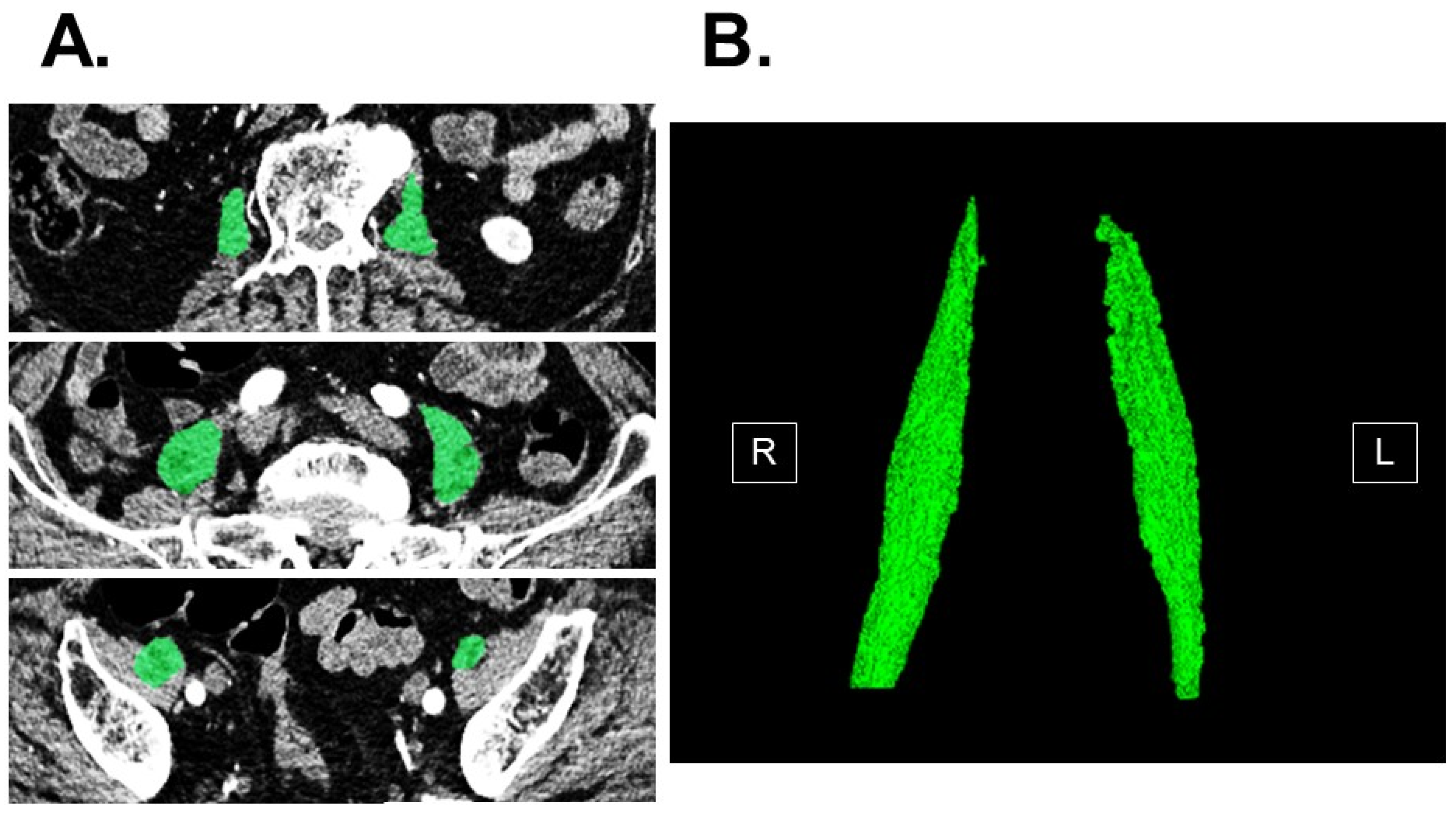
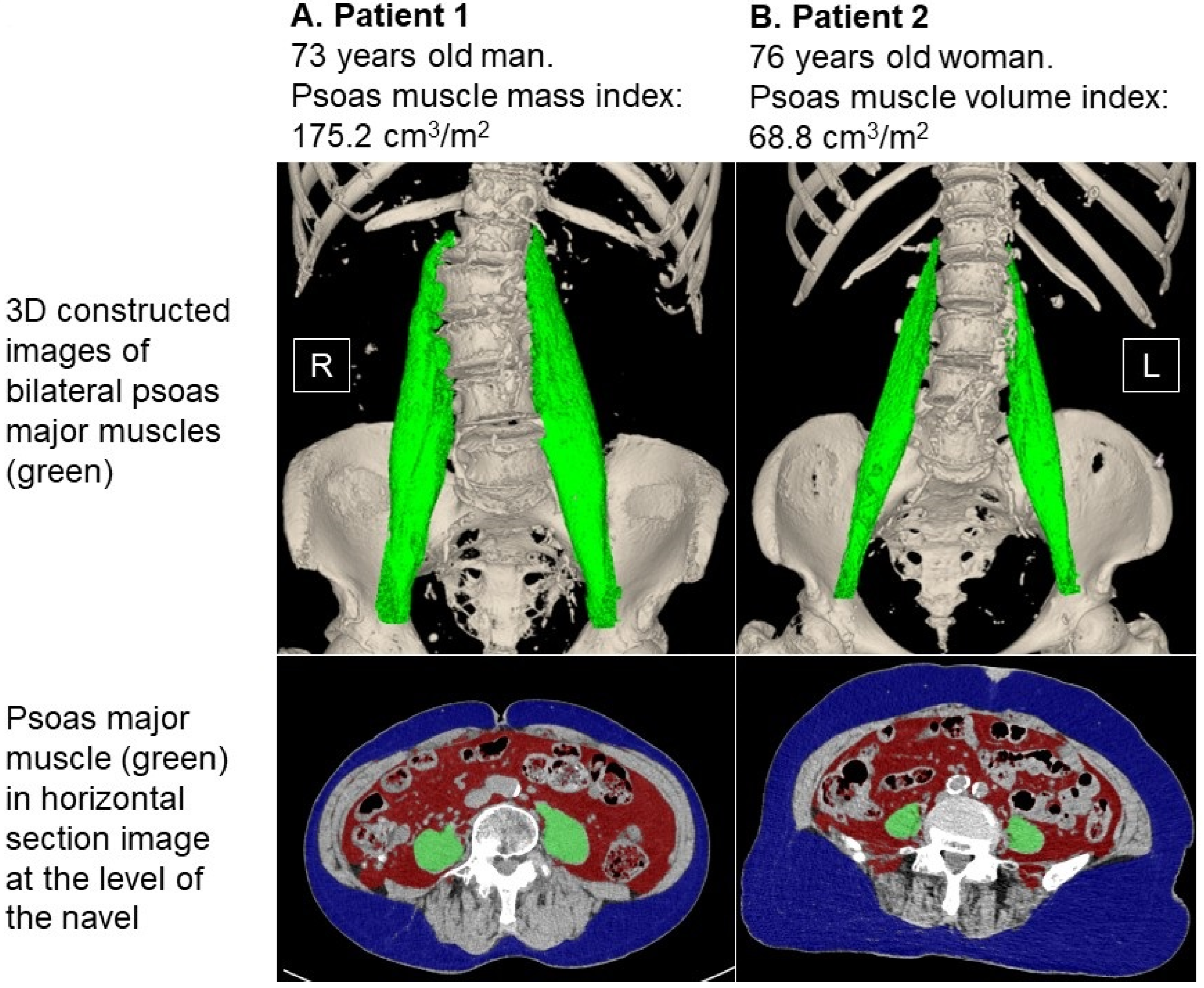
2.2. Measurement of Psoas Muscle Mass
2.3. TAVR Procedure
2.4. Independent Variable and the Primary Outcome
2.5. Other Clinical Variables
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics at Index Discharge
3.2. Prognostic Impact of Baseline Characteristics including Psoas Muscle Volume
3.3. Profile of Patients with Low Psoas Muscle Mass
4. Discussion
4.1. Psoas Muscle Mass and Sarcopenia
4.2. Prognostic Impact of Psoas Muscle Mass following TAVR
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B. Transcatheter Aortic-Valve Replacement in Low-Risk Patients. Reply. N. Engl. J. Med. 2019, 381, 684–685. [Google Scholar] [CrossRef]
- Svensson, L.G.; Blackstone, E.H.; Rajeswaran, J.; Brozzi, N.; Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; et al. Comprehensive analysis of mortality among patients undergoing TAVR: Results of the PARTNER trial. J. Am. Coll. Cardiol. 2014, 64, 158–168. [Google Scholar] [CrossRef]
- Afilalo, J.; Lauck, S.; Kim, D.H.; Lefevre, T.; Piazza, N.; Lachapelle, K.; Martucci, G.; Lamy, A.; Labinaz, M.; Peterson, M.D.; et al. Frailty in Older Adults Undergoing Aortic Valve Replacement: The FRAILTY-AVR Study. J. Am. Coll. Cardiol. 2017, 70, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Matsue, Y.; Kamiya, K.; Saito, H.; Saito, K.; Ogasahara, Y.; Maekawa, E.; Konishi, M.; Kitai, T.; Iwata, K.; Jujo, K.; et al. Prevalence and prognostic impact of the coexistence of multiple frailty domains in elderly patients with heart failure: The FRAGILE-HF cohort study. Eur. J. Heart Fail. 2020, 22, 2112–2119. [Google Scholar] [CrossRef]
- Arnold, S.V.; Zhao, Y.; Leon, M.B.; Sathananthan, J.; Alu, M.; Thourani, V.H.; Smith, C.R.; Mack, M.J.; Cohen, D.J. Impact of Frailty and Prefrailty on Outcomes of Transcatheter or Surgical Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2022, 15, e011375. [Google Scholar] [CrossRef]
- Kiani, S.; Stebbins, A.; Thourani, V.H.; Forcillo, J.; Vemulapalli, S.; Kosinski, A.S.; Babaliaros, V.; Cohen, D.; Kodali, S.K.; Kirtane, A.J.; et al. The Effect and Relationship of Frailty Indices on Survival After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2020, 13, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Brouessard, C.; Bobet, A.S.; Mathieu, M.; Manigold, T.; Arrigoni, P.P.; Le Tourneau, T.; De Decker, L.; Boureau, A.S. Impact of Severe Sarcopenia on Rehospitalization and Survival One Year after a TAVR Procedure in Patients Aged 75 and Older. Clin. Interv. Aging 2021, 16, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Beaudart, C.; McCloskey, E.; Bruyere, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; Araujo de Carvalho, I.; Amuthavalli Thiyagarajan, J.; Bautmans, I.; Bertiere, M.C.; et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef]
- Matsubara, Y.; Nakamura, K.; Matsuoka, H.; Ogawa, C.; Masuyama, H. Pre-treatment psoas major volume is a predictor of poor prognosis for patients with epithelial ovarian cancer. Mol. Clin. Oncol. 2019, 11, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Shimada, Y.; Makino, Y.; Kudo, Y.; Maehara, S.; Yamada, T.; Hagiwara, M.; Kakihana, M.; Ohira, T.; Ikeda, N. Clinical utility of psoas muscle volume in assessment of sarcopenia in patients with early-stage non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Takano, M.; Miyamoto, M.; Yajima, I.; Shimizu, Y.; Aizawa, Y.; Suguchi, Y.; Moriiwa, M.; Aoyama, T.; Soyama, H.; et al. Psoas muscle volume as a predictor of peripheral neurotoxicity induced by primary chemotherapy in ovarian cancers. Cancer Chemother. Pharmacol. 2017, 80, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Zargar, H.; Almassi, N.; Kovac, E.; Ercole, C.; Remer, E.; Rini, B.; Stephenson, A.; Garcia, J.A.; Grivas, P. Change in Psoas Muscle Volume as a Predictor of Outcomes in Patients Treated with Chemotherapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer. Bladder Cancer 2017, 3, 57–63. [Google Scholar] [CrossRef]
- Osnabrugge, R.L.; Mylotte, D.; Head, S.J.; Van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, A.J.; Piazza, N.; Kappetein, A.P. Aortic stenosis in the elderly: Disease prevalence and number of candidates for transcatheter aortic valve replacement: A meta-analysis and modeling study. J. Am. Coll. Cardiol. 2013, 62, 1002–1012. [Google Scholar] [CrossRef]
- Roberts, S.; Collins, P.; Rattray, M. Identifying and Managing Malnutrition, Frailty and Sarcopenia in the Community: A Narrative Review. Nutrients 2021, 13, 2316. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Rollins, K.E.; Gopinath, A.; Awwad, A.; Macdonald, I.A.; Lobo, D.N. Computed tomography-based psoas skeletal muscle area and radiodensity are poor sentinels for whole L3 skeletal muscle values. Clin. Nutr. 2020, 39, 2227–2232. [Google Scholar] [CrossRef]
- So, S.P.; Lee, B.S.; Kim, J.W. Psoas Muscle Volume as an Opportunistic Diagnostic Tool to Assess Sarcopenia in Patients with Hip Fractures: A Retrospective Cohort Study. J. Pers. Med. 2021, 11, 1338. [Google Scholar] [CrossRef]
- Prado, C.M.; Wells, J.C.; Smith, S.R.; Stephan, B.C.; Siervo, M. Sarcopenic obesity: A Critical appraisal of the current evidence. Clin. Nutr. 2012, 31, 583–601. [Google Scholar] [CrossRef]
- Kodama, S.; Togami, W.; Miyamoto, T. Psoas Major Skeletal Muscle Mass Is a Predictive Factor for Independent Walking After Living Donor Liver Transplantation. Transplant. Proc. 2022, 54, 2285–2294. [Google Scholar] [CrossRef]
- Patel, E.; Varghese, J.J.; Garg, M.; Yacob, O.; Sanchez, J.S.; Garcia-Garcia, H.M. Comparison of Body Mass Index (Four Categories) to In-Hospital Outcomes in Patients Who Underwent Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2023, 192, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Kleczynski, P.; Tokarek, T.; Dziewierz, A.; Sorysz, D.; Bagienski, M.; Rzeszutko, L.; Dudek, D. Usefulness of Psoas Muscle Area and Volume and Frailty Scoring to Predict Outcomes After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2018, 122, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Otaka, N.; Shibata, R.; Ohashi, K.; Uemura, Y.; Kambara, T.; Enomoto, T.; Ogawa, H.; Ito, M.; Kawanishi, H.; Maruyama, S.; et al. Myonectin is an Exercise-Induced Myokine that Protects the Heart from Ischemia-Reperfusion Injury. Circ. Res. 2018, 123, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Saji, M.; Lim, D.S.; Ragosta, M.; LaPar, D.J.; Downs, E.; Ghanta, R.K.; Kern, J.A.; Dent, J.M.; Ailawadi, G. Usefulness of Psoas Muscle Area to Predict Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement. Am. J. Cardiol. 2016, 118, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Heidari, B.; Al-Hijji, M.A.; Moynagh, M.R.; Takahashi, N.; Welle, G.; Eleid, M.; Singh, M.; Gulati, R.; Rihal, C.S.; Lerman, A. Transcatheter aortic valve replacement outcomes in patients with sarcopaenia. EuroIntervention 2019, 15, 671–677. [Google Scholar] [CrossRef]
- Pollari, F.; Hitzl, W.; Vogt, F.; Cuomo, M.; Schwab, J.; Sohn, C.; Kalisnik, J.M.; Langhammer, C.; Bertsch, T.; Fischlein, T.; et al. Aortic valve calcification as a risk factor for major complications and reduced survival after transcatheter replacement. J. Cardiovasc. Comput. Tomogr. 2020, 14, 307–313. [Google Scholar] [CrossRef]

| Total (N = 322) | Deceased (N = 36) | Alive (N = 286) | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 85 (83, 88) | 86 (84, 89) | 85 (82, 88) | 0.12 |
| Male sex | 95 (30%) | 13 (36%) | 82 (29%) | 0.23 |
| Body surface area, m2 | 1.39 (1.29, 1.52) | 1.41 (1.37, 1.51) | 1.30 (1.25, 1.49) | 0.30 |
| Body mass index | 21.7 (19.4, 24.4) | 21.8 (19.7, 23.7) | 21.7 (19.2, 24.4) | 0.85 |
| Systolic BP, mmHg | 117 (106, 128) | 109 (106, 118) | 112 (105, 119) | 0.49 |
| Pulse rate, bpm | 70 (63, 78) | 72 (63, 82) | 71 (62, 79) | 0.40 |
| STS score | 4.6 (3.9, 6.1) | 6.6 (3.8, 9.7) | 4.3 (3.2, 5.8) | <0.001 * |
| Comorbidity | ||||
| Coronary artery disease | 87 (27%) | 6 (17%) | 81 (28%) | 0.096 |
| Peripheral artery disease | 71 (22%) | 15 (42%) | 56 (20%) | 0.004 * |
| Diabetes mellitus | 58 (18%) | 3 (8%) | 55 (19%) | 0.078 |
| Atrial fibrillation | 42 (13%) | 9 (25%) | 33 (12%) | 0.029 * |
| Laboratory data | ||||
| Hemoglobin, g/dL | 10.4 (9.6, 11.2) | 10.5 (8.4, 11.3) | 10.6 (10.0, 11.0) | 0.46 |
| Serum albumin, g/dL | 3.4 (3.1, 3.6) | 3.5 (3.0, 3.6) | 3.5 (3.2, 3.9) | 0.093 |
| Serum sodium, mEq/L | 139 (137, 141) | 140 (137, 142) | 139 (138, 140) | 0.95 |
| Total cholesterol, mg/dL | 154 (137, 172) | 144 (97, 176) | 163 (145, 175) | 0.33 |
| eGFR, mL/min/1.73 m2 | 51 (37, 64) | 37 (33, 46) | 57 (37, 67) | 0.055 |
| Plasma BNP, pg/mL | 101 (55, 210) | 156 (73, 178) | 103 (41, 157) | 0.012 * |
| Echocardiography | ||||
| LVDd, mm | 45 (40, 50) | 47 (43, 53) | 42 (39, 50) | 0.96 |
| LVEF, % | 64 (56, 70) | 68 (45, 71) | 63 (58, 70) | 0.77 |
| Left atrial diameter, mm | 41 (36, 52) | 41 (36, 45) | 38 (35, 47) | 0.98 |
| AV peak velocity, m/s | 2.2 (1.8, 2.4) | 2.2 (1.9, 2.5) | 2.1 (1.7, 2.4) | 0.65 |
| AV mean velocity, m/s | 11 (7, 12) | 10 (6, 13) | 11 (8, 12) | 0.77 |
| AV area, cm2 | 1.4 (1.2, 1.6) | 1.7 (1.3, 2.0) | 1.4 (1.2, 1.6) | 0.52 |
| Frailty data | ||||
| MMS | 26 (23, 28) | 22 (19, 279 | 27 (24, 28) | 0.076 |
| CSHA scale | 4 (3, 4) | 5 (4, 6) | 3 (3, 4) | 0.002 * |
| SAS, Mets | 5.0 (4.0, 5.8) | 4.5 (4.5, 5.3) | 5.5 (4.0, 6.0) | 0.89 |
| Barthel index | 100 (95, 100) | 95 (73, 100) | 100 (100, 100) | 0.11 |
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Hazard Ratio (95%CI) | p Value | Hazard Ratio (95%CI) | p Value | |
| STS score | 0.99 (0.98–1.01) | 0.87 | NA | NA |
| Peripheral artery disease | 2.05 (1.05–3.98) | 0.035 * | 1.55 (0.75–3.21) | 0.24 |
| Atrial fibrillation | 2.47 (1.16–5.26) | 0.019 * | 2.04 (0.93–4.45) | 0.075 |
| Logarithm of BNP, pg/mL | 2.54 (1.20–5.39) | 0.015 * | 1.92 (0.84–4.41) | 0.12 |
| CSHA scale | 1.58 (0.97–2.12) | 0.076 | NA | NA |
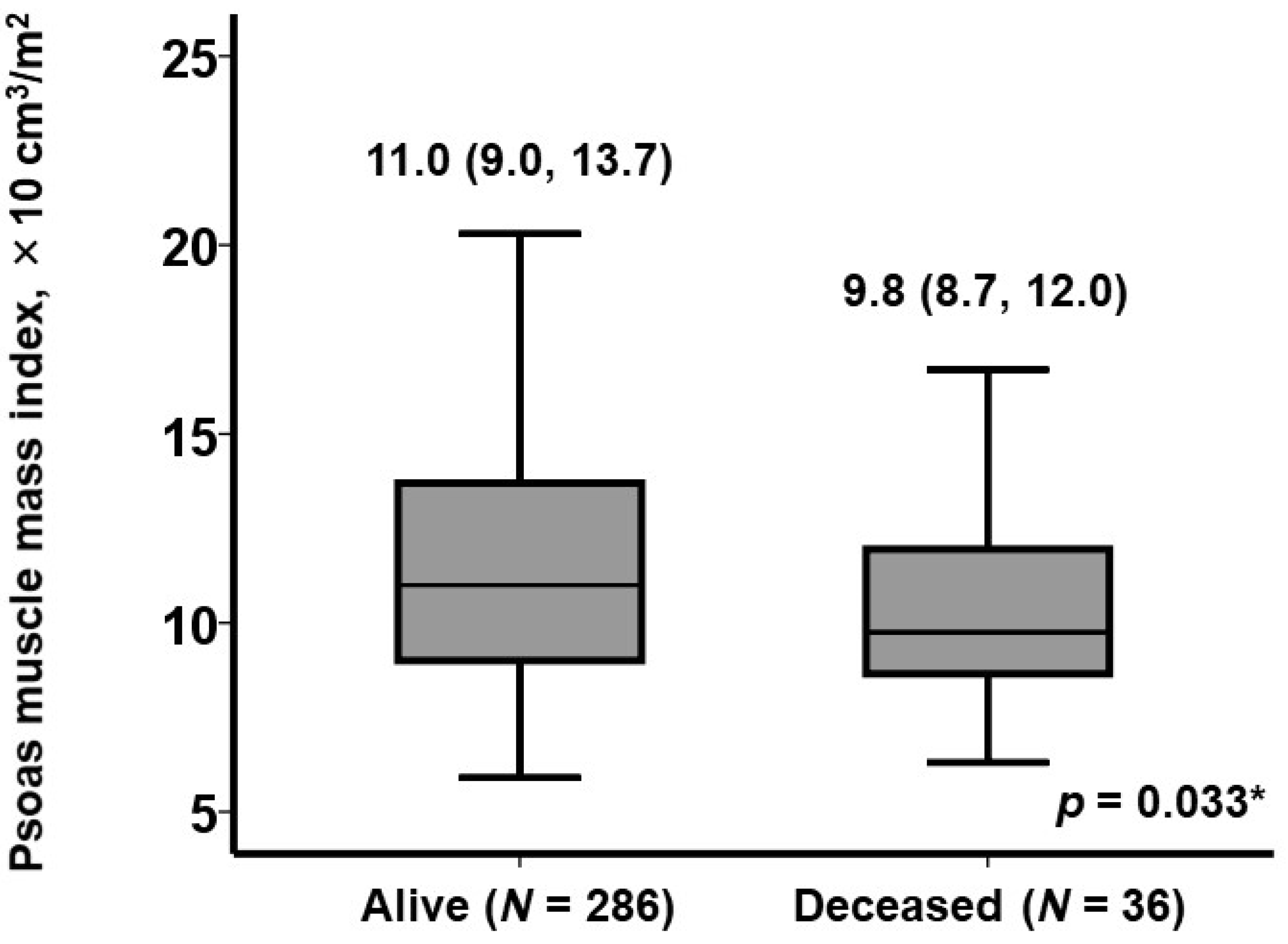
| Psoas muscle mass index, ×10 cm3/m2 | 0.89 (0.79–0.99) | 0.041 * | 0.88 (0.79–0.99) | 0.044 * |
| Small Psoas Muscle Mass Index | Large Psoas Muscle Mass Index | p Value | |
|---|---|---|---|
| Age, years | 85 (82, 89) | 86 (82, 89) | 0.076 |
| Body mass index | 22.6 (20.0, 24.5) | 20.9 (19.0, 23.5) | 0.004 * |
| Hemoglobin, g/dL | 10.4 (9.6, 11.5) | 10.6 (10.0, 10.9) | 0.006 * |
| Serum albumin, g/dL | 3.5 (3.2, 3.7) | 3.5 (3.4, 4.0) | 0.71 |
| Total cholesterol, mg/dL | 154 (131, 164) | 166 (146, 178) | 0.007 * |
| MMS | 24 (20, 27) | 28 (27, 30) | 0.008 * |
| CSHA score | 4 (3, 5) | 4 (3, 4) | 0.10 |
| SAS, Mets | 4.5 (3.9, 5.5) | 5.5 (4.3, 6.0) | 0.053 |
| Barthel index | 100 (95, 100) | 100 (95, 100) | 0.73 |
| Psoas mass index, ×10 cm3/m2 | 9.4 (8.1, 10.3) | 14.1 (12.1, 17.7) | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imamura, T.; Fujioka, H.; Ushijima, R.; Sobajima, M.; Fukuda, N.; Ueno, H.; Kinugawa, K. Prognostic Impact of Psoas Muscle Mass Index following Trans-Catheter Aortic Valve Replacement. J. Clin. Med. 2023, 12, 3943. https://doi.org/10.3390/jcm12123943
Imamura T, Fujioka H, Ushijima R, Sobajima M, Fukuda N, Ueno H, Kinugawa K. Prognostic Impact of Psoas Muscle Mass Index following Trans-Catheter Aortic Valve Replacement. Journal of Clinical Medicine. 2023; 12(12):3943. https://doi.org/10.3390/jcm12123943
Chicago/Turabian StyleImamura, Teruhiko, Hayato Fujioka, Ryuichi Ushijima, Mitsuo Sobajima, Nobuyuki Fukuda, Hiroshi Ueno, and Koichiro Kinugawa. 2023. "Prognostic Impact of Psoas Muscle Mass Index following Trans-Catheter Aortic Valve Replacement" Journal of Clinical Medicine 12, no. 12: 3943. https://doi.org/10.3390/jcm12123943
APA StyleImamura, T., Fujioka, H., Ushijima, R., Sobajima, M., Fukuda, N., Ueno, H., & Kinugawa, K. (2023). Prognostic Impact of Psoas Muscle Mass Index following Trans-Catheter Aortic Valve Replacement. Journal of Clinical Medicine, 12(12), 3943. https://doi.org/10.3390/jcm12123943