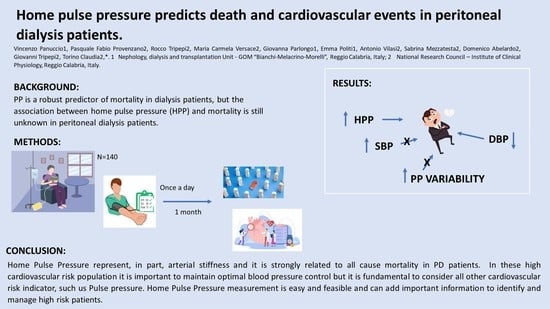
Home Pulse Pressure Predicts Death and Cardiovascular Events in Peritoneal Dialysis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Laboratory Measurements
2.3. Study End-Points
2.4. Statistical Analysis
3. Results
3.1. Survival Analysis
3.1.1. All-Cause Mortality
3.1.2. Combined Outcome—Death and Cardiovascular Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [CrossRef] [PubMed] [Green Version]
- De Nicola, L.; Chiodini, P.; Zoccali, C.; Borrelli, S.; Cianciaruso, B.; Di Iorio, B.; Santoro, D.; Giancaspro, V.; Abaterusso, C.; Gallo, C.; et al. Prognosis of CKD patients receiving outpatient nephrology care in Italy. Clin. J. Am. Soc. Nephrol. 2011, 6, 2421–2428. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, R.; Nissenson, A.R.; Batlle, D.; Coyne, D.W.; Trout, J.R.; Warnock, D.G. Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am. J. Med. 2003, 115, 291–297. [Google Scholar] [CrossRef]
- Rossignol, P.; Massy, Z.A.; Azizi, M.; Bakris, G.; Ritz, E.; Covic, A.; Goldsmith, D.; Heine, G.H.; Jager, K.J.; Kanbay, M.; et al. The double challenge of resistant hypertension and chronic kidney disease. Lancet 2015, 386, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, R.; Agarwal, R.; Borrelli, S.; Chiodini, P.; Bellizzi, V.; Nappi, F.; Cianciaruso, B.; Zamboli, P.; Conte, G.; Gabbai, F.B.; et al. Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease. Arch. Intern. Med. 2011, 171, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Tripepi, G.; Fagugli, R.M.; Dattolo, P.; Parlongo, G.; Mallamaci, F.; Buoncristiani, U.; Zoccali, C. Prognostic value of 24-hour ambulatory blood pressure monitoring and of night/day ratio in nondiabetic, cardiovascular events-free hemodialysis patients. Kidney Int. 2005, 68, 1294–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sytkowski, P.A.; D’Agostino, R.B.; Belanger, A.J.; Kannel, W.B. Secular trends in long-term sustained hypertension, long-term treatment, and cardiovascular mortality. The Framingham Heart Study 1950 to 1990. Circulation 1996, 93, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Page, M.R. The JNC 8 hypertension guidelines: An in-depth guide. Am. J. Manag. Care 2014, 20, E8. [Google Scholar]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar]
- Charra, B.; Calemard, E.; Ruffet, M.; Chazot, C.; Terrat, J.C.; Vanel, T.; Laurent, G. Survival as an index of adequacy of dialysis. Kidney Int. 1992, 41, 1286–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, M.M.; Bower, J. Hypertension in the hemodialysis population: Any relation to one-year survival? Am. J. Kidney Dis. 1996, 28, 737–740. [Google Scholar] [CrossRef]
- Mazzuchi, N.; Carbonell, E.; Fernández-Cean, J. Importance of blood pressure control in hemodialysis patient survival. Kidney Int. 2000, 58, 2147–2154. [Google Scholar] [CrossRef]
- Duranti, E.; Imperiali, P.; Sasdelli, M. Is hypertension a mortality risk factor in dialysis? Kidney Int. Suppl. 1996, 55, S173–S174. [Google Scholar]
- Salem, M.M. Hypertension in the haemodialysis population: Any relationship to 2-years survival? Nephrol. Dial. Transplant 1999, 14, 125–128. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Kilpatrick, R.D.; McAllister, C.J.; Greenland, S.; Kopple, J.D. Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: The 58th annual fall conference and scientific sessions. Hypertension 2005, 45, 811–817. [Google Scholar] [CrossRef] [Green Version]
- Port, F.K.; Robinson, B.M.; McCullough, K.P.; Morgenstern, H. Predialysis blood pressure on survival in hemodialysis patients. Kidney Int. 2017, 91, 755–756. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.M.; Tong, L.; Zhang, J.; Wolfe, R.A.; Goodkin, D.A.; Greenwood, R.N.; Kerr, P.G.; Morgenstern, H.; Li, Y.; Pisoni, R.L.; et al. Blood pressure levels and mortality risk among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2012, 82, 570–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torino, C.; Tripepi, R.; Versace, M.C.; Vilasi, A.; Tripepi, G.; Panuccio, V. Clinical Epidemiology of Systolic and Diastolic Orthostatic Hypotension in Patients on Peritoneal Dialysis. J. Clin. Med. 2021, 10, 3075. [Google Scholar] [CrossRef] [PubMed]
- Inrig, J.K.; Patel, U.D.; Toto, R.D.; Szczech, L.A. Association of blood pressure increases during hemodialysis with 2-year mortality in incident hemodialysis patients: A secondary analysis of the Dialysis Morbidity and Mortality Wave 2 Study. Am. J. Kidney Dis. 2009, 54, 881–890. [Google Scholar] [CrossRef] [Green Version]
- Inrig, J.K.; Oddone, E.Z.; Hasselblad, V.; Gillespie, B.; Patel, U.D.; Reddan, D.; Toto, R.; Himmelfarb, J.; Winchester, J.F.; Stivelman, J.; et al. Association of intradialytic blood pressure changes with hospitalization and mortality rates in prevalent ESRD patients. Kidney Int. 2007, 71, 454–461. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Gul, A.; Sarnak, M.J. Management of intradialytic hypertension: The ongoing challenge. Semin. Dial. 2006, 19, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R. Hypertension and survival in chronic hemodialysis patients--past lessons and future opportunities. Kidney Int. 2005, 67, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Lacson, E.; Lowrie, E.G.; Ofsthun, N.J.; Kuhlmann, M.K.; Lazarus, J.M.; Levin, N.W. The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am. J. Kidney Dis. 2006, 48, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Amar, J.; Vernier, I.; Rossignol, E.; Bongard, V.; Arnaud, C.; Conte, J.J.; Salvador, M.; Chamontin, B. Nocturnal blood pressure and 24-hour pulse pressure are potent indicators of mortality in hemodialysis patients. Kidney Int. 2000, 57, 2485–2491. [Google Scholar] [CrossRef] [Green Version]
- Foley, R.N.; Herzog, C.A.; Collins, A.J. Blood pressure and long-term mortality in United States hemodialysis patients: USRDS Waves 3 and 4 Study. Kidney Int. 2002, 62, 1784–1790. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Rhee, C.M.; Sim, J.J.; Kim, Y.L.; Ricks, J.; Streja, E.; Vashistha, T.; Tolouian, R.; Kovesdy, C.P.; Kalantar-Zadeh, K. A comparative effectiveness research study of the change in blood pressure during hemodialysis treatment and survival. Kidney Int. 2013, 84, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Zager, P.G.; Nikolic, J.; Brown, R.H.; Campbell, M.A.; Hunt, W.C.; Peterson, D.; Van Stone, J.; Levey, A.; Meyer, K.B.; Klag, M.J.; et al. “U” curve association of blood pressure and mortality in hemodialysis patients. Kidney Int. 1998, 54, 561–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.; Griffin, V.; Kumar, A.; Manzoor, F.; Wright, J.T.; Smith, M.C. A comparison of standardized versus “usual” blood pressure measurements in hemodialysis patients. Am. J. Kidney Dis. 2002, 39, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Andersen, M.J.; Bishu, K.; Saha, C. Home blood pressure monitoring improves the diagnosis of hypertension in hemodialysis patients. Kidney Int. 2006, 69, 900–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, R.; Lewis, R.R. Prediction of hypertension in chronic hemodialysis patients. Kidney Int. 2001, 60, 1982–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, R.; Peixoto, A.J.; Santos, S.F.F.; Zoccali, C. Pre- and postdialysis blood pressures are imprecise estimates of interdialytic ambulatory blood pressure. Clin. J. Am. Soc. Nephrol. 2006, 1, 389–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, R.; Brim, N.J.; Mahenthiran, J.; Andersen, M.J.; Sana, C. Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension 2006, 47, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Thompson, A.M.; Pickering, T.G. The role of ambulatory blood pressure monitoring in chronic and end-stage renal disease. Kidney Int. 2006, 70, 1000–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, N.; McCulloch, C.E.; Rahman, M.; Kusek, J.W.; Anderson, A.H.; Xie, D.; Townsend, R.R.; Lora, C.M.; Wright, J.; Go, A.S.; et al. Blood pressure and risk of all-cause mortality in advanced chronic kidney disease and hemodialysis: The chronic renal insufficiency cohort study. Hypertension 2015, 65, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Alborzi, P.; Patel, N.; Agarwal, R. Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin. J. Am. Soc. Nephrol. 2007, 2, 1228–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, R. Blood pressure and mortality among hemodialysis patients. Hypertension 2010, 55, 762–768. [Google Scholar] [CrossRef] [Green Version]
- Bansal, N.; McCulloch, C.E.; Lin, F.; Alper, A.; Anderson, A.H.; Cuevas, M.; Go, A.S.; Kallem, R.; Kusek, J.W.; Lora, C.M.; et al. Blood Pressure and Risk of Cardiovascular Events in Patients on Chronic Hemodialysis: The CRIC Study (Chronic Renal Insufficiency Cohort). Hypertension 2017, 70, 435–443. [Google Scholar] [CrossRef]
- Jardine, A.G. Con: Ambulatory blood pressure measurement in patients receiving haemodialysis: A sore arm and a waste of time? Nephrol. Dial. Transpl. 2015, 30, 1438–1441. [Google Scholar] [CrossRef] [Green Version]
- Zoccali, C.; Tripepi, R.; Torino, C.; Tripepi, G.; Mallamaci, F. Moderator’s view: Ambulatory blood pressure monitoring and home blood pressure for the prognosis, diagnosis and treatment of hypertension in dialysis patients. Nephrol. Dial. Transpl. 2015, 30, 1443–1448. [Google Scholar] [CrossRef] [Green Version]
- Shimbo, D.; Abdalla, M.; Falzon, L.; Townsend, R.R.; Muntner, P. Studies comparing ambulatory blood pressure and home blood pressure on cardiovascular disease and mortality outcomes: A systematic review. J. Am. Soc. Hypertens. 2016, 10, 224–234.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimbo, D.; Artinian, N.T.; Basile, J.N.; Krakoff, L.R.; Margolis, K.L.; Rakotz, M.K.; Wozniak, G. Self-Measured Blood Pressure Monitoring at Home: A Joint Policy Statement from the American Heart Association and American Medical Association. Circulation 2020, 142, E42–E63. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, M.M.; Durcan, M.; Kinsella, S.M.; Griffin, M.D.; Reddan, D.N.; Lappin, D.W. Blood pressure measurement in peritoneal dialysis: Which method is best? Perit. Dial. Int. 2013, 33, 544–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.C.; Tseng, C.C.; Tsai, W.C.; Huang, J.J. Blood pressure and left ventricular hypertrophy in patients on different peritoneal dialysis regimens. Perit. Dial. Int. 2001, 21, 36–42. [Google Scholar] [CrossRef]
- Vaios, V.; Georgianos, P.I.; Vareta, G.; Dounousi, E.; Dimitriadis, C.; Eleftheriadis, T.; Papagianni, A.; Zebekakis, P.E.; Liakopoulos, V. Clinic and Home Blood Pressure Monitoring for the Detection of Ambulatory Hypertension Among Patients on Peritoneal Dialysis. Hypertension 2019, 74, 998–1004. [Google Scholar] [CrossRef]
- Fang, W.; Yang, X.; Bargman, J.M.; Oreopoulos, D.G. Association between pulse pressure and mortality in patients undergoing peritoneal dialysis. Perit. Dial. Int. 2009, 29, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Udayaraj, U.P.; Steenkamp, R.; Caskey, F.J.; Rogers, C.; Nitsch, D.; Ansell, D.; Tomson, C.R.V. Blood pressure and mortality risk on peritoneal dialysis. Am. J. Kidney Dis. 2009, 53, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Chen, C.C.; Wang, S.M.; Chou, C.Y.; Liu, Y.L.; Kuo, H.L.; Lin, H.H.; Wang, I.K.; Yang, Y.F.; Huang, C.C. Association between pulse pressure and 30-month all-cause mortality in peritoneal dialysis patients. Am. J. Hypertens. 2008, 21, 1318–1323. [Google Scholar] [CrossRef] [Green Version]
- London, G.M.; Marchais, S.J.; Safar, M.E.; Genest, A.F.; Guerin, A.P.; Metivier, F.; Chedid, K.; London, A.M. Aortic and large artery compliance in end-stage renal failure. Kidney Int. 1990, 37, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Barenbrock, M.; Spieker, C.; Laske, V.; Heidenreich, S.; Hohage, H.; Bachmann, J.; Hoeks, A.P.G.; Rahn, K.H. Studies of the vessel wall properties in hemodialysis patients. Kidney Int. 1994, 45, 1397–1400. [Google Scholar] [CrossRef] [Green Version]
- Kannel, W.B.; Dawber, T.R.; McGee, D.L. Perspectives on systolic hypertension. The Framingham study. Circulation 1980, 61, 1179–1182. [Google Scholar] [CrossRef] [Green Version]
- Klassen, P.S.; Lowrie, E.G.; Reddan, D.N.; DeLong, E.R.; Coladonato, J.A.; Szczech, L.A.; Michael Lazarus, J.; Owen, W.F. Association between pulse pressure and mortality in patients undergoing maintenance hemodialysis. JAMA 2002, 287, 1548–1555. [Google Scholar] [CrossRef] [Green Version]
- Tozawa, M.; Iseki, K.; Iseki, C.; Takishita, S. Pulse pressure and risk of total mortality and cardiovascular events in patients on chronic hemodialysis. Kidney Int. 2002, 61, 717–726. [Google Scholar] [CrossRef] [Green Version]
- Da Nichols, W.W.; O’Rourke, M.F. Mcdonald’s Blood Flow in Arteries: Theoretic, Experimental and Clinical Principles; Anybook Ltd.: London, UK, 1998. [Google Scholar]
- Benetos, A.; Rudnichi, A.; Safar, M.; Guize, L. Pulse pressure and cardiovascular mortality in normotensive and hypertensive subjects. Hypertension 1998, 32, 560–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domanski, M.J.; Mitchell, G.F.; Norman, J.E.; Exner, D.V.; Pitt, B.; Pfeffer, M.A. Independent prognostic information provided by sphygmomanometrically determined pulse pressure and mean arterial pressure in patients with left ventricular dysfunction. J. Am. Coll. Cardiol. 1999, 33, 951–958. [Google Scholar] [CrossRef] [Green Version]
- Chae, C.U.; Pfeffer, M.A.; Glynn, R.J.; Mitchell, G.F.; Taylor, J.O.; Hennekens, C.H. Increased pulse pressure and risk of heart failure in the elderly. JAMA 1999, 281, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Domanski, M.J.; Davis, B.R.; Pfeffer, M.A.; Kastantin, M.; Mitchell, G.F. Isolated systolic hypertension: Prognostic information provided by pulse pressure. Hypertension 1999, 34, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Franklin, S.S.; Khan, S.A.; Wong, N.D.; Larson, M.G.; Levy, D. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation 1999, 100, 354–360. [Google Scholar] [CrossRef] [Green Version]
- Port, F.K.; Hulbert-Shearon, T.E.; Wolfe, R.A.; Bloembergen, W.E.; Golper, T.A.; Agodoa, L.Y.C.; Young, E.W.; Salem, M. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am. J. Kidney Dis. 1999, 33, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E. Pulse pressure, arterial stiffness, and cardiovascular risk. Curr. Opin. Cardiol. 2000, 15, 258–263. [Google Scholar] [CrossRef]
- Celentano, A.; Palmieri, V.; Di Palma Esposito, N.; Pietropaolo, I.; Arezzi, E.; Mureddu, G.F.; De Simone, G. Relations of pulse pressure and other components of blood pressure to preclinical echocardiographic abnormalities. J. Hypertens. 2002, 20, 531–537. [Google Scholar] [CrossRef]
- Dai, S.; Chen, Y.; Shang, D.; Ge, X.; Xie, Q.; Hao, C.M.; Zhu, T. Association of Ambulatory Blood Pressure with All-Cause Mortality and Cardiovascular Outcomes in Peritoneal Dialysis Patients. Kidney Blood Press. Res. 2020, 45, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lv, D.; Zheng, H.; Zhang, X.; Han, F.; Chen, J. The associations of blood pressure parameters with all-cause and cardiovascular mortality in peritoneal dialysis patients: A cohort study in China. J. Hypertens. 2020, 38, 2252–2260. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.F.; Parise, H.; Benjamin, E.J.; Larson, M.G.; Keyes, M.J.; Vita, J.A.; Vasan, R.S.; Levy, D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: The Framingham Heart Study. Hypertension 2004, 43, 1239–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, G.F.; Wang, N.; Palmisano, J.N.; Larson, M.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J.; Vasan, R.S. Hemodynamic correlates of blood pressure across the adult age spectrum: Noninvasive evaluation in the Framingham Heart Study. Circulation 2010, 122, 1379–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackenzie, I.S.; Wilkinson, I.B.; Cockcroft, J.R. Assessment of arterial stiffness in clinical practice. QJM 2002, 95, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgianos, P.I.; Sarafidis, P.A.; Lasaridis, A.N. Arterial stiffness: A novel cardiovascular risk factor in kidney disease patients. Curr. Vasc. Pharmacol. 2015, 13, 229–238. [Google Scholar] [CrossRef] [PubMed]
- London, G.M.; Safar, M.E.; Pannier, B. Aortic Aging in ESRD: Structural; Hemodynamic; and Mortality Implications. J. Am. Soc. Nephrol. 2016, 27, 1837–1846. [Google Scholar] [CrossRef] [Green Version]


| Whole Group (n = 140) | |
|---|---|
| Age (years) | 67 ± 14 |
| BMI (kg/m2) | 27 ± 4 |
| Male sex n. (%) | 83 (59.3) |
| Current smokers n. (%) | 14 (10) |
| Past smokers n. (%) | 35 (25) |
| Diabetics n. (%) | 51 (36.4) |
| With cardiovascular comorbidities 1 n. (%) | 66 (47.1) |
| On anti-hypertensive treatment n. (%) | 106 (75.7) |
| Home Pulse Pressure (mmHg) | 62 ± 16 |
| Systolic Blood Pressure (mmHg) | 139 ± 16 |
| Diastolic Blood Pressure (mmHg) | 77 ± 10 |
| Cholesterol (mg/dL) | 171 ± 40 |
| Hemoglobin (g/dL) | 11 ± 1.6 |
| PTH (pg/mL) | 202 ± 173 |
| CRP (mg/L) | 3.7 (3.2–10.3) |
| Calcium (mg/dL) | 8.9 ± 1.3 |
| Phosphate (mg/dL) | 5.6 ± 1.6 |
| Kt/V | 1.9 ± 0.4 |
| Variables | R | p |
|---|---|---|
| Age (years) | 0.435 | <0.001 |
| Previous cardiovascular comorbidities | 0.234 | 0.006 |
| Diabetes | 0.357 | <0.001 |
| NYHA Score | 0.281 | 0.001 |
| Kt/V | −0.147 | 0.083 |
| Hemoglobin | −0.114 | 0.182 |
| C-reactive protein | 0.053 | 0.548 |
| Phosphate | −0.162 | 0.057 |
| PTHi | −0.066 | 0.449 |
| VitD | 0.057 | 0.501 |
| Use of blood-pressure-lowering therapy | 0.257 | 0.003 |
| Use of erythropoiesis-stimulating agents | −0.105 | 0.218 |
| Lipid-lowering therapy | −0.053 | 0.532 |
| Variables (Units of Increase) | Crude Analysis | Fully Adjusted Analysis | Over-Adjusted Analysis |
|---|---|---|---|
| Pulse Pressure (5 mmHg) | 1.17 (1.08–1.26), p < 0.001 | 1.31 (1.12–1.52), p = 0.001 | 1.30 (1.11–1.51), p = 0.001 |
| Age (1 year) | 1.05 (1.01–1.08), p = 0.004 | 1.04 (1.01–1.08), p = 0.005 | |
| Gender (0 = female; 1 = male) | 0.82 (0.44–1.53), p = 0.53 | 0.79 (0.42–1.47), p = 0.45 | |
| Diabetes (0 = no; 1 = yes) | 0.88 (0.51–1.51), p = 0.63 | 0.93 (0.93–1.62), p = 0.80 | |
| Kt/V (1 unit) | 1.01 (0.51–2.01), p = 0.97 | 1.01 (0.51–1.99), p = 0.98 | |
| Systolic Blood Pressure (1 mmHg) | 0.96 (0.93–0.99), p = 0.009 | 0.96 (0.93–0.99), p = 0.01 | |
| Cardiovascular comorbidities (0 = no; 1 = yes) | 1.28 (0.72–2.28), p = 0.40 |
| Variables (Units of Increase) | Crude Analysis | Fully Adjusted Analysis | Over-Adjusted Analysis |
|---|---|---|---|
| Pulse Pressure (1 mmHg) | 1.17 (1.08–1.26), p < 0.001 | 1.28 (1.11–1.47), p = 0.001 | 1.25 (1.08–1.44), p = 0.003 |
| Age (1 year) | 1.04 (1.01–1.06), p = 0.01 | 1.04 (1.01–1.06), p = 0.01 | |
| Gender (0 = female; 1 = male) | 0.98 (0.54–1.74), p = 0.93 | 0.87 (0.48–1.58), p = 0.65 | |
| Diabetes (0 = no; 1 = yes) | 0.93 (0.55–1.56), p = 0.77 | 1.01 (0.60–1.70), p = 0.98 | |
| Kt/V (1 unit) | 0.74 (0.37–1.49), p = 0.40 | 0.67 (0.33–1.35), p = 0.26 | |
| Systolic Blood Pressure (1 mmHg) | 0.96 (0.94–0.99); p = 0.01 | 0.97 (0.94–0.99); p = 0.02 | |
| Cardiovascular comorbidities (0 = no; 1 = yes) | 1.60 (0.92–2.81), p = 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panuccio, V.; Provenzano, P.F.; Tripepi, R.; Versace, M.C.; Parlongo, G.; Politi, E.; Vilasi, A.; Mezzatesta, S.; Abelardo, D.; Tripepi, G.L.; et al. Home Pulse Pressure Predicts Death and Cardiovascular Events in Peritoneal Dialysis Patients. J. Clin. Med. 2023, 12, 3904. https://doi.org/10.3390/jcm12123904
Panuccio V, Provenzano PF, Tripepi R, Versace MC, Parlongo G, Politi E, Vilasi A, Mezzatesta S, Abelardo D, Tripepi GL, et al. Home Pulse Pressure Predicts Death and Cardiovascular Events in Peritoneal Dialysis Patients. Journal of Clinical Medicine. 2023; 12(12):3904. https://doi.org/10.3390/jcm12123904
Chicago/Turabian StylePanuccio, Vincenzo, Pasquale Fabio Provenzano, Rocco Tripepi, Maria Carmela Versace, Giovanna Parlongo, Emma Politi, Antonio Vilasi, Sabrina Mezzatesta, Domenico Abelardo, Giovanni Luigi Tripepi, and et al. 2023. "Home Pulse Pressure Predicts Death and Cardiovascular Events in Peritoneal Dialysis Patients" Journal of Clinical Medicine 12, no. 12: 3904. https://doi.org/10.3390/jcm12123904
APA StylePanuccio, V., Provenzano, P. F., Tripepi, R., Versace, M. C., Parlongo, G., Politi, E., Vilasi, A., Mezzatesta, S., Abelardo, D., Tripepi, G. L., & Torino, C. (2023). Home Pulse Pressure Predicts Death and Cardiovascular Events in Peritoneal Dialysis Patients. Journal of Clinical Medicine, 12(12), 3904. https://doi.org/10.3390/jcm12123904