Diagnostic Performance of Dynamic Whole-Body Patlak [18F]FDG-PET/CT in Patients with Indeterminate Lung Lesions and Lymph Nodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. PET/CT Examination Protocol
2.3. Reconstruction and Postprocessing
2.4. Image Evaluation and Segmentation
2.5. Ground Truth
2.6. Statistical Analysis
3. Results
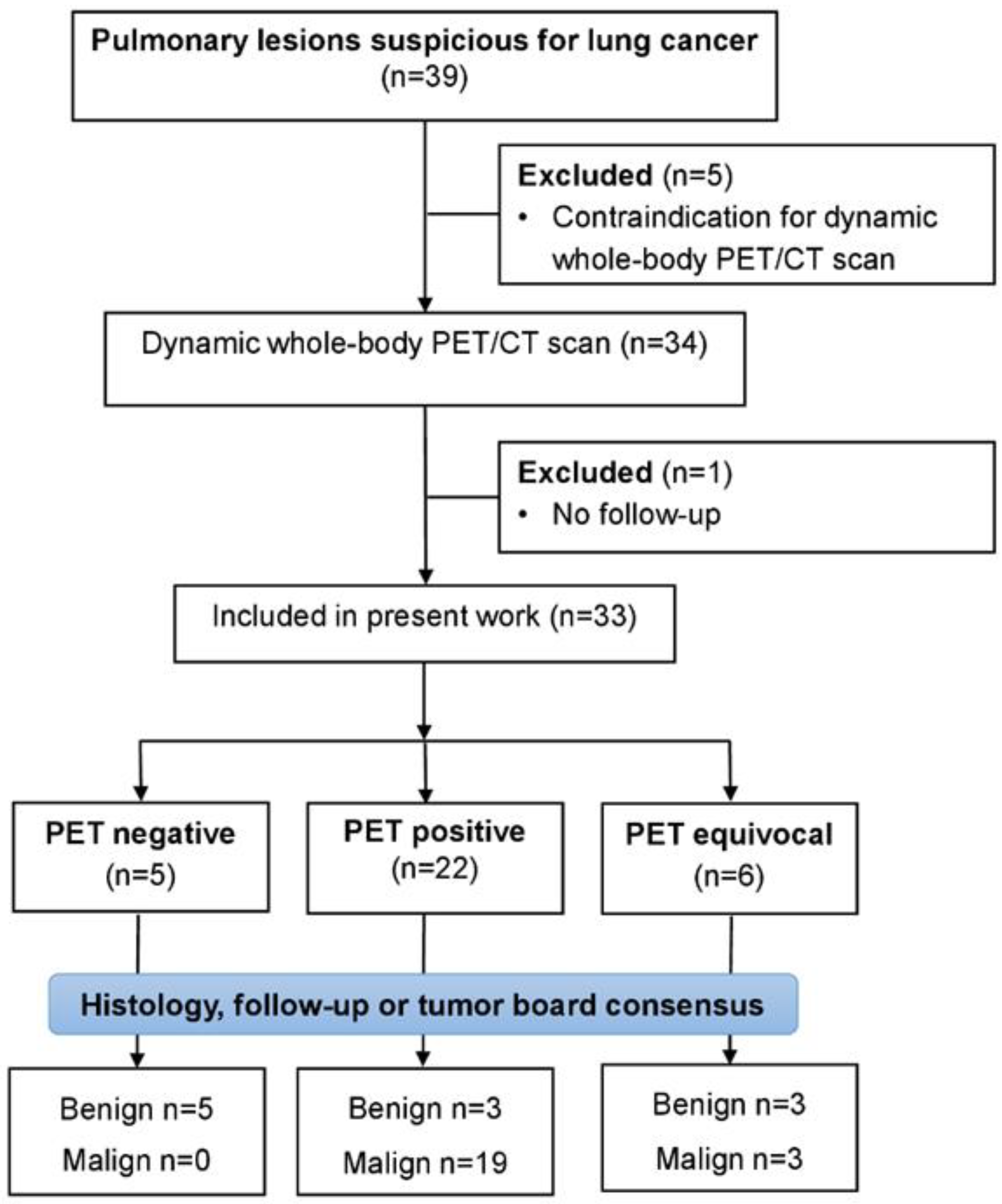
3.1. Patient Cohort
3.2. Pulmonary Lesions
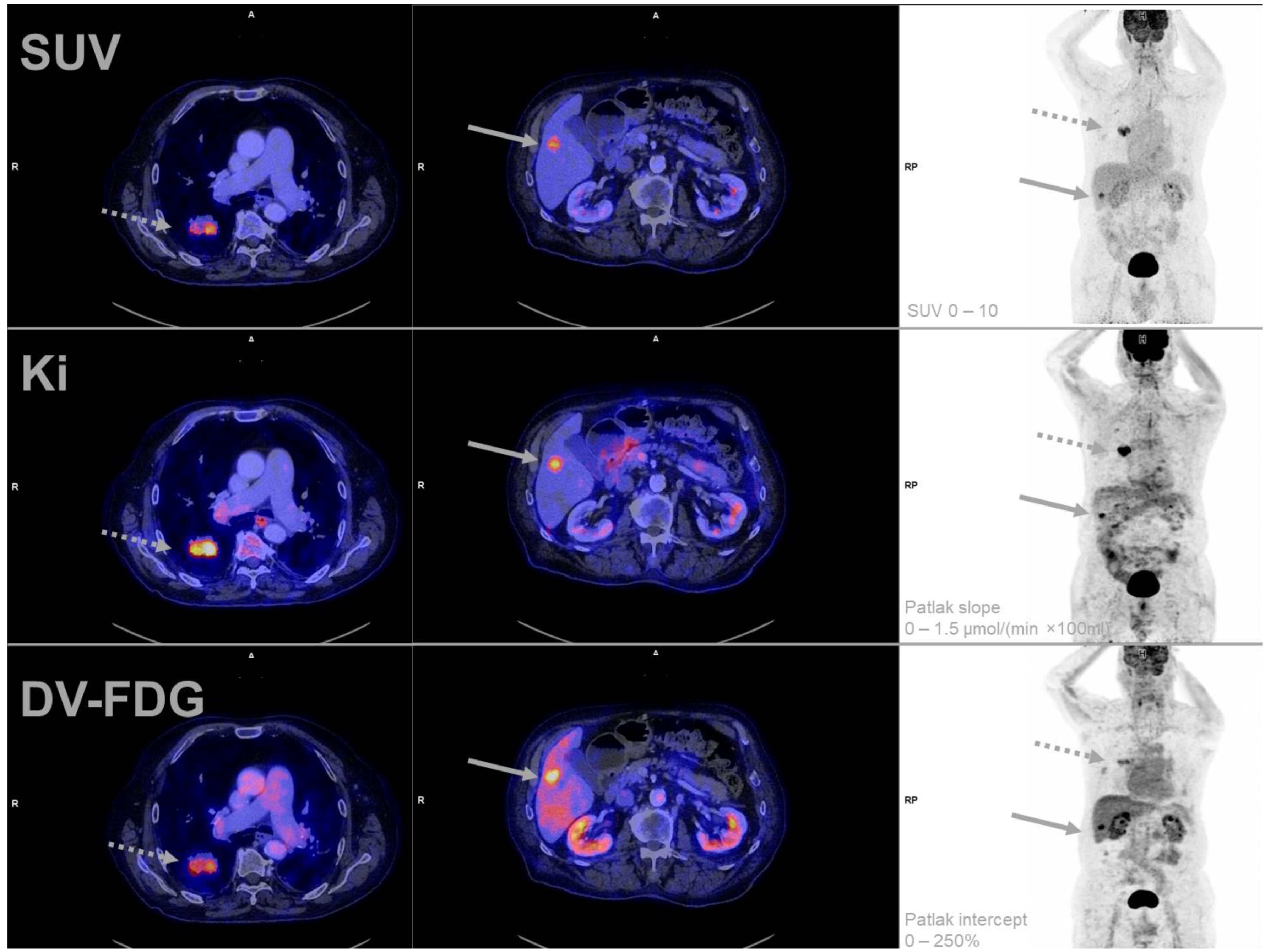
3.3. Feasibility of Patlak-PET Data Acquisition
3.4. Effect of Quantification Method on Diagnostic Accuracy
3.5. Lymph Nodes Characteristics
3.6. Patlak FDG-PET: Dynamic Parameter Evaluation
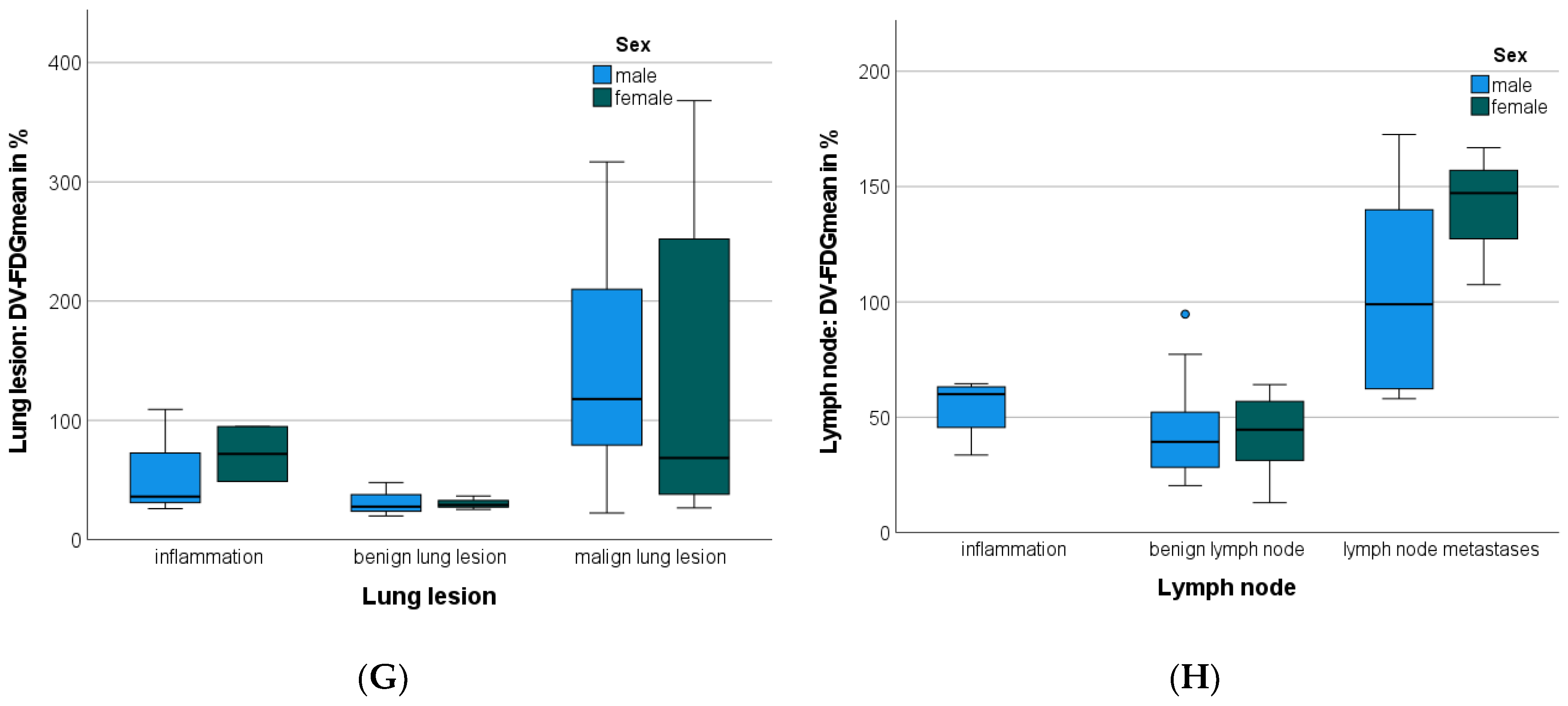
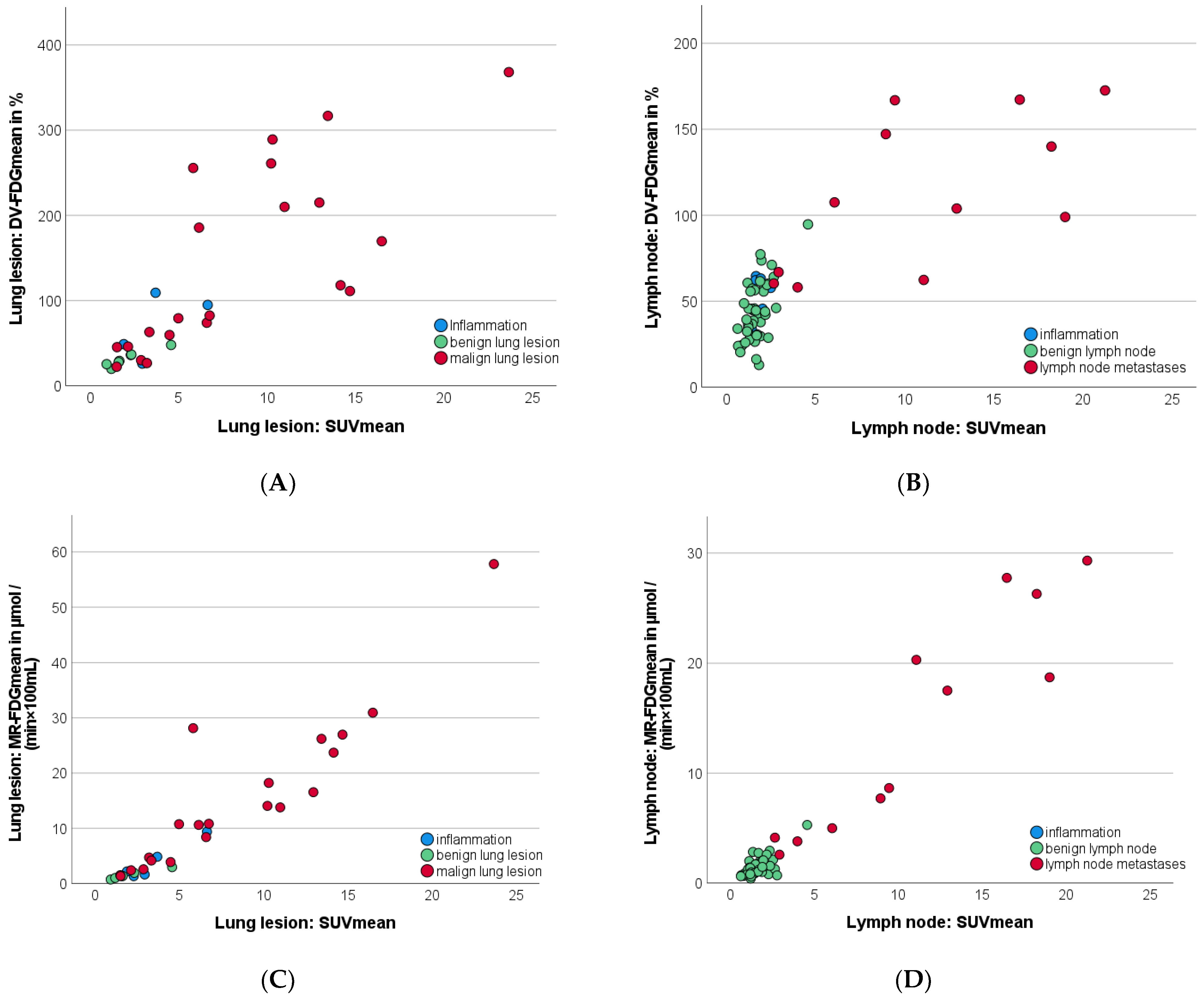
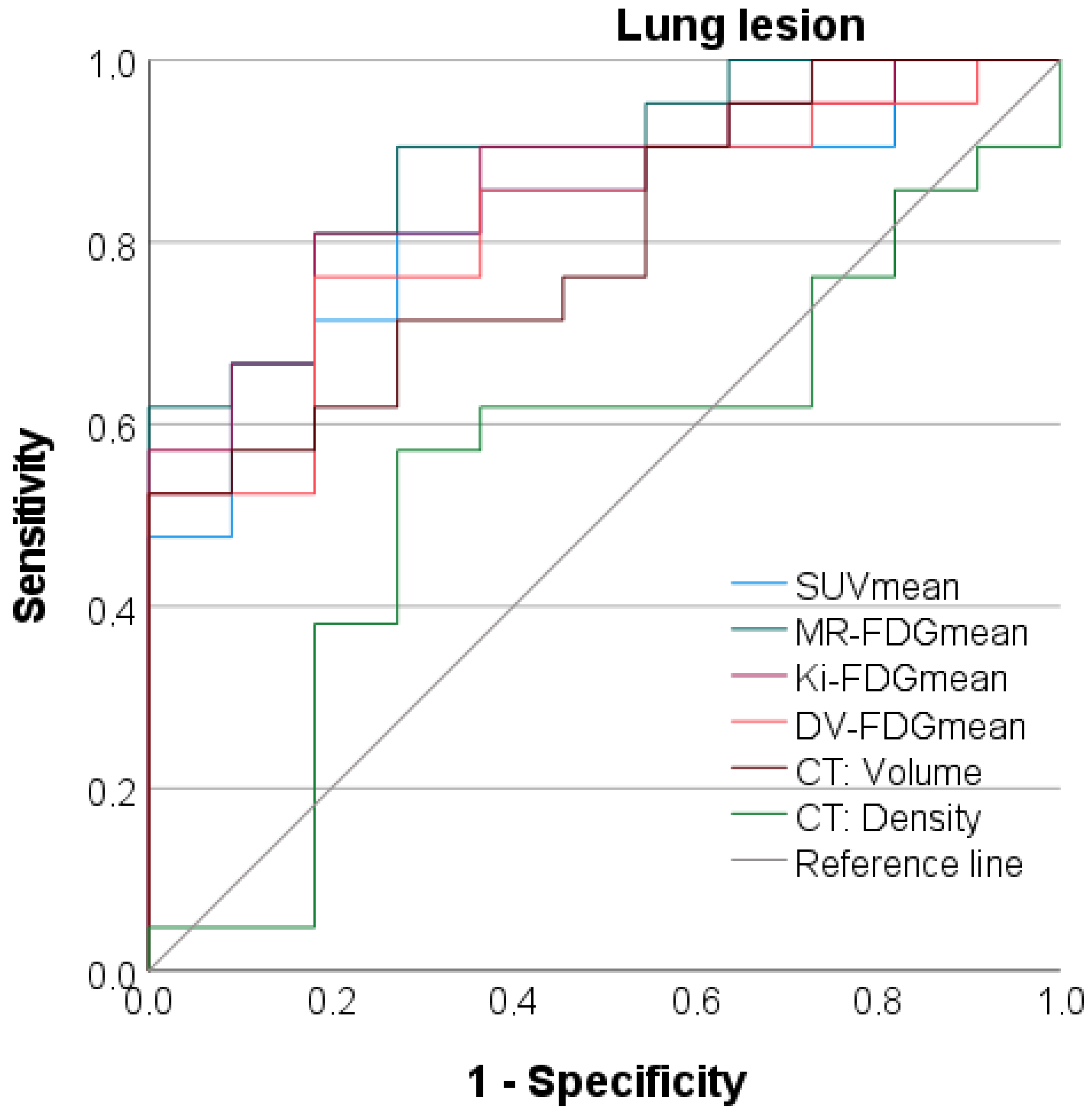
3.7. Discriminatory Power between Benign and Malignant Lung Lesions
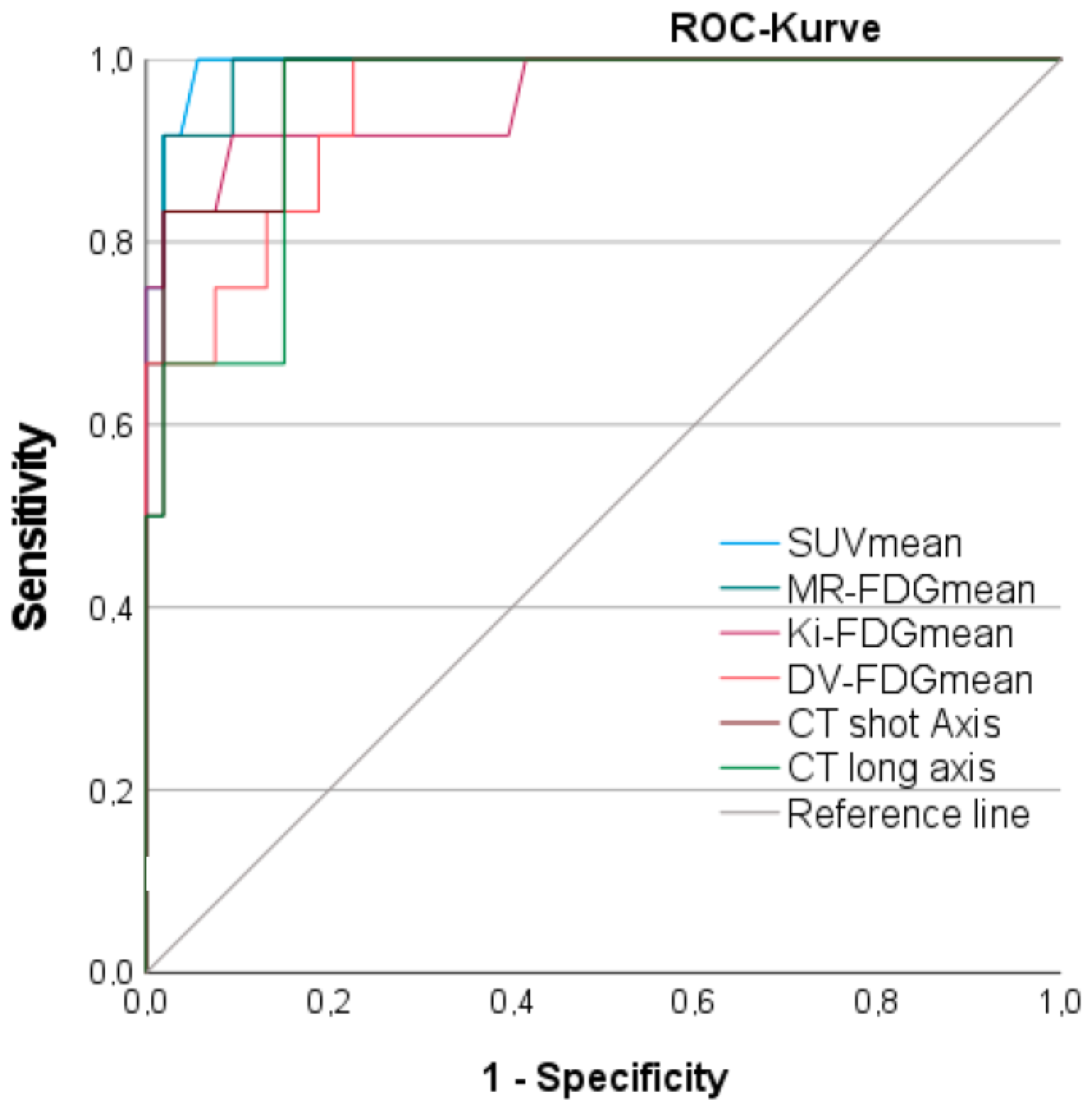
3.8. Discriminatory Power between Benign and Malignant Lymph Nodes
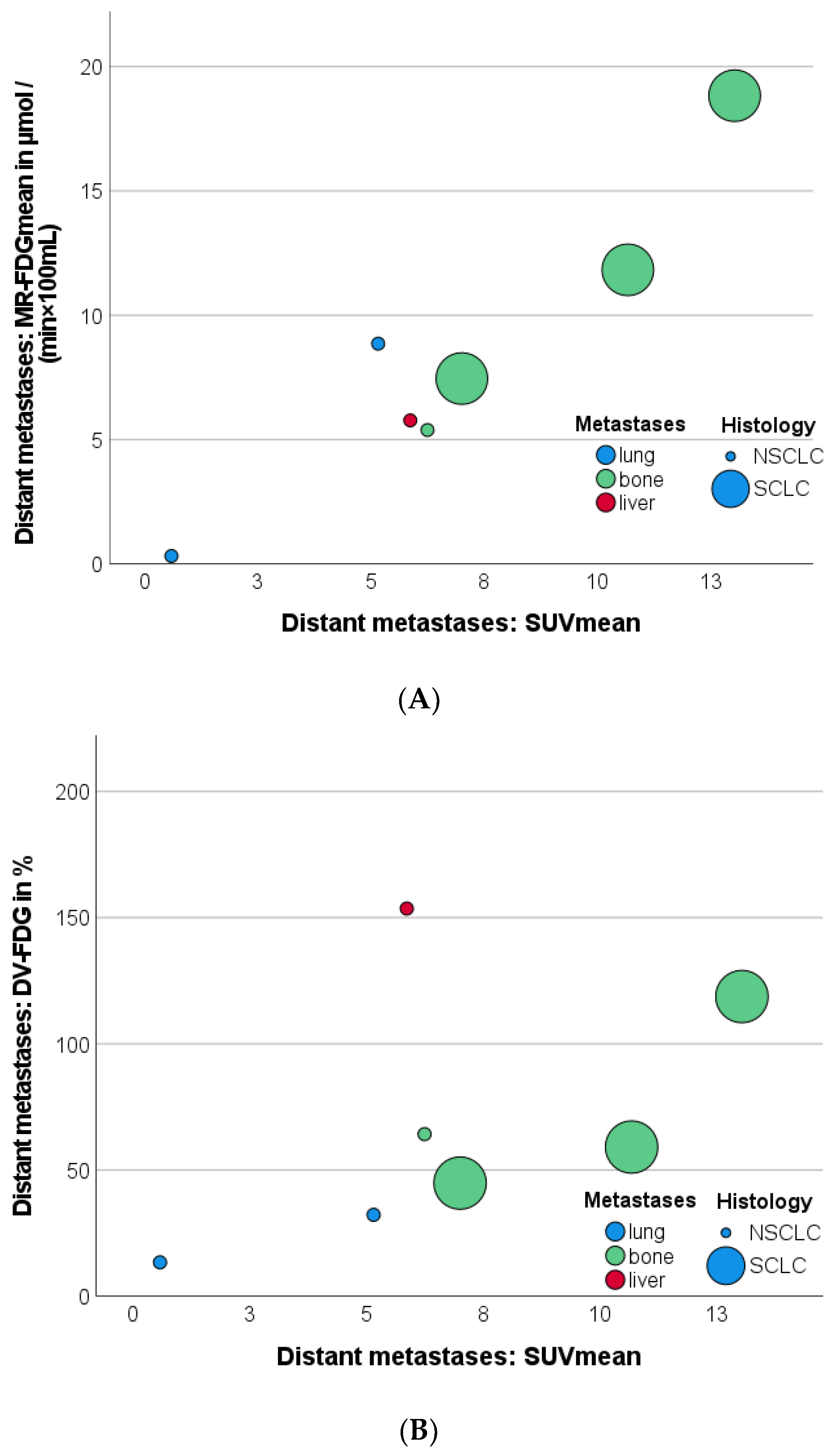
3.9. Effect of Distant Metastases on SUVmean, Patlak Kimean, and DV-FDGmean Values of Primary Tumor and LNM
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wankhede, D. Evaluation of Eighth AJCC TNM Sage for Lung Cancer NSCLC: A Meta-analysis. Ann. Surg. Oncol. 2021, 28, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef] [PubMed]
- Kalemkerian, G.P.; Loo, B.W.; Akerley, W.; Attia, A.; Bassetti, M.; Boumber, Y.; Decker, R.; Dobelbower, C.; Dowlati, A.; Grecula, J.C.; et al. NCCN Guidelines Insights: Small Cell Lung Cancer, Version 2.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Coello, C.; Fisk, M.; Mohan, D.; Wilson, F.J.; Brown, A.P.; Polkey, M.I.; Wilkinson, I.; Tal-Singer, R.; Murphy, P.S.; Cheriyan, J.; et al. Quantitative analysis of dynamic (18)F-FDG PET/CT for measurement of lung inflammation. EJNMMI Res. 2017, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lin, Z.; Xu, Z.; Li, D.; Lv, W.; Yang, S.; Liu, Y.; Cao, Y.; Cao, Q.; Jin, H. Influx rate constant of (18)F-FDG increases in metastatic lymph nodes of non-small cell lung cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Hu, S.; Deng, H.; Jia, Z. Diagnostic value of dual time-point 18 F-FDG PET/CT versus single time-point imaging for detection of mediastinal nodal metastasis in non-small cell lung cancer patients: A meta-analysis. Acta Radiol. 2015, 56, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Nogami, Y.; Banno, K.; Irie, H.; Iida, M.; Masugi, Y.; Murakami, K.; Aoki, D. Efficacy of 18-FDG PET-CT dual-phase scanning for detection of lymph node metastasis in gynecological cancer. Anticancer Res. 2015, 35, 2247–2253. [Google Scholar] [PubMed]
- Shinya, T.; Rai, K.; Okumura, Y.; Fujiwara, K.; Matsuo, K.; Yonei, T.; Sato, T.; Watanabe, K.; Kawai, H.; Sato, S.; et al. Dual-Time-Point F-18 FDG PET/CT for Evaluation of Intrathoracic Lymph Nodes in Patients With Non-Small Cell Lung Cancer. Clin. Nucl. Med. 2009, 34, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Karakatsanis, N.A.; Lodge, M.A.; Tahari, A.K.; Zhou, Y.; Wahl, R.L.; Rahmim, A. Dynamic whole-body PET parametric imaging: I. Concept, acquisition protocol optimization and clinical application. Phys. Med. Biol. 2013, 58, 7391–7418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakatsanis, N.A.; Lodge, M.A.; Zhou, Y.; Wahl, R.L.; Rahmim, A. Dynamic whole-body PET parametric imaging: II. Task-oriented statistical estimation. Phys. Med. Biol. 2013, 58, 7419–7445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmim, A.; Lodge, M.A.; Karakatsanis, N.A.; Panin, V.Y.; Zhou, Y.; McMillan, A.; Cho, S.; Zaidi, H.; Casey, M.E.; Wahl, R.L. Dynamic whole-body PET imaging: Principles, potentials and applications. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 501–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, A.H.; Pedersen, M.F.; Danielsen, H.; Munk, O.L.; Gormsen, L.C. Clinical feasibility and impact of fully automated multiparametric PET imaging using direct Patlak reconstruction: Evaluation of 103 dynamic whole-body (18)F-FDG PET/CT scans. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 837–850. [Google Scholar] [CrossRef] [PubMed]
- Patlak, C.S.; Blasberg, R.G. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J. Cereb. Blood Flow Metab. 1985, 5, 584–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patlak, C.S.; Blasberg, R.G.; Fenstermacher, J.D. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J. Cereb. Blood Flow Metab. 1983, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Spottiswoode, B.S.; Vijay; Hu, J.; von Gall, C. FlowMotion Multiparametric PET Suite—The Patlak Model; Siemens Medical Solutions USA, Inc.: Princeton, NJ, USA, 2018. [Google Scholar]
- Ye, Q.; Wu, J.; Lu, Y.; Naganawa, M.; Gallezot, J.D.; Ma, T.; Liu, Y.; Tanoue, L.; Detterbeck, F.; Blasberg, J. Improved discrimination between benign and malignant LDCT screening-detected lung nodules with dynamic over static (18)F-FDG PET as a function of injected dose. Phys. Med. Biol. 2018, 63, 175015. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.H.; Hansen, A.K.; Munk, O.L.; Gormsen, L.C. Normal values for (18)F-FDG uptake in organs and tissues measured by dynamic whole body multiparametric FDG PET in 126 patients. EJNMMI Res. 2022, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Laffon, E.; Marthan, R. Is Patlak y-intercept a relevant metrics? Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1287–1290. [Google Scholar] [CrossRef] [PubMed]

| Study-ID | Sex | Age at PET | Final Diagnosis of Lung Lesion | Diagnosis Confirmation | Tumor Stage | ||
|---|---|---|---|---|---|---|---|
| T | N | M | |||||
| 1 | f | 54 | Inflammation | Follow-up | |||
| 2 | m | 81 | CLL | Biopsy | |||
| 3 | f | 56 | Benign | Follow-up | |||
| 4 | m | 75 | NSCLC | Surgery | T4 | N2 | M1a |
| 5 | m | 61 | Hematoma | Follow-up | |||
| 6 | m | 58 | NSCLC | Surgery | pT3 | pN0 | cM0 |
| 7 | m | 64 | Inflammation | Follow-up | |||
| 8 | m | 78 | NSCLC | Biopsy | cT3 | cN2 | cM0 |
| 9 | f | 50 | NSCLC | Biopsy | cT4 | cN3 | cM1 |
| 10 | m | 82 | Benign | Follow-up | |||
| 11 | m | 66 | NSCLC | Biopsy | pT2a | N0 | M0 |
| 12 | m | 79 | Inflammatory myofibroblastic tumor | Surgery | |||
| 13 | m | 69 | SCLC | Biopsy | cT4 | cN3 | cM1c |
| 14 | f | 71 | NSCLC | Surgery | pT2a | pN0 | cM0 |
| 15 | f | 73 | NSCLC | Surgery | pT1b | pN0 | cM0 |
| 16 | m | 77 | Malign | Interdisciplinary Tumor board | cT1b | N0 | M0 |
| 17 | f | 76 | NET | Surgery | pT2a | pN0 | pM0 |
| 18 | f | 41 | Benign | Interdisciplinary Tumor board | |||
| 20 | m | 69 | NSCLC | Surgery | pT1b | pN0 | pM0 |
| 21 | f | 57 | NSCLC | Surgery | pT1c | pN0 | pM0 |
| 22 | f | 56 | Hamartoma | Follow-up | |||
| 23 | f | 56 | Sarcoidosis | Biopsy | |||
| 24 | f | 73 | Regredient Lesion | Interdisciplinary Tumor board | |||
| 25 | f | 57 | NSCLC | Biopsy | cT3c | cN1 | pM1a |
| 26 | m | 52 | Inflammation | Follow-up | |||
| 28 | m | 61 | Primary Lung Tumor | Interdisciplinary Tumor board | cT1b | cN0 | cM0 |
| 29 | m | 66 | Primary Lung Tumor | Interdisciplinary Tumor board | cT4 | cN2 | cM1b |
| 30 | f | 69 | NSCLC | Biopsy | cT4 | cN0 | cM0 |
| 31 | m | 59 | NSCLC | Surgery | pT2b | pN0 | cM0 |
| 32 | f | 54 | NSCLC | Surgery | pT2a pT1a | pN1 pN0 | pMx pMx |
| 33 | m | 73 | NSCLC | Biopsy | T2b | Nx | M1 |
| 35 | m | 54 | Inflammation | Biopsy | |||
| 36 | m | 65 | NSCLC | Biopsy | cT2a | cN2 | cM0 |
| Total | Malign | Benign | Inflammation | |
|---|---|---|---|---|
| Lung lesions | n = 32 | n = 21 | n = 6 | n = 5 |
| Volume (mL) | 33.41 ± 58.63 | 48.34 ± 67.09 | 1.85 ± 1.71 * | 8.63 ± 5.80 * |
| Density (HU) | 19.55 ± 28.93 | 20.78 ± 30.18 | 3.72 ± 23.26 * | 33.40 ± 25.16 |
| SUVmean | 6.45 ± 5.56 | 8.40 ± 5.89 | 2.05 ± 1.33 * | 3.50 ± 1.89 |
| Patlak Kimean (mL/(min × 100 mL)) | 1.93 ± 2.1 | 2.67 ± 2.26 | 0.30 ± 0.17 * | 0.78 ± 0.56 |
| MR-FDGmean (µmol/(min × 100 mL)) | 10.82 ± 12.62 | 15.01 ± 13.68 | 1.56 ± 0.80 * | 3.88 ± 3.38 |
| DV-FDGmean (%) | 110.35 ± 99.56 | 114.25 ± 106.95 | 31.13 ± 9.87 * | 63.03 ± 26.89 |
| Lymph nodes | n = 65 | n = 6 | n = 47 | n = 12 |
| Short-axis (mm) | 9.38 ± 5.75 | 17.73 ± 8.22 | 7.61 ± 2.73 * | 6.57 ± 0.54 * |
| Long-axis (mm) | 15.95 ± 7.97 | 26.52 ± 10.35 | 12.32 ± 4.27 * | 12.45 ± 3.93 * |
| Volume (mL) | 2.05 ± 6.40 | 8.17 ± 13.63 | 0.65 ± 7.22 | 0.77 ± 0.66 |
| SUVmean | 3.43 ± 4.60 | 11.09 ± 6.54 | 1.67 ± 0.68 * | 1.86 ± 0.38 * |
| Patlak Kimean (mL/(min × 100 mL) | 0.70 ± 1.14 | 2.47 ± 1.80 | 0.28 ± 0.16 * | 0.40 ± 0.69 * |
| MR-FDGmean (µmol/(min × 100 mL)) | 3.85 ± 6.58 | 14.31 ± 10.13 | 1.50 ± 0.83 * | 1.32 ± 0.30 * |
| DV-FDGmean (%) | 57.02 ± 36.00 | 112.69 ± 44.81 | 43.13 ± 17.24 * | 54.50 ± 12.35 * |
| Metastases | n = 7 | |||
| SUVmean | 6.94 ± 4.00 | |||
| Patlak Kimean (mL/(min × 100 mL)) | 1.47 ± 1.03 | |||
| MR-FDGmean (µmol/(min × 100 mL)) | 8.37 ± 5.82 | |||
| DV-FDGmean (%) | 69.45 ± 49.63 |
| AUC | Std. Error | 95% CI | p-Value | Cut-off Value | Sens. | Spez. | |
|---|---|---|---|---|---|---|---|
| PET: SUVmean | 0.827 | 0.073 | 0.684–0.970 | 0.003 | 3.08 | 81.0% | 72.7% |
| PET: MR-FDGmean | 0.887 | 0.057 | 0.775–1.000 | <0.001 | 61.7 (µmol/(min × 100 mL)) | 81.0% | 81.8% |
| PET: Patlak Ki-FDGmean | 0.861 | 0.065 | 0.735–0.988 | 0.001 | 0.68 (mL/(min × 100 mL)) | 81.0% | 81.8% |
| PET: DV-FDGmean | 0.818 | 0.075 | 0.671–0.965 | 0.004 | 54.3% | 76.2% | 81.8% |
| Ratio: SUVmean lesion/SUVmean blood pool | 0.835 | 0.070 | 0.698–0.973 | 0.002 | 1.86 | 71.4% | 72.7% |
| Ratio: SUVmean lesion/SUVmean liver tissue | 0.838 | 0.071 | 0.699–0.977 | 0.002 | 1.38 | 71.4% | 72.7% |
| CT: Lesion volume | 0.797 | 0.078 | 0.643–0.950 | 0.007 | 5.6 mL | 71.4% | 72.7% |
| CT: Lesion density | 0.550 | 0.109 | 0.335–0.764 | 0.648 | 17.0 HU | 61.9% | 63.6% |
| CT: Lesion SD density | 0.677 | 0.103 | 0.475–0.880 | 0.104 | 15.1 HU | 76.2% | 63.2% |
| AUC | Std. Error | 95% CI | p-Value | Cut-off Value | Sens. | Spez. | |
|---|---|---|---|---|---|---|---|
| PET: SUVmean | 0.993 | 0.007 | 0.979–1.000 | <0.001 | 2.61 | 100% | 94.3% |
| PET: MR-FDGmean | 0.987 | 0.011 | 0.966–1.000 | <0.001 | 2.58 (µmol/(min × 100 mL)) | 91.7% | 90.6% |
| PET: Patlak Ki-FDGmean | 0.958 | 0.034 | 0.891–1.000 | <0.001 | 0.49 (mL/(min × 100 mL)) | 83.3% | 92.5% |
| PET: DV-FDGmean | 0.948 | 0.028 | 0.893–1.000 | <0.001 | 60.5 % | 83.3% | 81.1% |
| CT: short axis | 0.969 | 0.020 | 0.929–1.000 | <0.001 | 10.5 mm | 91.7% | 84.9% |
| CT: long axis | 0.947 | 0.028 | 0.893–1.000 | <0.001 | 16.1 mm | 83.3% | 84.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weissinger, M.; Atmanspacher, M.; Spengler, W.; Seith, F.; Von Beschwitz, S.; Dittmann, H.; Zender, L.; Smith, A.M.; Casey, M.E.; Nikolaou, K.; et al. Diagnostic Performance of Dynamic Whole-Body Patlak [18F]FDG-PET/CT in Patients with Indeterminate Lung Lesions and Lymph Nodes. J. Clin. Med. 2023, 12, 3942. https://doi.org/10.3390/jcm12123942
Weissinger M, Atmanspacher M, Spengler W, Seith F, Von Beschwitz S, Dittmann H, Zender L, Smith AM, Casey ME, Nikolaou K, et al. Diagnostic Performance of Dynamic Whole-Body Patlak [18F]FDG-PET/CT in Patients with Indeterminate Lung Lesions and Lymph Nodes. Journal of Clinical Medicine. 2023; 12(12):3942. https://doi.org/10.3390/jcm12123942
Chicago/Turabian StyleWeissinger, Matthias, Max Atmanspacher, Werner Spengler, Ferdinand Seith, Sebastian Von Beschwitz, Helmut Dittmann, Lars Zender, Anne M. Smith, Michael E. Casey, Konstantin Nikolaou, and et al. 2023. "Diagnostic Performance of Dynamic Whole-Body Patlak [18F]FDG-PET/CT in Patients with Indeterminate Lung Lesions and Lymph Nodes" Journal of Clinical Medicine 12, no. 12: 3942. https://doi.org/10.3390/jcm12123942
APA StyleWeissinger, M., Atmanspacher, M., Spengler, W., Seith, F., Von Beschwitz, S., Dittmann, H., Zender, L., Smith, A. M., Casey, M. E., Nikolaou, K., Castaneda-Vega, S., & la Fougère, C. (2023). Diagnostic Performance of Dynamic Whole-Body Patlak [18F]FDG-PET/CT in Patients with Indeterminate Lung Lesions and Lymph Nodes. Journal of Clinical Medicine, 12(12), 3942. https://doi.org/10.3390/jcm12123942







