Therapeutic Effects of Apremilast on Enthesitis and Dactylitis in Real Clinical Setting: An Italian Multicenter Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Patients
2.3. Data
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
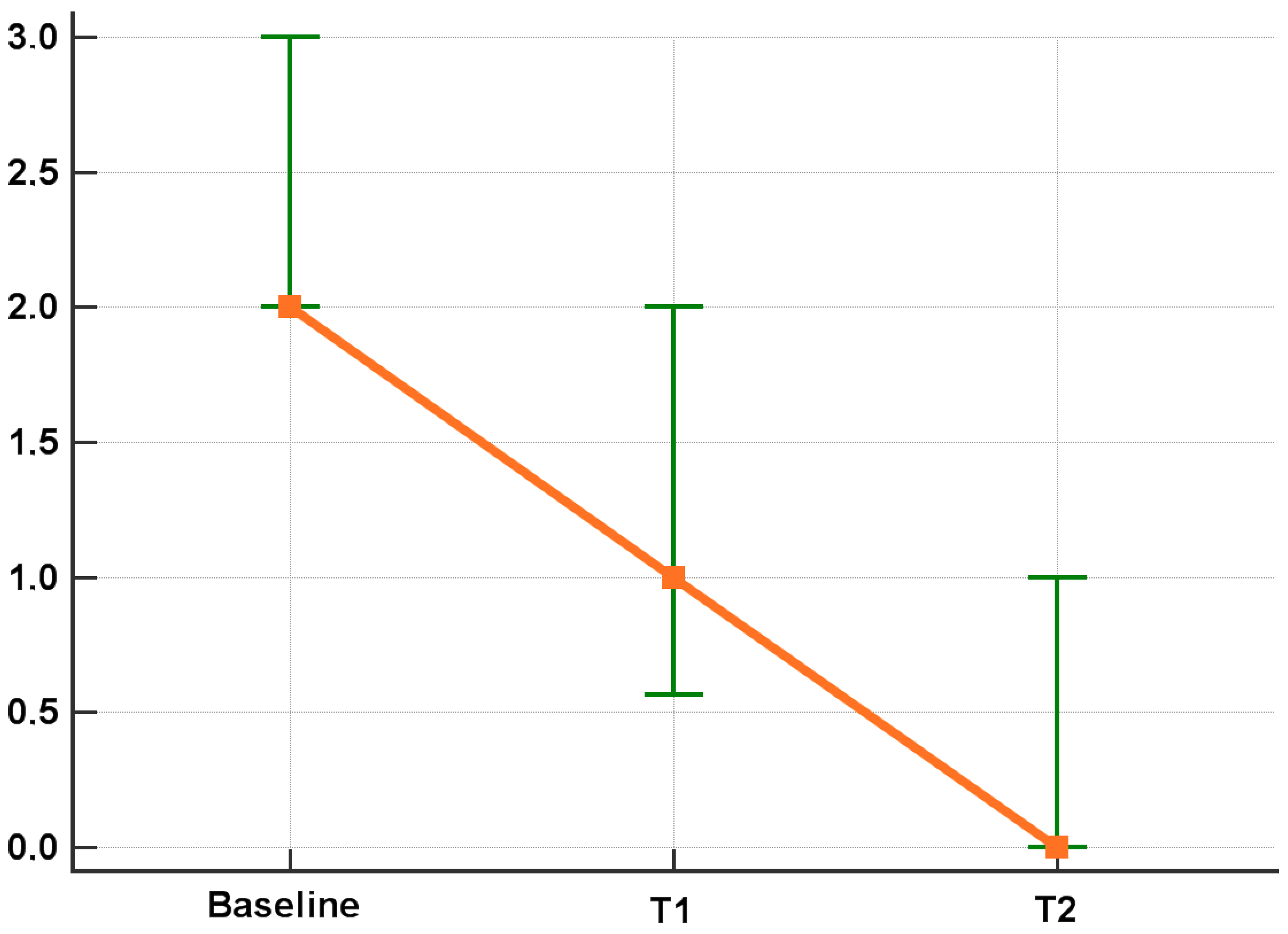
3.2. Efficacy Results
3.3. Predictors of Remission
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ritchlin, C.T.; Colbert, R.A.; Gladman, D.D. Psoriatic Arthritis. N. Engl. J. Med. 2017, 376, 957–970. [Google Scholar] [CrossRef] [Green Version]
- Gladman, D.D.; Antoni, C.; Mease, P.; Clegg, D.O.; Nash, P. Psoriatic arthritis: Epidemiology, clinical features, course, and out-come. Ann. Rheum. Dis. 2005, 64 (Suppl. S2), ii14–ii17. [Google Scholar] [PubMed]
- Kaeley, G.S.; Eder, L.; Aydin, S.Z.; Gutierrez, M.; Bakewell, C. Dactylitis: A hallmark of psoriatic arthritis. Semin. Arthritis Rheum. 2018, 48, 263–273. [Google Scholar] [CrossRef]
- Kaeley, G.S.; Eder, L.; Aydin, S.Z.; Gutierrez, M.; Bakewell, C. Enthesitis: A hallmark of psoriatic arthritis. Semin. Arthritis Rheum. 2018, 48, 35–43. [Google Scholar] [CrossRef]
- Polachek, A.; Li, S.; Chandran, V.; Gladman, D.D. Clinical Enthesitis in a Prospective Longitudinal Psoriatic Arthritis Cohort: Incidence, Prevalence, Characteristics, and Outcome. Arthritis Care Res. 2017, 69, 1685–1691. [Google Scholar] [CrossRef] [Green Version]
- Orbai, A.-M.; Weitz, J.; Siegel, E.L.; Siebert, S.; Savage, L.J.; Aydin, S.Z.; Luime, J.J.; Elkayam, O.; Neerinckx, B.; Urbancek, S.; et al. Systematic review of treatment effectiveness and outcome measures for enthesitis in psoriatic arthritis. J. Rheumatol. 2014, 41, 2290–2294. [Google Scholar] [CrossRef] [Green Version]
- Rose, S.; Toloza, S.; Bautista-Molano, W.; Helliwell, P.S.; Group, G.D.S. Comprehensive treatment of dactylitis in psoriatic arthritis. J. Rheumatol. 2014, 41, 2295–2300. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, S.; Bortoluzzo, A.; Gonçalves, C.; da Silva, J.A.B.; Ximenes, A.C.; Bértolo, M.; Ribeiro, S.L.; Keiserman, M.; Skare, T.; Menin, R.; et al. Effect of enthesitis on 1505 Brazilian patients with spondyloarthritis. J. Rheumatol. 2013, 40, 1719–1725. [Google Scholar] [CrossRef]
- Brockbank, J.E.; Stein, M.; Schentag, C.T.; Gladman, D.D. Dactylitis in psoriatic arthritis: A marker for disease severity? Ann. Rheum. Dis. 2005, 64, 188–190. [Google Scholar] [CrossRef] [Green Version]
- Kavanaugh, A.; Helliwell, P.; Ritchlin, C.T. Psoriatic Arthritis and Burden of Disease: Patient Perspectives from the Population-Based Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) Survey. Rheumatol. Ther. 2016, 3, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Nash, P.; Clegg, D.O. Psoriatic arthritis therapy: NSAIDs and traditional DMARDs. Ann. Rheum. Dis. 2005, 64 (Suppl. S2), ii74–ii77. [Google Scholar] [CrossRef]
- Kavanaugh, A.; Mease, P.J.; Gomez-Reino, J.J.; Adebajo, A.O.; Wollenhaupt, J.; Gladman, D.D.; Lespessailles, E.; Hall, S.; Hochfeld, M.; Hu, C.; et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann. Rheum. Dis. 2014, 73, 1020–1026. [Google Scholar] [CrossRef]
- Kavanaugh, A.; Mease, P.J.; Gomez-Reino, J.J.; Adebajo, A.O.; Wollenhaupt, J.; Gladman, D.D.; Hochfeld, M.; Teng, L.L.; Schett, G.; Lespessailles, E.; et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J. Rheumatol. 2015, 42, 479–488. [Google Scholar] [CrossRef] [Green Version]
- Cutolo, M.; Myerson, G.E.; Fleischmann, R.M.; Lioté, F.; Diaz-Gonzalez, F.; Bosch, F.V.D.; Marzo-Ortega, H.; Feist, E.; Shah, K.; Hu, C.; et al. A Phase III, Randomized, Controlled Trial of Apremilast in Patients with Psoriatic Arthritis: Results of the PALACE 2 Trial. J. Rheumatol. 2016, 43, 1724–1734. [Google Scholar] [CrossRef]
- Edwards, C.J.; Blanco, F.J.; Crowley, J.; Birbara, C.A.; Jaworski, J.; Aelion, J.; Stevens, R.M.; Vessey, A.; Zhan, X.; Bird, P. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: A phase III, randomised, controlled trial (PALACE 3). Ann. Rheum. Dis. 2016, 75, 1065–1073. [Google Scholar] [CrossRef]
- Ariani, A.; Parisi, S.; Del Medico, P.; Farina, A.; Visalli, E.; Colella, A.B.M.; Lumetti, F.; Caccavale, R.; Scolieri, P.; Andracco, R.; et al. Apremilast retention rate in clinical practice: Observations from an Italian multi-center study. Clin. Rheumatol. 2022, 41, 3219–3225. [Google Scholar] [CrossRef]
- Hackett, S.; Coates, L.C. Outcome measures in psoriatic arthritis: Where next? Musculoskelet. Care 2022, 20 (Suppl. S1), S22–S31. [Google Scholar] [CrossRef]
- Healy, P.J.; Helliwell, P.S. Measuring clinical enthesitis in psoriatic arthritis: Assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum. 2008, 59, 686–691. [Google Scholar] [CrossRef]
- de Vlam, K.; Toukap, A.N.; Kaiser, M.J.; Vanhoof, J.; Remans, P.; Van den Berghe, M.; Di Romana, M.; Van den Bosch, F.; Lories, R. Real-World Efficacy and Safety of Apre-milast in Belgian Patients with Psoriatic Arthritis: Results from the Prospective Observational APOLO Study. Adv. Ther. 2022, 39, 1055–1067. [Google Scholar] [CrossRef]
- Mease, P.J.; Genovese, M.C.; Greenwald, M.W.; Ritchlin, C.T.; Beaulieu, A.D.; Deodhar, A.; Newmark, R.; Feng, J.; Erondu, N.; Nirula, A. Brodalumab, an anti-IL17RA mono-clonal antibody, in psoriatic arthritis. N. Engl. J. Med. 2014, 370, 2295–2306. [Google Scholar] [CrossRef] [Green Version]
- Dubash, S.; Alabas, O.A.; Michelena, X.; Garcia-Montoya, L.; Wakefield, R.J.; Helliwell, P.S.; Emery, P.; McGonagle, D.G.; Tan, A.L.; Marzo-Ortega, H. Dactylitis is an indicator of a more severe phenotype independently associated with greater SJC, CRP, ultrasound synovitis and erosive damage in DMARD-naïve early psoriatic arthritis. Ann. Rheum. Dis. 2022, 81, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Gladman, D.D.; Collier, D.H.; Ritchlin, C.T.; Helliwell, P.S.; Liu, L.; Kricorian, G.; Chung, J.B. Etanercept and Methotrexate as Monotherapy or in Combination for Psoriatic Arthritis: Primary Results From a Randomized, Controlled Phase III Trial. Arthritis Rheumatol. 2019, 71, 1112–1124. [Google Scholar] [CrossRef] [Green Version]
- Gladman, D.D.; Kavanaugh, A.; Gómez-Reino, J.J.; Wollenhaupt, J.; Cutolo, M.; Schett, G.; Lespessailles, E.; Guerette, B.; Delev, N.; Teng, L.; et al. Therapeutic benefit of apremilast on enthesitis and dactylitis in patients with psoriatic arthritis: A pooled analysis of the PALACE 1-3 studies. RMD Open. 2018, 4, e000669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abignano, G.; Fadl, N.; Merashli, M.; Wenham, C.; Freeston, J.; McGonagle, D.; Marzo-Ortega, H. Apremilast for the treatment of active psoriatic arthritis: A single-centre real-life experience. Rheumatology 2018, 57, 578–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favalli, E.G.; Conti, F.; Selmi, C.; Iannone, F.; Bucci, R.; D’Onofrio, F.; Carlino, G.; Santo, L.; Semeraro, A.; Zuccaro, C.; et al. Retrospective evaluation of patient profiling and effectiveness of apremilast in an Italian multicentric cohort of psoriatic arthritis patients. Clin. Exp. Rheumatol. 2020, 38, 19–26. [Google Scholar]
- Sfikakis, P.P.; Vassilopoulos, D.; Katsifis, G. Apremilast for biologic-naïve, peripheral psoriatic arthritis, including patients with early disease: Results from the APROACH observational prospective study. Rheumatol. Int. 2023, 43, 889–902. [Google Scholar] [CrossRef]
- Gossec, L.; Baraliakos, X.; Kerschbaumer, A.; de Wit, M.; McInnes, I.; Dougados, M.; Primdahl, J.; McGonagle, D.G.; Aletaha, D.; Balanescu, A.; et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann. Rheum. Dis. 2020, 79, 700–712. [Google Scholar] [CrossRef]
- Appani, S.K.; Devarasetti, P.K.; Irlapati, R.V.P.; Rajasekhar, L. Methotrexate achieves major cDAPSA response, and improvement in dactylitis and functional status in psoriatic arthritis. Rheumatology 2019, 58, 869–873. [Google Scholar] [CrossRef] [Green Version]
- Mourad, A.; Gniadecki, R. Treatment of Dactylitis and Enthesitis in Psoriatic Arthritis with Biologic Agents: A Systematic Review and Metaanalysis. J. Rheumatol. 2020, 47, 59–65. [Google Scholar] [CrossRef]
- Becciolini, A.; Parisi, S.; Del Medico, P.; Farina, A.; Visalli, E.; Colella, A.B.M.; Lumetti, F.; Caccavale, R.; Scolieri, P.; Andracco, R.; et al. Predictors of DAPSA Response in Psoriatic Arthritis Patients Treated with Apremilast in a Retrospective Observational Multi-Centric Study. Biomedicines 2023, 11, 433. [Google Scholar] [CrossRef]
- Mathew, A.J.; Sutton, M.; Pereira, D.; Gladman, D.D.; Chandran, V. Effectiveness of Disease-Modifying Antirheumatic Drugs for Enthesitis in a Prospective Longitudinal Psoriatic Arthritis Cohort. J. Rheumatol. 2022, 49, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Saber, T.P.; Ng, C.T.; Renard, G.; Lynch, B.M.; Pontifex, E.; Walsh, C.A.; Grier, A.; Molloy, M.; Bresnihan, B.; FitzGerald, O.; et al. Remission in psoriatic arthritis: Is it possible and how can it be predicted? Arthritis Res. Ther. 2010, 12, R94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glintborg, B.; Østergaard, M.; Dreyer, L.; Krogh, N.S.; Tarp, U.; Hansen, M.S.; Rifbjerg-Madsen, S.; Lorenzen, T.; Hetland, M.L. Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor α therapy: Results from the nationwide Danish DANBIO registry. Arthritis Rheum. 2011, 63, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Salvarani, C.; Cantini, F.; Olivieri, I.; Macchioni, P.; Padula, A.; Niccoli, L.; Catanoso, M.G.; Scocco, G.L.; Boiardi, L. Efficacy of infliximab in resistant psoriatic arthritis. Arthritis Rheum. 2003, 49, 541–545. [Google Scholar] [CrossRef]
- Girolimetto, N.; Macchioni, P.; Possemato, N.; Tinazzi, I.; Bascherini, V.; Citriniti, G.; McConnell, R.; Marchetta, A.; Peluso, R.; Sabbatino, V.; et al. Musculoskeletal Ultrasound in Monitor-ing Clinical Response to Treatment in Acute Symptomatic Psoriatic Dactylitis: Results from a Multicentre Prospective Observa-tional Study. J. Clin. Med. 2020, 9, 3127. [Google Scholar] [CrossRef] [PubMed]


| Baseline Characteristic | Dactylitis Subgroup | Enthesitic Subgroup | |
|---|---|---|---|
| N | 96 | 118 | |
| M:F | 39:57 | 46:72 | |
| Age, median (IQR) yrs | 58 (50–64) | 58 (51–65) | |
| Smokers, n (%) | Yes Former No Unknown | 20 (20.8) 12 (12.5) 63 (65.6) 1 (1.1) | 22 (18.6) 17 (14.4) 78 (66.1) 1 (0.9) |
| Body Mass Index, median (IQR) kg/m2 | 25.7 (23.4–29.0) (*) | 24.9 (23.0–29.0) (**) | |
| PsA Duration, median (IQR), months | 44 (17–85) | 42 (14–83) | |
| PsO Duration, median (IQR), months | 57 (19–128) | 59 (15–139) | |
| SJC, median (IQR) | 4 (2–4) | 3 (2–4) | |
| TJC, median (IQR) | 6 (4–10) | 8 (4–12) | |
| LEI, median (IQR), | - | 3 (1–4) | |
| Dactylitis, median (IQR), fingers | 1 (1–2) | - | |
| CRP, median (IQR), mg/dL | 2.3 (1.0–5.0) | 3.0 (1.0–7.0) | |
| PGA Patient (0–10), median (IQR) | 7 (6–8) | 6 (5–8) | |
| VAS pain (0–10), median (IQR) | 7 (6–8) | 7 (6–8) | |
| DAPSA, median (IQR) | 25.7 (20.2–33.0) | 27.6 (22.6–36.0) | |
| Concomitant csDMARDs use, n (%) | 27 (28.1) | 30 (25.4) | |
| Prior bDMARDs use, n (%) | 34 (35.4) | 34 (28.8) | |
| Concomitant relevant disease, n (%) | 51 (53.1) | 45 (38.1) |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age | 0.99 (0.95–1.02) | 0.5 | ||
| Sex | 2.34 (1.07–5.12) | 0.03 | 2.01 (0.86–4.68) | 0.11 |
| BMI | 1.11 (1.02–1.22) | 0.02 | 1.09 (0.99–1.20) | 0.09 |
| Smoke habit | 0.89 (0.33–2.40) | 0.80 | ||
| PsA duration | 1.00 (0.99–1.01) | 0.90 | ||
| Relevant comorbidity | 0.76 (0.35–1.65) | 0.49 | ||
| Concomitant csDMARDs | 0.97 (0.27–3.44) | 0.97 | ||
| LEI, baseline | 0.70 (0.54–0.91) | 0.007 | 0.76 (0.58–0.98) | 0.03 |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age | 1.04 (1.00–1.08) | 0.04 | 1.05 (1.00–1.09) | 0.04 |
| Sex | 1.18 (0.52–2.68) | 0.7 | ||
| BMI | 1.06 (0.97–1.16) | 0.18 | ||
| Smoke habit | 0.63 (0.22–1.76) | 0.38 | ||
| PsA duration | 0.99 (0.98–1.00) | 0.15 | ||
| Relevant comorbidity | 0.63 (0.28–1.43) | 0.27 | ||
| Concomitant csDMARDs | 2.41 (0.97–5.97) | 0.06 | 3.84 (1.30–11.31) | 0.01 |
| Dactylitis, baseline | 0.45 (0.24–0.85) | 0.014 | 0.41 (0.21–0.78) | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Gullo, A.; Becciolini, A.; Parisi, S.; Del Medico, P.; Farina, A.; Visalli, E.; Dal Bosco, Y.; Molica Colella, A.B.; Lumetti, F.; Caccavale, R.; et al. Therapeutic Effects of Apremilast on Enthesitis and Dactylitis in Real Clinical Setting: An Italian Multicenter Study. J. Clin. Med. 2023, 12, 3892. https://doi.org/10.3390/jcm12123892
Lo Gullo A, Becciolini A, Parisi S, Del Medico P, Farina A, Visalli E, Dal Bosco Y, Molica Colella AB, Lumetti F, Caccavale R, et al. Therapeutic Effects of Apremilast on Enthesitis and Dactylitis in Real Clinical Setting: An Italian Multicenter Study. Journal of Clinical Medicine. 2023; 12(12):3892. https://doi.org/10.3390/jcm12123892
Chicago/Turabian StyleLo Gullo, Alberto, Andrea Becciolini, Simone Parisi, Patrizia Del Medico, Antonella Farina, Elisa Visalli, Ylenia Dal Bosco, Aldo Biagio Molica Colella, Federica Lumetti, Rosalba Caccavale, and et al. 2023. "Therapeutic Effects of Apremilast on Enthesitis and Dactylitis in Real Clinical Setting: An Italian Multicenter Study" Journal of Clinical Medicine 12, no. 12: 3892. https://doi.org/10.3390/jcm12123892
APA StyleLo Gullo, A., Becciolini, A., Parisi, S., Del Medico, P., Farina, A., Visalli, E., Dal Bosco, Y., Molica Colella, A. B., Lumetti, F., Caccavale, R., Scolieri, P., Andracco, R., Girelli, F., Bravi, E., Colina, M., Volpe, A., Ianniello, A., Ditto, M. C., Nucera, V., ... Ariani, A. (2023). Therapeutic Effects of Apremilast on Enthesitis and Dactylitis in Real Clinical Setting: An Italian Multicenter Study. Journal of Clinical Medicine, 12(12), 3892. https://doi.org/10.3390/jcm12123892









