A Neuropsychiatric Assessment of Children with Previous SARS-CoV-2 Infection
Abstract
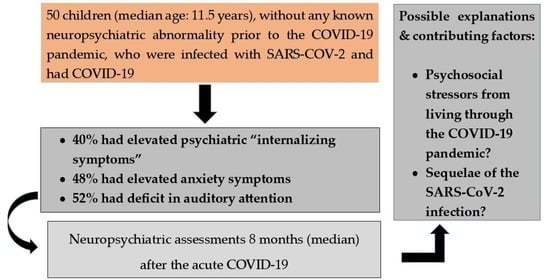
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Assessments
2.4. Statistical Analyses
3. Results
3.1. Sample Demographics and Clinical Characteristics
3.2. Neuropsychiatric Follow-Up Assessments
3.2.1. Neurological Assessment
3.2.2. Sleep
3.2.3. Psychiatric Assessment
3.2.4. Neurocognitive Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boldrini, M.; Canoll, P.D.; Klein, R.S. How COVID-19 Affects the Brain. JAMA Psychiatry 2021, 78, 682. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nalleballe, K.; Onteddu, S.R.; Sharma, R.; Dandu, V.; Brown, A.; Jasti, M.; Yadala, S.; Veerapaneni, K.; Siddamreddy, S.; Avula, A.; et al. Spectrum of neuropsychiatric manifestations in COVID-19. Brain Behav. Immun. 2020, 88, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Molteni, E.; Sudre, C.H.; Canas, L.S.; Bhopal, S.S.; Hughes, R.C.; Antonelli, M.; Murray, B.; Kläser, K.; Kerfoot, E.; Chen, L.; et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc. Health 2021, 5, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Dufort, E.M.; Koumans, E.H.; Chow, E.; Rosenthal, E.M.; Muse, A.; Rowlands, J.; Barranco, M.A.; Maxted, A.M.; Rosenberg, E.S.; Easton, D.; et al. Multisystem Inflammatory Syndrome in Children in New York State. N. Engl. J. Med. 2020, 383, 347–358. [Google Scholar] [CrossRef]
- LaRovere, K.L.; Riggs, B.J.; Poussaint, T.Y.; Young, C.C.; Newhams, M.M.; Maamari, M.; Walker, T.C.; Singh, A.R.; Dapul, H.; Hobbs, C.V.; et al. Neurologic Involvement in Children and Adolescents Hospitalized in the United States for COVID-19 or Multisystem Inflammatory Syndrome. JAMA Neurol. 2021, 78, 536. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and Predictors of Long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Pereira, S.M.P.; Shafran, R.; Nugawela, M.D.; Panagi, L.; Hargreaves, D.; Ladhani, S.N.; Bennett, S.D.; Chalder, T.; Dalrymple, E.; Ford, T.; et al. Natural course of health and well-being in non-hospitalised children and young people after testing for SARS-CoV-2: A prospective follow-up study over 12 months. Lancet Reg. Health Eur. 2023, 25. [Google Scholar] [CrossRef]
- Borch, L.; Holm, M.; Knudsen, M.; Ellermann-Eriksen, S.; Hagstroem, S. Long COVID symptoms and duration in SARS-CoV-2 positive children—A nationwide cohort study. Eur. J. Pediatr. 2022, 181, 1597–1607. [Google Scholar] [CrossRef]
- Berg, S.K.; Palm, P.; Nygaard, U.; Bundgaard, H.; Petersen, M.N.S.; Rosenkilde, S.; Thorsted, A.B.; Ersbøll, A.K.; Thygesen, L.C.; Nielsen, S.D.; et al. Long COVID symptoms in SARS-CoV-2-positive children aged 0–14 years and matched controls in Denmark (LongCOVIDKidsDK): A national, cross-sectional study. Lancet Child Adolesc. Health 2022, 6, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Simon, N.M. Bridging Knowledge Gaps in the Diagnosis and Management of Neuropsychiatric Sequelae of COVID-19. JAMA Psychiatry 2022, 79, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef]
- Ferrucci, R.; Dini, M.; Groppo, E.; Rosci, C.; Reitano, M.R.; Bai, F.; Poletti, B.; Brugnera, A.; Silani, V.; Monforte, A.D.; et al. Long-Lasting Cognitive Abnormalities after COVID-19. Brain Sci. 2021, 11, 235. [Google Scholar] [CrossRef]
- Savino, R.; Polito, A.N.; Arcidiacono, G.; Poliseno, M.; Caputo, S.L. Neuropsychiatric Disorders in Pediatric Long COVID-19: A Case Series. Brain Sci. 2022, 12, 514. [Google Scholar] [CrossRef]
- Buonsenso, D.; Pazukhina, E.; Gentili, C.; Vetrugno, L.; Morello, R.; Zona, M.; De Matteis, A.; D’Ilario, F.; Lanni, R.; Rongai, T.; et al. The prevalence, characteristics and risk factors of persistent symptoms in non-hospitalized and hospitalized children with SARS-CoV-2 infection followed-up for up to 12 months: A prospective, cohort study in Rome, Italy. J. Clin. Med. 2022, 11, 6772. [Google Scholar] [CrossRef] [PubMed]
- Troitskaya, L.A.; Plotnikova, I.A.; Avakyan, G.G.; Erokhina, V.A.; Badalyan, O.L.; Muraveva, A.V.; Zelentsova, V.L.; Khodko, O.K.; Safarova, S.T.; Shirokova, E.I.; et al. Neuropsychological evaluation of cognitive disorders in children after COVID-19. Eur. J. Transl. Myol. 2022, 32, 10685. [Google Scholar] [CrossRef] [PubMed]
- Avittan, H.; Kustovs, D. Cognition and Mental Health in Pediatric Patients Following COVID-19. Int. J. Environ. Res. Public Health 2023, 20, 5061. [Google Scholar] [CrossRef]
- Frigerio, A.; Rucci, P.; Goodman, R.; Ammaniti, M.; Carlet, O.; Cavolina, P.; De Girolamo, G.; Lenti, C.; Lucarelli, L.; Mani, E.; et al. Prevalence and correlates of mental disorders among adolescents in Italy: The PrISMA study. Eur. Child Adolesc. Psychiatry 2009, 18, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Ivarsson, T.; Svalander, P.; Litlere, O. The Children’s Depression Inventory (CDI) as measure of depression in Swedish adolescents. A normative study. Nord. J. Psychiatry 2006, 60, 220–226. [Google Scholar] [CrossRef]
- Ivarsson, T. Normative data for the Multidimensional Anxiety Scale for Children (MASC) in Swedish adolescents. Nord. J. Psychiatry 2006, 60, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Polanczyk, G.V.; Salum, G.A.; Sugaya, L.S.; Caye, A.; Rohde, L.A. Annual Research Review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J. Child Psychol. Psychiatry 2015, 56, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Livingston, R.D.; Shockey, D.; Morton, L.; Rao, S. Pediatric Headache Management and Use of the PedMIDAS. J. Dr. Nurs. Pract. 2019, 12, 24–30. [Google Scholar] [CrossRef]
- Bruni, O.; Ottaviano, S.; Guidetti, V.; Romoli, M.; Innocenzi, M.; Cortesi, F.; Giannotti, F. The Sleep Disturbance Scale for Children (SDSC) Construct ion and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J. Sleep Res. 1996, 5, 251–261. [Google Scholar] [CrossRef] [PubMed]
- March, J.S. Multidimensional Anxiety Scale for Children, 2nd ed.; Multi-Health Systems: Toronto, ON, Canada, 2013. [Google Scholar]
- Kovács, M. The Children’s Depression Inventory, Edition 2 (CDI-2); Technical Manual; Multi-Health Systems Inc.: Toronto, ON, Canada, 2011. [Google Scholar]
- Achenbach, T.M. Child Behavior Checklist. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Esposito, M.; Antinolfi, L.; Carotenuto, M. Neuropsychological Profile in Pediatric Migraine without Aura: A Pilot Study. Brain Sci. 2021, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, R.; Dini, M.; Rosci, C.; Capozza, A.; Groppo, E.; Reitano, M.R.; Allocco, E.; Poletti, B.; Brugnera, A.; Bai, F.; et al. One-year cognitive follow-up of COVID-19 hospitalized patients. Eur. J. Neurol. 2022, 29, 2006–2014. [Google Scholar] [CrossRef]
- Dalsgaard, S.; Thorsteinsson, E.; Trabjerg, B.B.; Schullehner, J.; Plana-Ripoll, O.; Brikell, I.; Wimberley, T.; Thygesen, M.; Madsen, K.B.; Timmerman, A.; et al. Incidence Rates and Cumulative Incidences of the Full Spectrum of Diagnosed Mental Disorders in Childhood and Adolescence. JAMA Psychiatry 2020, 77, 155–164. [Google Scholar] [CrossRef]
- Strawn, J.R.; Mills, J.A.; Schroeder, H.K.; Neptune, Z.A.; Specht, A.; Keeshin, S.W. The Impact of COVID-19 Infection and Characterization of Long COVID in Adolescents With Anxiety Disorders: A Prospective Longitudinal Study. J. Am. Acad. Child Adolesc. Psychiatry 2023. [Google Scholar] [CrossRef]
- Jones, E.A.K.; Mitra, A.K.; Bhuiyan, A.R. Impact of COVID-19 on Mental Health in Adolescents: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 2470. [Google Scholar] [CrossRef]
- Areza-Fegyveres, R.; Kairalla, R.A.; Carvalho, C.R.R.; Nitrini, R. Cognition and chronic hypoxia in pulmonary diseases. Dement. Neuropsychol. 2010, 4, 14–22. [Google Scholar] [CrossRef]
- Mizrahi, B.; Sudry, T.; Flaks-Manov, N.; Yehezkelli, Y.; Kalkstein, N.; Akiva, P.; Ekka-Zohar, A.; Ben David, S.S.; Lerner, U.; Bivas-Benita, M.; et al. Long covid outcomes at one year after mild SARS-CoV-2 infection: Nationwide cohort study. BMJ 2023, 380, e072529. [Google Scholar] [CrossRef] [PubMed]
- Steardo, L.; Verkhratsky, A. Psychiatric face of COVID-19. Transl. Psychiatry 2020, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Straburzyński, M.; Kuca-Warnawin, E.; Waliszewska-Prosół, M. COVID-19-related headache and innate immune response—A narrative review. Neurol. Neurochir. Polska 2023, 57, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Almeria, M.; Cejudo, J.C.; Sotoca, J.; Deus, J.; Krupinski, J. Cognitive profile following COVID-19 infection: Clinical predictors leading to neuropsychological impairment. Brain Behav. Immun. Health 2020, 9, 100163. [Google Scholar] [CrossRef]
- Davico, C.; Ghiggia, A.; Marcotulli, D.; Ricci, F.; Amianto, F.; Vitiello, B. Psychological Impact of the COVID-19 Pandemic on Adults and Their Children in Italy. Front. Psychiatry 2021, 12, 572997. [Google Scholar] [CrossRef]
- Meherali, S.; Punjani, N.; Louie-Poon, S.; Abdul Rahim, K.; Das, J.K.; Salam, R.A.; Lassi, Z.S. Mental Health of Children and Adolescents Amidst COVID-19 and Past Pandemics: A Rapid Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 3432. [Google Scholar] [CrossRef]
| N = 50 | |
|---|---|
| Males [n (%)] | 28 (56%) |
| Females [n (%)] | 22 (44%) |
| Hospitalized [n (%)] | 35 (70%) |
| Treated at home [n (%)] | 15 (30%) |
| MIS-C [n (%)] | 13 (26%) |
| Age at the time of the evaluation, years, min–max (median) | 8–16 (11.5) |
| Symptoms during the acute infection [n (%)]: | |
| Fever | 32 (70%) |
| Cough | 13 (26%) |
| Sore throat | 13 (26%) |
| Abdominal pain | 13 (26%) |
| Skin rash | 12 (24%) |
| Conjunctivitis | 11 (22%) |
| Headache | 11 (22%) |
| Vomiting | 11 (22%) |
| Diarrhea | 10 (20%) |
| Asthenia | 10 (20%) |
| Dyspnea | 10 (20%) |
| Anosmia/ageusia | 7 (14%) |
| Arthromyalgia | 7 (14%) |
| Chest pain | 5 (10%) |
| Cold | 20 (10%) |
| Seizures | 1 (2%) |
| Aphthous stomatitis | 1 (2%) |
| Intensive care | 5 (10%) |
| Oxygen therapy | 13 (26%) |
| Time since the acute infection, months, min–max (median) | 1–18 (8) |
| In Normal Range | In Borderline/Clinical Range | ||||
|---|---|---|---|---|---|
| n | % | n | % | ||
| SDSC | Total score | 36 | 72 | 14 | 28 |
| Disorders of excessive sleepiness (DES) | 35 | 70 | 15 | 30 | |
| Disorders of initiating and maintaining sleep (DIMS) | 37 | 74 | 13 | 26 | |
| Sleep breathing disorders (SBD) | 44 | 88 | 6 | 12 | |
| Sleep wake transition disorders (SWTD) | 44 | 88 | 6 | 12 | |
| Disorders of arousal nightmares (DA) | 45 | 90 | 5 | 10 | |
| Sleep hyperhidrosis (SHY) | 47 | 94 | 3 | 6 | |
| Ped-MIDAS | Disability Grade | 44 | 88 | 6 | 12 |
| MASC-2 | Total score | 26 | 52 | 24 | 48 |
| Public Performance Fears | 22 | 44 | 28 | 56 | |
| Separation Anxiety | 24 | 48 | 26 | 52 | |
| Harm Avoidance | 25 | 50 | 25 | 50 | |
| Generalized Anxiety Disorder | 27 | 54 | 23 | 46 | |
| Social Anxiety | 29 | 58 | 21 | 42 | |
| Panic | 29 | 58 | 21 | 42 | |
| Obsessions and compulsions | 30 | 60 | 20 | 40 | |
| Physical Symptoms | 31 | 62 | 19 | 38 | |
| Tense/Restless | 33 | 66 | 17 | 34 | |
| Humiliation/Rejection | 34 | 68 | 16 | 32 | |
| CDI-2 | Total score | 42 | 84 | 8 | 16 |
| Ineffectiveness | 38 | 76 | 12 | 24 | |
| Functional Problems | 40 | 80 | 10 | 20 | |
| Negative Mood | 45 | 90 | 5 | 10 | |
| Emotional Problems | 46 | 92 | 4 | 8 | |
| Interpersonal Problems | 46 | 92 | 4 | 8 | |
| Negative Self-Esteem | 46 | 92 | 4 | 8 | |
| CBCL | Total score | 40 | 80 | 10 | 20 |
| Internalizing problems | 30 | 60 | 20 | 40 | |
| Anxiety | 37 | 74 | 13 | 26 | |
| Somatic complaints | 38 | 76 | 12 | 24 | |
| Affective Problems | 39 | 78 | 11 | 22 | |
| Anxiety Problems | 40 | 80 | 10 | 202 | |
| Somatic Problems | 40 | 80 | 10 | 0 | |
| Inhibition/Depression | 43 | 86 | 7 | 14 | |
| Thought Problems | 43 | 86 | 7 | 14 | |
| Externalizing Problems | 44 | 88 | 6 | 12 | |
| Attention Problems | 47 | 94 | 3 | 6 | |
| Aggressive Behavior | 47 | 94 | 3 | 6 | |
| Conduct Problems | 47 | 94 | 3 | 6 | |
| Social Problems | 47 | 94 | 3 | 6 | |
| Attention Deficit/Hyperactivity | 47 | 94 | 3 | 6 | |
| Oppositional Defiant Problems | 48 | 96 | 2 | 4 | |
| Rule-breaking Behavior | 49 | 98 | 1 | 2 | |
| NEPSY-II | Response set | 20 | 40 | 30 | 60 |
| Auditory attention | 24 | 48 | 26 | 52 | |
| Inhibition A time | 24 | 48 | 26 | 52 | |
| Design fluency | 25 | 50 | 25 | 50 | |
| List memory | 25 | 50 | 25 | 50 | |
| Visual attention | 30 | 60 | 20 | 40 | |
| Inhibition B time | 30 | 60 | 20 | 40 | |
| Drawing memory test | 30 | 60 | 20 | 40 | |
| Inhibition C time | 31 | 62 | 19 | 38 | |
| Animal sorting | 32 | 64 | 18 | 36 | |
| Inhibition C combined | 33 | 66 | 17 | 34 | |
| Inhibition C error | 33 | 66 | 17 | 34 | |
| Inhibition C error | 33 | 66 | 17 | 34 | |
| Inhibition A combined | 33 | 66 | 17 | 34 | |
| Inhibition B error | 36 | 72 | 14 | 28 | |
| Inhibition A error | 36 | 72 | 14 | 28 | |
| Inhibition B combined | 37 | 74 | 13 | 26 | |
| Word list interference–repetition | 40 | 80 | 10 | 20 | |
| Word list interference–re-enactment | 40 | 80 | 10 | 20 | |
| Multidimensional Anxiety Scale for Children—2nd edition (MASC-2) | |||||
|---|---|---|---|---|---|
| Estimate | Std. Error | t | p | ||
| Sex [Female] | −2.0827737 | 3.528333 | −0.5903 | 0.55808 | |
| Age | −0.3413707 | 0.710367 | −0.4806 | 0.63327 | |
| Hospitaliz [yes] | 3.078022 | 4.303286 | 0.7153 | 0.47831 | |
| MIS-C [yes] | −11.4110832 | 4.707216 | −2.4242 | 0.01962 | |
| PedMidas 2 | 2.08062 | 4.452401 | 0.4673 | 0.64264 | |
| Time 1 | −0.0029624 | 0.014049 | −0.2109 | 0.83399 | |
| R2 = 0.12 | |||||
| Child Depression Inventory 2nd edition (CDI-2) | |||||
| Estimate | Std. Error | t | p | ||
| Sex [Female] | −0.2752695 | 1.985363 | −0.1386 | 0.89037 | |
| Age | 0.181751 | 0.399717 | 0.4547 | 0.65161 | |
| Hospital. [yes] | 4.479153 | 2.421422 | 1.8498 | 0.07122 | |
| MIS-C 1 [yes] | −2.4021302 | 2.64871 | −0.9069 | 0.36951 | |
| PedMidas 2 | 2.280707 | 2.505328 | 0.9103 | 0.36772 | |
| Time 3 | −0.0054655 | 0.007905 | −0.6914 | 0.49303 | |
| R2 = 0.15 | |||||
| Child Behavior Checklist (CBCL) Internalizing Symptoms | |||||
| Estimate | Std. Error | t | p | ||
| Sex [Female] | −0.836063 | 3.220694 | −0.2596 | 0.7964 | |
| Age | −0.392845 | 0.648429 | −0.6058 | 0.5478 | |
| Hospital. [yes] | 3.314423 | 3.928078 | 0.8438 | 0.4035 | |
| MIS-C 1 [yes] | −2.379739 | 4.296789 | −0.5538 | 0.5826 | |
| PedMidas 2 | 6.128051 | 4.064191 | 1.5078 | 0.1389 | |
| Time 3 | −0.013699 | 0.012824 | −1.0683 | 0.2914 | |
| R2 = 0.09 | |||||
| Sleep Disturbance Scale for Children (SDSC) | |||||
| Estimate | Std. Error | t | p | ||
| Sex [Female] | 4.037301 | 2.90745 | 1.3886 | 0.1723 | |
| Age | 0.55399 | 0.597811 | 0.9267 | 0.3594 | |
| Hospital. [yes] | 1.992369 | 3.488743 | 0.5711 | 0.571 | |
| MIS-C 1 [yes] | −2.137154 | 3.816479 | −0.5600 | 0.5785 | |
| PedMidas 2 | 5.333019 | 3.61232 | 1.4763 | 0.1473 | |
| Time 3 | −0.012149 | 0.012516 | −0.9707 | 0.3373 | |
| R2 = 0.11 | |||||
| Neuropsychological Assessment (NEPSY-II) | |||||
| Visual attention | |||||
| Estimate | Std. Error | t | p | p adjusted 4 | |
| Sex [Female] | −0.5879919 | 1.018448 | −0.5773 | 0.56672 | 0.95 |
| Age | −0.5155826 | 0.205046 | −2.5145 | 0.01574 | 0.15 |
| Hospital. [yes] | −0.9192230 | 1.242137 | −0.7400 | 0.4633 | 0.95 |
| MIS-C1 [yes] | −0.0055350 | 1.35873 | −0.0041 | 0.99677 | 0.99 |
| PedMidas 2 | −0.8583192 | 1.285178 | −0.6679 | 0.50779 | 0.95 |
| Time 3 | 0.001186 | 0.004055 | 0.2925 | 0.77133 | 0.95 |
| R2 = 0.18 | |||||
| Graphic fluency | |||||
| Estimate | Std. Error | t | p | p adjusted 4 | |
| Sex [Female] | −0.0806714 | 1.058304 | −0.0762 | 0.9396 | 0.99 |
| Age | −0.4914407 | 0.211424 | −2.3244 | 0.025011 | 0.15 |
| Hospital. [yes] | 2.147341 | 1.291644 | 1.6625 | 0.103862 | 0.41 |
| MIS-C1 [yes] | −0.3787250 | 1.412905 | −0.2680 | 0.789975 | 0.95 |
| PedMidas 2 | 0.644733 | 1.32159 | 0.4878 | 0.628196 | 0.95 |
| Time 3 | 0.001579 | 0.004187 | 0.3771 | 0.708024 | 0.95 |
| R2 = 0.19 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarselli, V.; Calderoni, D.; Terrinoni, A.; Davico, C.; Pruccoli, G.; Denina, M.; Carducci, C.; Smarrazzo, A.; Martucci, M.; Presicce, M.; et al. A Neuropsychiatric Assessment of Children with Previous SARS-CoV-2 Infection. J. Clin. Med. 2023, 12, 3917. https://doi.org/10.3390/jcm12123917
Scarselli V, Calderoni D, Terrinoni A, Davico C, Pruccoli G, Denina M, Carducci C, Smarrazzo A, Martucci M, Presicce M, et al. A Neuropsychiatric Assessment of Children with Previous SARS-CoV-2 Infection. Journal of Clinical Medicine. 2023; 12(12):3917. https://doi.org/10.3390/jcm12123917
Chicago/Turabian StyleScarselli, Veronica, Dario Calderoni, Arianna Terrinoni, Chiara Davico, Giulia Pruccoli, Marco Denina, Chiara Carducci, Andrea Smarrazzo, Melania Martucci, Mariaelena Presicce, and et al. 2023. "A Neuropsychiatric Assessment of Children with Previous SARS-CoV-2 Infection" Journal of Clinical Medicine 12, no. 12: 3917. https://doi.org/10.3390/jcm12123917
APA StyleScarselli, V., Calderoni, D., Terrinoni, A., Davico, C., Pruccoli, G., Denina, M., Carducci, C., Smarrazzo, A., Martucci, M., Presicce, M., Marcotulli, D., Arletti, L., Ferrara, M., Garazzino, S., Mariani, R., Campana, A., & Vitiello, B. (2023). A Neuropsychiatric Assessment of Children with Previous SARS-CoV-2 Infection. Journal of Clinical Medicine, 12(12), 3917. https://doi.org/10.3390/jcm12123917