Associations of Serum CXCL12α and CK Levels with Skeletal Muscle Mass in Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Blood Sample Collection and Processing
2.3. Measurement of Candidate Biomarkers
2.4. Grip Strength Measurement
2.5. Bioelectrical Impedance Analysis
2.6. Statistical Analysis
3. Results
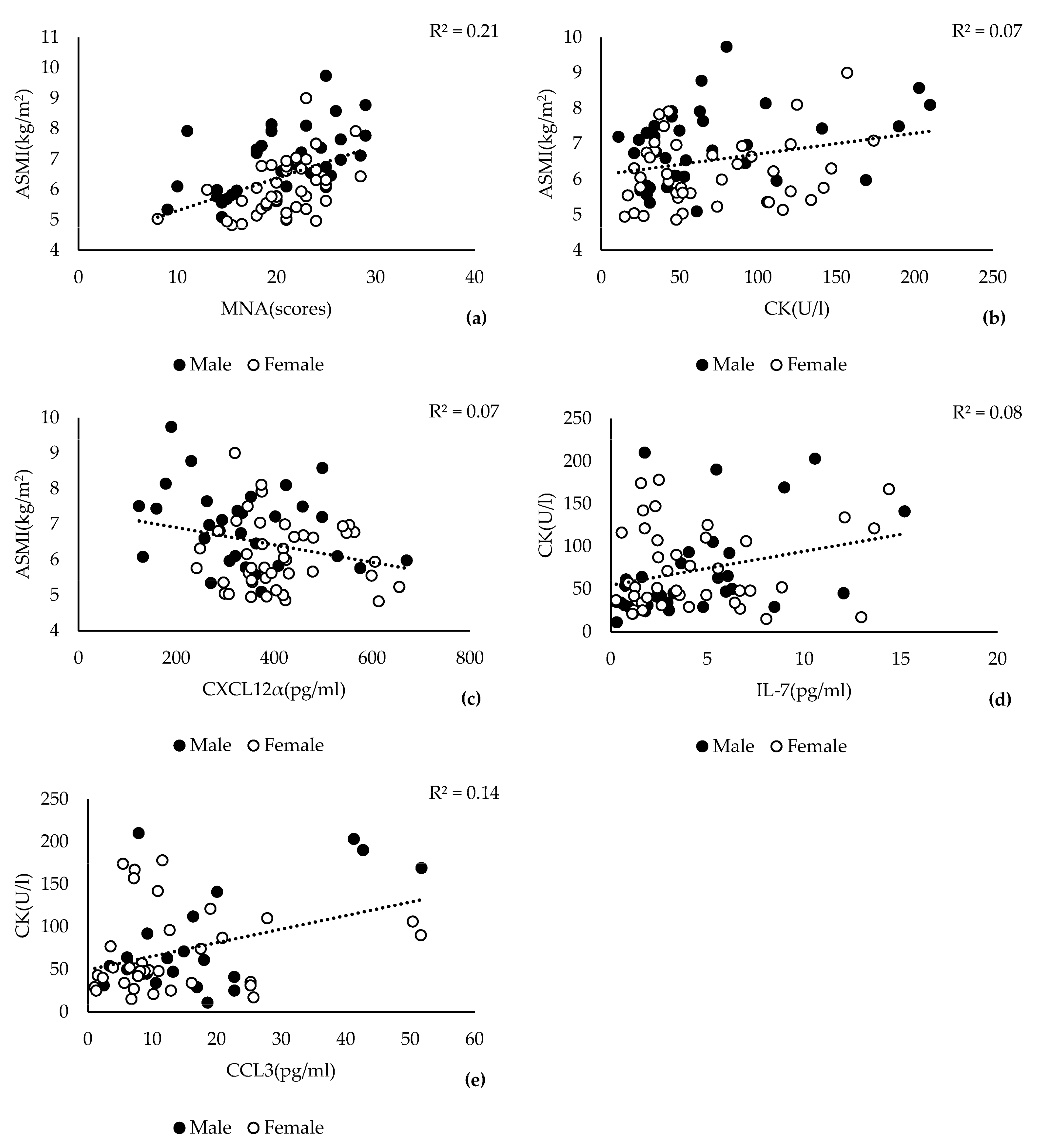
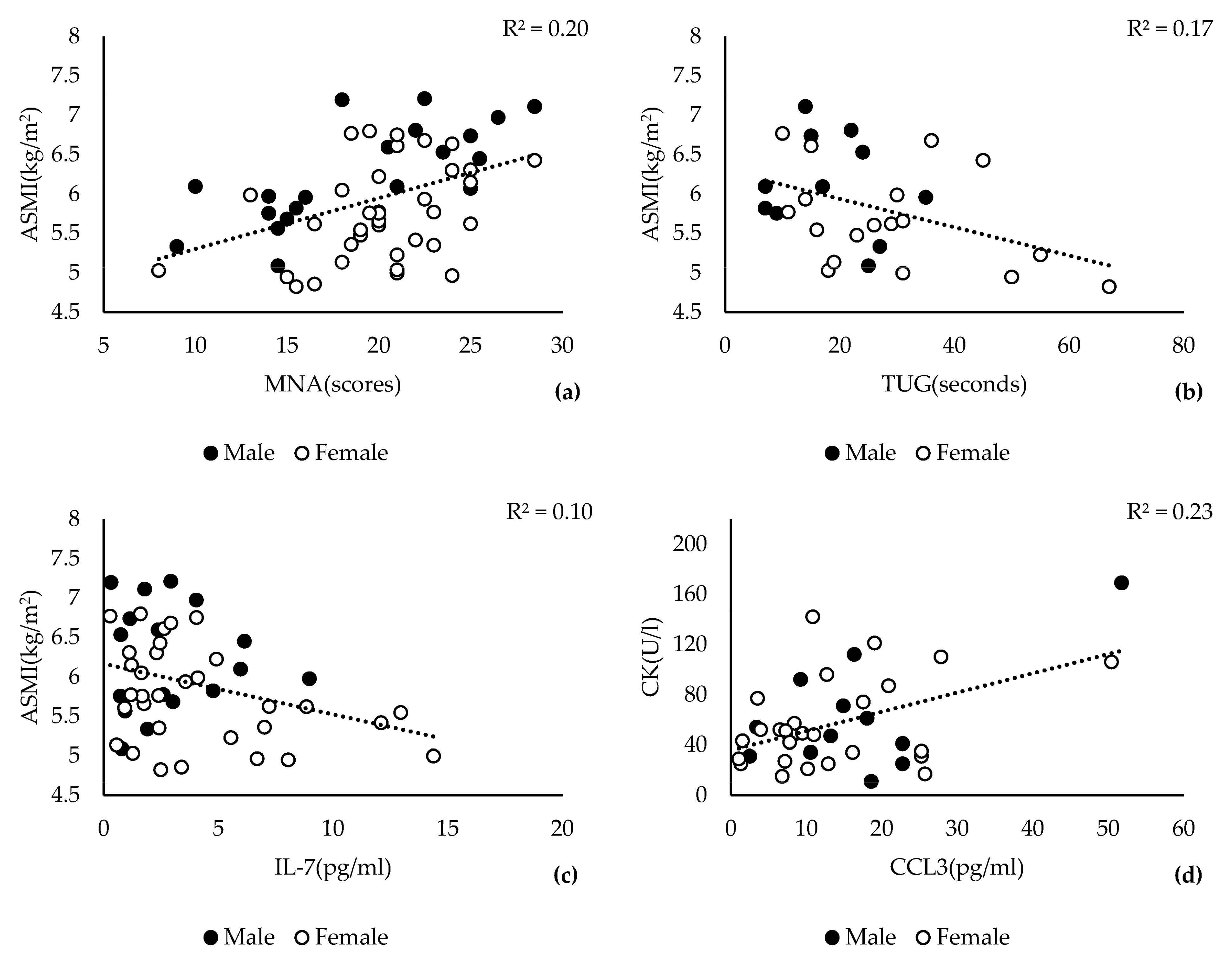
3.1. Association of Skeletal Muscle Mass Decline with CK and CXCL12α
3.2. Evaluation of ASMI Cut-Offs for the Sarcopenia Case Group
3.3. Low ASMI Is Significantly Associated with Lower CK and IL-7 Levels
3.4. Serum Levels of CK and CXCL12α Are Markers for Sarcopenia
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. People Writing Group for the European Working Group on Sarcopenia in Older, and Ewgsop the Extended Group for “Sarcopenia: Revised European Consensus on Definition and Diagnosis”. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the Icd-10 Code for Sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, E.; Salas, R.; Bouzas, C.; Pastor, R.; Tur, J.A. Comparison between Original and Reviewed Consensus of European Working Group on Sarcopenia in Older People: A Probabilistic Cross-Sectional Survey among Community-Dwelling Older People. Gerontology 2021, 68, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Messina, C.; Vitale, J.; Sconfienza, L.M. Imaging of Sarcopenia: Old Evidence and New Insights. Eur. Radiol. 2020, 30, 2199–2208. [Google Scholar] [CrossRef]
- Anusitviwat, C.; Vanitcharoenkul, E.; Chotiyarnwong, P.; Unnanuntana, A. Dual-Frequency Bioelectrical Impedance Analysis Is Accurate and Reliable to Determine Lean Muscle Mass in the Elderly. J. Clin. Densitom. 2023, 26, 90–96. [Google Scholar] [CrossRef]
- Oflazoglu, U.; Alacacioglu, A.; Varol, U.; Kucukzeybek, Y.; Salman, T.; Taskaynatan, H.; Yildiz, Y.; Ozdemir, O.; Tarhan, M. Prevalence and Related Factors of Sarcopenia in Newly Diagnosed Cancer Patients. Support. Care Cancer 2020, 28, 837–843. [Google Scholar] [CrossRef]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal Muscle Performance and Ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef]
- Koutedakis, Y.; Raafat, A.; Sharp, N.C.; Rosmarin, M.N.; Beard, M.J.; Robbins, S.W. Serum Enzyme Activities in Individuals with Different Levels of Physical Fitness. J. Sports Med. Phys. Fit. 1993, 33, 252–257. [Google Scholar]
- Zhu, L.-Y.; Chan, R.; Kwok, T.; Cheng, K.C.-C.; Ha, A.; Woo, J. Effects of Exercise and Nutrition Supplementation in Community-Dwelling Older Chinese People with Sarcopenia: A Randomized Controlled Trial. Age Ageing 2019, 48, 220–228. [Google Scholar] [CrossRef]
- Moghadam-Kia, S.; Oddis, C.V.; Aggarwal, R. Approach to Asymptomatic Creatine Kinase Elevation. Clevel. Clin. J. Med. 2016, 83, 37–42. [Google Scholar] [CrossRef]
- Malafarina, V.; Uriz-Otano, F.; Malafarina, C.; Martinez, J.A.; Zulet, M.A. Effectiveness of Nutritional Supplementation on Sarcopenia and Recovery in Hip Fracture Patients. A Multi-Centre Randomized Trial. Maturitas 2017, 101, 42–50. [Google Scholar] [CrossRef]
- Cannataro, R.; Carbone, L.; Petro, J.L.; Cione, E.; Vargas, S.; Angulo, H.; Forero, D.A.; Odriozola-Martinez, A.; Kreider, R.B.; Bonilla, D.A. Sarcopenia: Etiology, Nutritional Approaches, and Mirnas. Int. J. Mol. Sci. 2021, 22, 9724. [Google Scholar] [CrossRef]
- Wilhelm-Leen, E.R.; Hall, Y.N.; DeBoer, I.H.; Chertow, G.M. Vitamin D Deficiency and Frailty in Older Americans. J. Intern. Med. 2010, 268, 171–180. [Google Scholar] [CrossRef]
- Symonsi, T.B.; Sheffield-Moore, M.; Mamerow, M.M.; Wolfe, R.R.; Paddon-Jones, D. The Anabolic Response to Resistance Exercise and a Protein-Rich Meal Is Not Diminished by Age. J. Nutr. Health Aging 2011, 15, 376–381. [Google Scholar] [CrossRef]
- Papadopoulou, S.K. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef]
- Calvani, R.; Picca, A.; Marini, F.; Biancolillo, A.; Cesari, M.; Pesce, V.; Lezza, A.M.S.; Bossola, M.; Leeuwenburgh, C.; Bernabei, R.; et al. The “Biomarkers Associated with Sarcopenia and Physical Frailty in Elderly Persons” (Biosphere) Study: Rationale, Design and Methods. Eur. J. Intern. Med. 2018, 56, 19–25. [Google Scholar] [CrossRef]
- Marzetti, E.; Guerra, F.; Calvani, R.; Marini, F.; Biancolillo, A.; Gervasoni, J.; Primiano, A.; Coelho-Junior, H.J.; Landi, F.; Bernabei, R.; et al. Circulating Mitochondrial-Derived Vesicles, Inflammatory Biomarkers and Amino Acids in Older Adults with Physical Frailty and Sarcopenia: A Preliminary Biosphere Multi-Marker Study Using Sequential and Orthogonalized Covariance Selection—Linear Discriminant Analysis. Front. Cell Dev. Biol. 2020, 8, 564417. [Google Scholar] [CrossRef]
- Koshikawa, M.; Harada, M.; Noyama, S.; Kiyono, K.; Motoike, Y.; Nomura, Y.; Nishimura, A.; Izawa, H.; Watanabe, E.; Ozaki, Y. Association between Inflammation and Skeletal Muscle Proteolysis, Skeletal Mass and Strength in Elderly Heart Failure Patients and Their Prognostic Implications. BMC Cardiovasc. Disord 2020, 20, 228. [Google Scholar] [CrossRef]
- Hida, T.; Imagama, S.; Ando, K.; Kobayashi, K.; Muramoto, A.; Ito, K.; Ishikawa, Y.; Tsushima, M.; Nishida, Y.; Ishiguro, N.; et al. Sarcopenia and Physical Function Are Associated with Inflammation and Arteriosclerosis in Community-Dwelling People: The Yakumo Study. Mod. Rheumatol. 2018, 28, 345–350. [Google Scholar] [CrossRef]
- Wang, T. Searching for the Link between Inflammaging and Sarcopenia. Ageing Res. Rev. 2022, 77, 101611. [Google Scholar] [CrossRef] [PubMed]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal Muscle as Potential Central Link between Sarcopenia and Immune Senescence. eBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Mukund, K.; Subramaniam, S. Skeletal Muscle: A Review of Molecular Structure and Function, in Health and Disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, W.; Bragg, R.; Elmansi, A.M.; McGee-Lawrence, M.E.; Isales, C.M.; Hamrick, M.W.; Hill, W.D.; Fulzele, S. Stromal Cell-Derived Factor-1 (Cxcl12) and Its Role in Bone and Muscle Biology. Cytokine 2019, 123, 154783. [Google Scholar] [CrossRef] [PubMed]
- Bobadilla, M.; Sainz, N.; Abizanda, G.; Orbe, J.; Rodriguez, J.A.; Paramo, J.A.; Prosper, F.; Perez-Ruiz, A. The Cxcr4/Sdf1 Axis Improves Muscle Regeneration through Mmp-10 Activity. Stem Cells Dev. 2014, 23, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Lewin, S.A.; Mihalko, L.A.; Lesher-Perez, S.C.; Takayama, S.; Luker, K.E.; Luker, G.D. Secreted Cxcl12 (Sdf-1) Forms Dimers under Physiological Conditions. Biochem. J. 2012, 442, 433–442. [Google Scholar] [CrossRef]
- Maeda, Y.; Yonemochi, Y.; Nakajyo, Y.; Hidaka, H.; Ikeda, T.; Ando, Y. Cxcl12 and Osteopontin from Bone Marrow-Derived Mesenchymal Stromal Cells Improve Muscle Regeneration. Sci. Rep. 2017, 7, 3305. [Google Scholar] [CrossRef]
- Steyn, P.J.; Dzobo, K.; Smith, R.I.; Myburgh, K.H. Interleukin-6 Induces Myogenic Differentiation Via Jak2-Stat3 Signaling in Mouse C2c12 Myoblast Cell Line and Primary Human Myoblasts. Int. J. Mol. Sci. 2019, 20, 5273. [Google Scholar] [CrossRef]
- Tierney, M.T.; Aydogdu, T.; Sala, D.; Malecova, B.; Gatto, S.; Puri, P.L.; Latella, L.; Sacco, A. Stat3 Signaling Controls Satellite Cell Expansion and Skeletal Muscle Repair. Nat. Med. 2014, 20, 1182–1186. [Google Scholar] [CrossRef]
- Alemán-Mateo, H.; López-Teros, M.T.; Ruiz-Valenzuela, R.E.; Ramírez-Torres, M.; Urquidez-Romero, R. Sarcopenia: Influence of Regional Skeletal Muscle Cutoff Points and Fat-Free Mass in Older Mexican People-a Pilot Study. Curr. Gerontol. Geriatr. Res. 2020, 2020, 8037503. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Chow, S.K.; Hung, V.W.; Wong, C.H.; Wong, R.M.; Tsang, C.S.; Kwok, T.; Cheung, W.H. Diagnosis of Sarcopenia by Evaluating Skeletal Muscle Mass by Adjusted Bioimpedance Analysis Validated with Dual-Energy X-ray Absorptiometry. J. Cachexia Sarcopenia Muscle 2021, 12, 2163–2173. [Google Scholar] [CrossRef]
- Yamamoto, H.; Takeshima, F.; Haraguchi, M.; Akazawa, Y.; Matsushima, K.; Kitayama, M.; Ogihara, K.; Tabuchi, M.; Hashiguchi, K.; Yamaguchi, N.; et al. High Serum Concentrations of Growth Differentiation Factor-15 and Their Association with Crohn’s Disease and a Low Skeletal Muscle Index. Sci. Rep. 2022, 12, 6591. [Google Scholar] [CrossRef]
- Masanes, F.; Luque, I.; Rojano, X.; Salva, A.; Serra-Rexach, J.A.; Artaza, I.; Formiga, F.; Cuesta, F.; Soto, A.L.; Ruiz, D.; et al. Cut-Off Points for Muscle Mass—Not Grip Strength or Gait Speed—Determine Variations in Sarcopenia Prevalence. J. Nutr. Health Aging 2017, 21, 825–829. [Google Scholar] [CrossRef]
- Kowalski, K.; Archacki, R.; Archacka, K.; Streminska, W.; Paciorek, A.; Golabek, M.; Ciemerych, M.A.; Brzoska, E. Stromal Derived Factor-1 and Granulocyte-Colony Stimulating Factor Treatment Improves Regeneration of Pax7−/− Mice Skeletal Muscles. J. Cachexia Sarcopenia Muscle 2016, 7, 483–496. [Google Scholar] [CrossRef]
- Tian, J.; Chung, H.K.; Moon, J.S.; Nga, H.T.; Lee, H.Y.; Kim, J.T.; Chang, J.Y.; Kang, S.G.; Ryu, D.; Che, X.; et al. Skeletal Muscle Mitoribosomal Defects Are Linked to Low Bone Mass Caused by Bone Marrow Inflammation in Male Mice. J. Cachexia Sarcopenia Muscle 2022, 13, 1785–1799. [Google Scholar] [CrossRef]
- Carbone, L.D.; Bůžková, P.; Fink, H.A.; Robbins, J.A.; Bethel, M.; Hamrick, M.W.; Hill, W.D. Association of Plasma Sdf-1 with Bone Mineral Density, Body Composition, and Hip Fractures in Older Adults: The Cardiovascular Health Study. Calcif. Tissue Int. 2017, 100, 599–608. [Google Scholar] [CrossRef]
- Polito, A.; Barnaba, L.; Ciarapica, D.; Azzini, E. Osteosarcopenia: A Narrative Review on Clinical Studies. Int. J. Mol. Sci. 2022, 23, 5591. [Google Scholar] [CrossRef]
- Kurita, N.; Kamitani, T.; Wada, O.; Shintani, A.; Mizuno, K. Disentangling Associations between Serum Muscle Biomarkers and Sarcopenia in the Presence of Pain and Inflammation among Patients with Osteoarthritis: The Spss-Ok Study. J. Clin. Rheumatol. 2021, 27, 56–63. [Google Scholar] [CrossRef]
- Haugen, F.; Norheim, F.; Lian, H.; Wensaas, A.J.; Dueland, S.; Berg, O.; Funderud, A.; Skålhegg, B.S.; Raastad, T.; Drevon, C.A. Il-7 Is Expressed and Secreted by Human Skeletal Muscle Cells. Am. J. Physiol.-Cell Physiol. 2010, 298, C807–C816. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, L.; Xiao, Y.; Xie, S.; Long, Y.; Wei, Y.; Meng, Q.; Li, X.; Luo, H.; Zhu, H. The Expression of Cytokine Profiles and Related Receptors in Idiopathic Inflammatory Myopathies. Front. Pharmacol. 2022, 13, 852055. [Google Scholar] [CrossRef]

| Normal ASMI Male ≥ 7.0 kg/m2 Female ≥ 5.5 kg/m2 (n = 49) | Low ASMI Male < 7.0 kg/m2 Female < 5.5 kg/m2 (n = 31) | p | Control Group Male ≥ 7.3 kg/m2 Female ≥ 6.8 kg/m2 (n = 24) | Case Group Male < 7.3 kg/m2 Female < 6.8 kg/m2 (n = 56) | p | |
|---|---|---|---|---|---|---|
| Age (years) | 80.73 ± 6.27 | 82.58 ± 6.57 | >0.05 | 79.46 ± 5.81 | 82.30 ± 6.52 | >0.05 |
| Sex (F/M) | 32/17 | 13/18 | 0.041 | 10/14 | 35/21 | >0.05 |
| ASMI (kg/m2) | 7.01 ± 0.99 | 5.67 ± 0.63 | <0.001 | 7.79 ± 0.70 | 5.93 ± 0.65 | <0.001 |
| Weight (kg) | 76.78 ± 15.47 | 62.22 ± 10.65 | <0.001 | 81.81 ± 13.39 | 66.56 ± 14.07 | <0.001 |
| BFR (%) | 34.41 ± 8.59 | 31.90 ± 7.15 | >0.05 | 29.95 ± 8.37 | 34.93 ± 7.58 | 0.011 |
| MNA (scores) | 25.28 ± 16.07 | 18.52 ± 4.92 | 0.026 | 22.83 ± 4.27 | 19.80 ± 4.57 | 0.012 |
| Monocyte (%) | 9.06 ± 4.21 | 8.53 ± 2.86 | >0.05 | 7.73 ± 2.39 | 9.34 ± 4.09 | 0.042 |
| IL-7 (pg/mL) | 4.35 ± 3.71 | 4.33 ± 3.67 | >0.05 | 5.63 ± 4.06 | 3.74 ± 3.34 | 0.040 |
| CK(U/L) | 73.94 ± 52.44 | 69.13 ± 46.60 | >0.05 | 91.38 ± 59.27 | 63.80 ± 43.50 | 0.017 |
| OR | 95% CI | p | |
|---|---|---|---|
| Age (years) | |||
| ≤83 | 1.00 | ||
| >83 | 27.57 | 2.04–371.85 | 0.012 |
| MNA (scores) | |||
| >24 | 1.00 | ||
| ≤24 | 5.78 | 1.11–30.16 | 0.038 |
| CK (U/L) | |||
| >120 | 1.00 | ||
| ≤120 | 6.44 | 1.05–39.36 | 0.044 |
| CXCL12α (pg/mL) | |||
| ≤240 | 1.00 | ||
| >240 | 19.13 | 1.35–271.38 | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Laurentius, T.; Fait, Y.; Müller, A.; Mückter, E.; Bollheimer, L.C.; Nourbakhsh, M. Associations of Serum CXCL12α and CK Levels with Skeletal Muscle Mass in Older Adults. J. Clin. Med. 2023, 12, 3800. https://doi.org/10.3390/jcm12113800
Chen Z, Laurentius T, Fait Y, Müller A, Mückter E, Bollheimer LC, Nourbakhsh M. Associations of Serum CXCL12α and CK Levels with Skeletal Muscle Mass in Older Adults. Journal of Clinical Medicine. 2023; 12(11):3800. https://doi.org/10.3390/jcm12113800
Chicago/Turabian StyleChen, Ze, Thea Laurentius, Yvonne Fait, Aline Müller, Eva Mückter, Leo Cornelius Bollheimer, and Mahtab Nourbakhsh. 2023. "Associations of Serum CXCL12α and CK Levels with Skeletal Muscle Mass in Older Adults" Journal of Clinical Medicine 12, no. 11: 3800. https://doi.org/10.3390/jcm12113800
APA StyleChen, Z., Laurentius, T., Fait, Y., Müller, A., Mückter, E., Bollheimer, L. C., & Nourbakhsh, M. (2023). Associations of Serum CXCL12α and CK Levels with Skeletal Muscle Mass in Older Adults. Journal of Clinical Medicine, 12(11), 3800. https://doi.org/10.3390/jcm12113800








