Abstract
Background: The most effective treatment for end-stage liver diseases is liver transplantation, which is impeded by the shortage of donor livers. Split liver transplantation (SLT) is important for addressing the donor liver shortage. However, full-right full-left SLT for two adult recipients is globally rarely conducted. This study aimed to investigate the clinical outcomes of this technique. Methods: We retrospectively analyzed the clinical data of 22 recipients who underwent full-right full-left SLT at Shulan (Hangzhou) Hospital between January, 2021 and September, 2022. The graft-to-recipient weight ratio (GRWR), cold ischemia time, operation time, length of the anhepatic phase, intraoperative blood loss, and red blood cell transfusion amount were all analyzed. The differences in liver function recovery after transplantation were compared between the left and right hemiliver groups. The postoperative complications and prognosis of the recipients were also analyzed. Results: The livers of 11 donors were transplanted into 22 adult recipients. The GRWR ranged from 1.16–1.65%, the cold ischemia time was 282.86 ± 134.87 min, the operation time was 371.32 ± 75.36 min, the anhepatic phase lasted 60.73 ± 19.00 min, the intraoperative blood loss was 759.09 ± 316.84 mL, and the red blood cell transfusion amount was 695.45 ± 393.67 mL. No significant difference in the levels of liver function markers, total bilirubin, aspartate aminotransferase, or alanine aminotransferase between left and right hemiliver groups at 1, 3, 5, 7, 14, and 28 d postoperatively was observed (both p > 0.05). One recipient developed bile leakage 10 d after transplantation, which improved with endoscopic retrograde cholangiopancreatography-guided nasobiliary drainage and stent placement. Another developed portal vein thrombosis 12 d after transplantation and underwent portal vein thrombolytic therapy and stenting to restore portal vein blood flow. A color Doppler ultrasound performed 2 d after transplantation revealed hepatic artery thrombosis in one patient, and thrombolytic therapy was administered to restore hepatic artery blood flow. The liver function of other patients recovered quickly after transplantation. Conclusions: Full-right full-left SLT for two adult patients is an efficient way to increase the donor pool. It is safe and feasible with careful donor and recipient selection. Transplant hospitals with highly experienced surgeons in SLT are recommended to promote using full-right full-left SLT for two adult recipients.
1. Introduction
The most effective treatment for end-stage liver diseases is liver transplantation. However, a global shortage of donor livers is impeding the advancement of liver transplantation. Split liver transplantation (SLT) is the process of dividing a donor liver into two or more independent structural and functional grafts for two or more recipients. It is a method used to mitigate the scarcity of donor livers [1]. In clinical practice, donor livers have most commonly been split into the left lateral segment and the right trisegment, or right and left hemilivers, enabling two recipients to receive liver transplants from a single donor and increasing the utilization rate of the liver. The liver is usually divided in SLT into a left lateral segment for a child and a right trisegment for an adult. A donor liver is usually split into two full grafts for two adult recipients. Full-right full-left SLT for two adult recipients is a complex procedure requiring careful recipient and donor selection. Moreover, the allocation of the middle hepatic vein (MHV) has been controversial, and this technique is rarely used at home or abroad. This study aimed to investigate the clinical outcomes of the full-right full-left SLT technique. From January, 2021 to September, 2022, we used 11 donor livers to perform full-right full-left SLT on 22 adult recipients. We describe our experience with this technique in this study.
2. Methods and Patients
2.1. Ethical Consideration
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Furthermore, the institutional ethics board of Shulan (Hangzhou) Hospital approved the study.
2.2. Donor Characteristics
Overall, 11 donors were included. Ten of the 11 donors were classified as China Category I (international standard donation after brain death), and one as China Category III (transitional period of donation after brain death followed by circulatory death). There were ten men and one woman, with a mean age of 41.36 ± 10.49 years and a mean body mass index (BMI) of 23.27 ± 2.37 kg/m2. The causes of death were cerebrovascular accidents in five cases, craniocerebral trauma in three cases, and hypoxic-ischemic encephalopathy in three cases. The length of stay in the intensive care unit (ICU) was 4.91 ± 1.92 d. At the time of donation, the total bilirubin (TBIL), aspartate aminotransferase (AST), alanine aminotransferase (ALT), cholinesterase enzyme, and serum sodium levels in donors were 22.36 ± 6.31 μmol/L, 30.55 ± 16.92 U/L, 39.45 ± 17.26 U/L U/L, 4265.09 ± 1866.03 U/L, and 139.00 ± 4.29 mmol/L, respectively. All donors had a stable hemodynamic status, were not on or received low-dose vasopressors, such as dopamine, and had no history of cardiac arrest. Before donation, the liver function of the donors was essentially normal. A liver biopsy using ultrasound guidance revealed no steatosis. Table 1 summarizes the characteristics of the donors.

Table 1.
Donor characteristics.
2.3. Recipient Characteristics
Overall, 22 liver transplant recipients were included in the study. There were sixteen men and six women. The mean age of recipients was 50.68 ± 11.98 years, the mean BMI was 22.62 ± 3.67 kg/m2, their preoperative Child–Pugh score was 9.91 ± 1.77, and their model for end-stage liver disease (MELD) score was 22.36 ± 11.51. Hepatocellular carcinoma was the primary disease in five cases, intrahepatic cholangiocarcinoma in two, hepatitis B virus (HBV)-related decompensated cirrhosis in eight, decompensated alcoholic cirrhosis in two, and decompensated primary biliary cirrhosis in five. In addition, three recipients had previously undergone a transjugular intrahepatic portosystemic shunt, one underwent splenectomy for splenomegaly with hypersplenism, and one underwent cholecystectomy. The blood types of the donors and recipients were in accordance with the principles of blood transfusion.
2.4. Donor Liver Harvest—Splitting Technique
Nine out of the eleven donor livers were split in situ. The MHV trunk and vena cava for the left hemiliver and hepatic vein branches in segments V and VIII for the right hemiliver were preserved, followed by angioplasty and reconstruction (Figure 1A–D). Preoperative color Doppler ultrasound was used to assess hepatic steatosis, portal vein, and hepatic vein variations. To assess anatomic variation in the bile ducts, intraoperative cholangiography was performed. The surgical procedure was the same as when harvesting the right hemiliver without the MHV during living donor liver transplantation. The gallbladder was removed, and intraoperative cholangiography was performed through the cystic duct. The right and left hepatic ducts, the main trunk and branches of the hepatic artery, and the right and left branches of the portal vein were separated and exposed after the first hepatic hilum was dissected. The clamping of the right portal vein and the right hepatic artery revealed the right hemihepatic ischemic line. The splitting plane was determined along the MHV-hepatic ischemia line-gallbladder fossa-confluence of right and left hepatic ducts. To expose the MHV, the liver parenchyma was split. The donor liver was perfused, the blood vessels were transected, and the liver was harvested after dissecting the parenchyma. For reconstruction, the MHV was kept for the left hemiliver, and the hepatic veins of Segments V and VIII were kept for the right hemiliver. Furthermore, when small blood vessels and bile ducts were encountered, ligation was performed using titanium clips, silk, or vascular sutures, which were then transected. The hepatic veins of Segments V and VIII had to be reconstructed using iliac vessels from the same donor as much as possible.
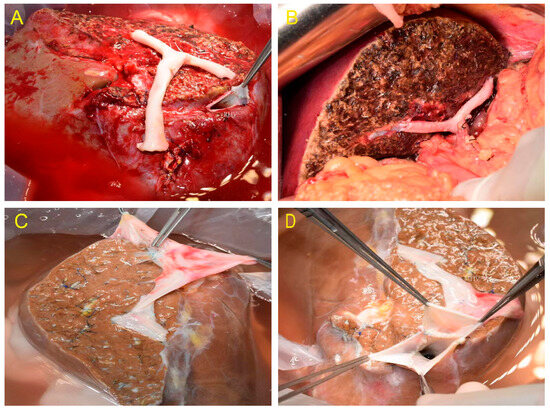
Figure 1.
(A) The hepatic veins of Segments V and VIII were retained in the right hemiliver and reconstructed with iliac artery. (B) No obvious ischemia and congestion were seen after implantation. (C) The hepatic veins of Segments V and VIII were retained in the right hemiliver and reconstructed with iliac vein. (D) MHV and RHV reconstruction.
Two out of the eleven donor livers were divided by complete splitting and reconstruction of the MHV and IVC, resulting in a right and left hemiliver graft that included the MHV and IVC ex situ (Figure 2A–D). The hepatic and portal veins were explored after the donor liver, and iliac vessels were harvested using the combined liver–kidney harvesting technique. The volume of the right and left hemilivers was moderate, and cholangiography and hepatic arteriography were performed. The MHV anterior wall was completely dissected and exposed, and the MHV and IVC were split midline. The portal vein, hepatic artery, and bile duct in the first hepatic hilum were transected sequentially, followed by a complete division of the right and left hemilivers. The right hemiliver included the right hepatic duct, the right hepatic artery, and the right branches of the portal vein. The left hemiliver included the common bile duct, the proper hepatic artery, and the main portal vein. The iliac vein from the same donor was used to reconstruct the MHV and IVC in both the left and right hemilivers.
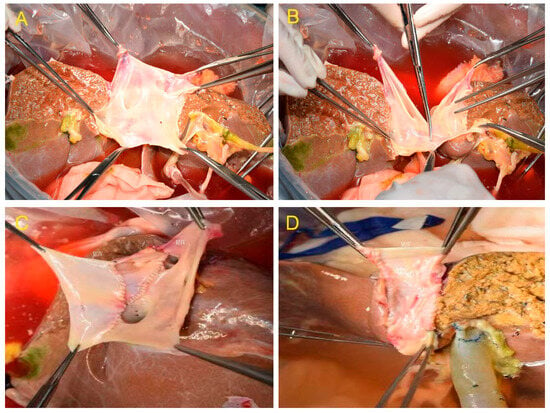
Figure 2.
(A) After transection of the liver parenchyma and bile duct. (B) The retrohepatic inferior vena cava (IVC) was divided by longitudinal transection of the front and back walls. (C) Reconstruction of the MHV and IVC in the right hemiliver graft. (D) Reconstruction of the MHV and IVC in the left hemiliver graft.
2.5. Recipient Surgery
The diseased liver was removed, and the new liver was implanted. Left and right hemiliver grafts were mainly implanted using piggyback or a modified piggyback technique based on the actual matching status of the split donor liver with the recipient. The portal vein, hepatic artery, and bile ducts were reconstructed sequentially. The condition of the bile ducts should be considered when deciding whether to implant a T-tube.
2.6. Postoperative Management and Follow-Up
Following transplantation, the following immunosuppressive regimens were used alone or in combination, depending on the condition of the recipients: cyclosporine/tacrolimus/rapamycin, mycophenolate mofetil, and glucocorticoids. Immunosuppressive blood levels were monitored dynamically to maintain stable immunosuppressive blood levels. Routine medication regimens, such as anti-infection, acid-suppressive, liver protection, anticoagulant, anti-HBV, and nutritional support therapies, were also used. Anti-HBV drugs were administered orally, and, if necessary, two antiviral drugs were administered for the anti-HBV treatment. Immune globulin was also administered during hospitalization. Ultrasound examination was used to dynamically monitor liver blood flow and changes in liver function. Following transplantation, all patients were followed up regularly and underwent re-examinations, including routine blood, biochemical, and blood coagulation tests, monitoring of immunosuppressive drug levels in the blood, color Doppler ultrasound of the transplanted liver, abdominal CT, and CT angiography.
2.7. Statistical Analysis
The measurement data are expressed as the mean ± SD, and the t-test was used to compare groups. All statistical analyses were performed using SPSS software 22.0. Statistical significance was defined as a p-value < 0.05.
3. Results
The piggyback or modified piggyback technique was used to perform 22 transplants. The mean graft weight was 855.05 ± 152.18 g, the graft-to-recipient weight ratio (GRWR) was 1.16–1.65%, the cold ischemia time was 282.86 ± 134.87 min, and the operation time was 371.32 ± 75.36 min, the length of the anhepatic phase was 60.73 ± 19.00 min, the intraoperative blood loss was 759.09 ± 316.84 mL, and the amount of red blood cell transfusion was 695.45 ± 393.67 mL. Table 2 shows the general characteristics of the recipients in the left and right hemiliver groups. Table 3 shows the status of the liver function recovery after the implantation of the right and left hemilivers. There was no significant difference in the levels of liver function markers (TBIL, AST, or ALT) between the left and right hemiliver groups at 1, 3, 5, 7, 14, and 28 d postoperatively (both p > 0.05).

Table 2.
Characteristics of recipients in left and right hemiliver groups.

Table 3.
Liver function status of the recipients after full-right full-left split liver transplantation.
Two of the eleven donors had Type III portal vein variation. Furthermore, one of the cases was separately transected because the portal vein was H-shaped, and the right anterior and right posterior portal veins were far apart during the splitting. During the MHV splitting, Segments V and VIII were transected and reconstructed with iliac vessels; recipients recovered well after transplantation (Figure 3A–D).
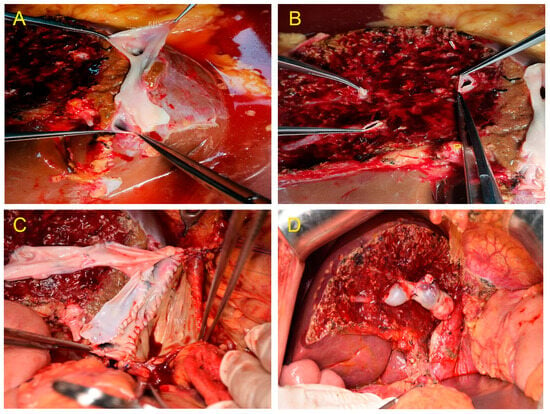
Figure 3.
(A) Venoplasty of the right hepatic vein and inferior right hepatic vein.(B) Segments V and VIII were separately transected during the middle hepatic vein (MHV) splitting. (C) Middle hepatic veins (Segments V and VIII) were bridged and reconstructed. (D) The MHV drainage of the right hemiliver graft was good, and no obvious ischemia and congestion were noted after implantation.
One of the twenty-two recipients developed bile leakage 10 d after transplantation, which improved with endoscopic retrograde cholangiopancreatography (ERCP)-guided nasobiliary drainage and stent placement. Owing to thrombus formation in the right anterior and right posterior branches of the portal vein 12 d after transplantation, one patient underwent portal vein thrombolytic therapy and stenting, and the portal vein blood flow was then restored. A color Doppler ultrasound performed 2 d after transplantation revealed hepatic artery thrombosis in one recipient. A local filling defect in the hepatic artery and its anastomosis were confirmed with emergency percutaneous super selective digital subtraction angiography. The patient then received catheter-directed thrombolysis. Furthermore, the patient was administered anticoagulation therapy with low-molecular-weight heparin, vasodilator, and microcirculation therapy. The hepatic artery blood flow was restored following treatment. The specifics of post-operative complications and treatments of recipients in left and right hemiliver groups are described in Table 4. The liver function of the remaining recipients recovered quickly after transplantation. Before discharge, a contrast-enhanced abdominal CT examination revealed no obvious ischemia or congestion in the hepatic segments of the left and right hemilivers near the splitting border (Figure 4A,B). All patients were followed up via outpatient visits, and the transplanted livers functioned normally during the follow-up period.

Table 4.
Post-operative complications and treatments of recipients in left and right hemiliver groups.
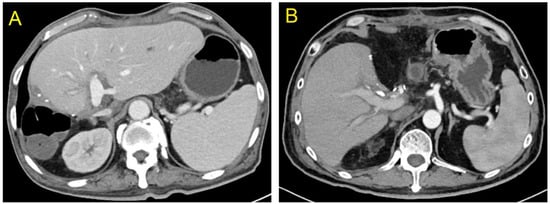
Figure 4.
At the time of discharge, no obvious ischemia and congestion were seen in the segments of both the left and right hemilivers near the splitting plane ((A) Left hemiliver; (B) Right hemiliver).
4. Discussion
SLT is one of the most common methods for increasing organ utilization; it is used to divide a whole cadaveric donor liver into two or more anatomical functional units based on the principle of liver functional segmentation, allowing for the transplantation of one donor liver into two or more recipients [2,3,4,5,6]. SLT is no longer considered a risk factor affecting liver function recovery, postoperative incidence complications are significantly reduced following SLT, and the efficacy of SLT performed by surgeons in hospitals experienced in this technique is comparable to that of whole liver transplantation [7,8,9,10,11,12,13]. SLT has the potential of increasing the number of donor livers by 15–28% and contributes towards expanding the high-quality donor pool [14,15,16,17,18].
Full-right full-left SLT for two adult recipients is uncommon worldwide, and the quality requirements for the split donor liver are also stringent. Therefore, donor selection criteria differ between transplant centers [19]. The following are the donor selection criteria for SLT: (1) Age < 50 years old, BMI < 26 kg/m2, ICU length of stay < 5 d; (2) Hepatic steatosis ratio < 10%, AST/ALT level < 3 times the upper limit of normal, TBIL level < 2 times the upper limit of normal; (3) Serum sodium level < 160 mmol/L, cold ischemia time < 10 h. The following are the recommended recipient selection criteria and the main principles for donor/recipient matching: (1) Adult recipient GRWR > 1.2% and pediatric recipient GRWR of 2–4% [20,21]; (2) No history of complex upper abdominal surgery; (3) Critically ill adult recipients with a MELD score of <30, and critically ill pediatric recipients with a pediatric end-stage liver disease score of <30 [22]. In this study, the mean age of the donors was 41.36 ± 10.49 years, the mean BMI was 23.27 ± 2.37 kg/m2, and TBIL, ALT, AST serum levels, and serum sodium in the donors at the time of donation were 22.36 ± 6.31 μmol/L, 30.55± 16.92 U/L, 39.45 ± 17.26 U/L, and 139.00 ± 4.29 mmol/L, respectively. Furthermore, the ICU length of stay was 4.91 ± 1.92 d, indicating that the donors basically met the above-mentioned selection criteria. The GRWR was 1.16% in one of the 22 recipients included in this study, which was higher than the 1.2% in the remaining recipients, and the MELD score was 22.36 ± 11.51. The recipient essentially met the above-mentioned selection criteria. After prompt and active symptomatic treatment, all 22 recipients in this study recovered well. Additionally, no significant difference in the levels of liver function markers, TBIL, AST, and ALT, was found between the left and right hemiliver groups at 1, 3, 5, 7, 14, and 28 d after transplantation.
The allocation of the MHV and IVC in full-right full-left hemiliver splitting depends on the status of the donor and recipient [23,24,25]. The full-right full-left SLT is a complex technique to master. The donor liver can be split in several ways. The allocation of the outflow tracts, i.e., the MHV and IVC allocation, is the key technical point of donor liver splitting. The effective volume of the transplanted liver graft could be ensured by optimal venous drainage. The allocation principle maximizes donor liver utilization. The caudate lobe veins drain directly into the IVC, while Segments IV, V, and VIII drain into the MHV. Patients who received a left hemiliver graft during full-right full-left SLT for two adult recipients had a relatively high risk of developing small-for-size syndrome after transplantation. Venous drainage of Hepatic Segment IV is primarily through the MHV, whereas venous drainage of Hepatic Segment I is primarily through the short hepatic veins. The MHV and IVC were allocated to the left hemiliver in the study of nine donor livers. This has the potential of improving the functional integrity of the left hemiliver grafts [26]. To maximize the use of the donor liver, the hepatic vein branches in Segments V and VIII were retained and reconstructed for the right hemiliver. The GRWR was 1.16–1.65% in recipients included in this study, suggesting that the risk of the small-for-size syndrome was reduced.
Chan et al. [27] believe that donor liver transplantation with a right hemiliver graft without the MHV trunk could produce satisfactory results. Patients who underwent venous reconstruction had a similar prognosis as those who did not undergo vein reconstruction. Gyu et al. [28] also suggested that Segment V and VIII tributaries of the MHV with diameters > 5 mm should be reconstructed and drained into the IVC. Our previous study also revealed that reconstructing the MHV could help patients recover after transplantation by ensuring the patency of the outflow tracts. Hence, the MHV tributaries should be reconstructed as much as possible to expand the hepatic vein anastomosis and reduce liver congestion [29]. However, Fan et al. believe that [30] even if the main branches of the MHV were reconstructed, adequate hepatic venous drainage for the right anterior sector of the graft would still be lacking. This is because the MHV has many branches, and it is impossible to reconstruct all of them. A retained graft with MHV can undoubtedly improve venous drainage during full-right full-left hemiliver splitting. Broering et al. [31] reported a procedure for outflow tract allocation during liver splitting in 2005. The MHV and retrohepatic IVC were split longitudinally and then separately reconstructed using iliac vessels from donors, resulting in full-right full-left hemiliver grafts. This more reasonable procedure allows retaining the MHV drainage of both the left and right hemiliver grafts. The method mentioned above was used in four recipients in this study. The MHV and IVC were split in the midline during the procedure, and many vein orifices on the lateral wall can be seen (Figure 2A–D). The iliac vessels were used to reconstruct the MHV and retrohepatic IVC. This method maximizes the effective volume of the donor liver. In addition, it reduces the degree of graft congestion by preserving the hepatic venous drainage at the transection plane of the left and right hemilivers. These four recipients recovered well after transplantation, with no outflow tract-related complications, biliary complications, or small-for-size syndrome.
The donor portal veins need to be allocated according to the characteristics of the recipient portal vein for full-right full-left liver splitting. The length of the recipient and donor portal veins, the degree of diameter matching, and the reconstruction difficulty should all be considered when deciding whether to keep the portal vein trunk in the left or right hemilivers. Two donors in this study had Type III portal vein variation. We transected them separately because one of the cases was H-shaped, and the right anterior and right posterior portal veins were far apart during the splitting. Additionally, Segments V and VIII were separately transected during the splitting of the MHV and reconstructed with iliac vessels. After transplantation, the blood supply to the left and right hemiliver was good, and the recipients recovered well.
Sneiders et al. and Chan et al. believe that [1,3] full-right full-left SLT for adult recipients may increase the availability of liver grafts, reducing waitlist time. Long-term patient and graft survival appear acceptable and justify transplant benefit in selected patients, which was consistent with our research. One of the twenty-two recipients developed bile leakage 10 d after transplantation, which improved with ERCP-guided nasobiliary drainage and stent placement. One recipient developed portal vein thrombosis 12 d after transplantation. The portal venous blood flow in this patient was restored after undergoing portal vein thrombolytic therapy and stenting. A color Doppler ultrasound performed 2 d after transplantation revealed hepatic artery thrombosis in one recipient, and thrombolytic therapy was used to restore hepatic artery blood flow. Most technical problems encountered with SLT can be solved by strengthening multidisciplinary (such as anesthesiology, artificial liver, ERCP, and angiographic interventions) and multicenter cooperation, improving the level of the SLT technique. No outflow tract-related complications or biliary complications occurred after transplantation. Nonetheless, the sample size of this study was small; hence, large-scale studies are needed to evaluate the efficacy of the MHV allocation procedure used in this study. Furthermore, the follow-up period in this study was relatively brief. In the future, we will continue to promote the use of full-right full-left SLT for two adult recipients and further strengthen follow-up and efficacy evaluation.
Author Contributions
Conceptualization, S.Z.; methodology, L.Z.; formal analysis, L.D., X.Y. and R.Z.; data curation, L.D., X.Y. and R.Z.; writing—original draft preparation, L.D., X.Y., R.Z., J.Q., W.Z. and Q.W.; writing—review and editing, S.Z. and Z.Y.; supervision, S.Z.; All authors have read and agreed to the published version of the manuscript.
Funding
A Project Supported by Scientific Research Fund of Zhejiang Provincial Education Department (Y202250768).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Shulan (Hangzhou) Hospital (No. KY2022030, Approved on 25 May 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| SLT | Split liver transplantation |
| GRWR | Graft-to-recipient weight ratio |
| MHV | Middle hepatic vein |
| BMI | Body mass index |
| ICU | Intensive care unit |
| TBIL | Total bilirubin |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| MELD | Model for end-stage liver disease |
| HBV | Hepatitis B virus |
| IVC | Inferior vena cava |
| ERCP | Endoscopic retrograde cholangiopancreatography |
| RBD | Right bile duct |
| LBD | Left bile duct |
| V5 | Liver segment 5 |
| V8 | Liver segment 8 |
| RHV | Right hepatic vein |
| LHV | Left hepatic vein |
| RAP | Right anterior portal vein |
| RPP | Right posterior portal vein |
| S5 | Venae hepatis 5th stage of reflux |
| S8 | Venae hepatis 8th stage of reflux |
| PV | Portal vein |
| MHV-IVC | MHV and retrohepatic IVC were split longitudinally and then separately reconstructed using iliac vessels from donors |
References
- Sneiders, D.; van Dijk, A.R.M.; Polak, W.G.; Mirza, D.F.; Perera, M.T.P.R.; Hartog, H. Full-left-full-right split liver transplantation for adult recipients: A systematic review and meta-analysis. Transpl. Int. 2021, 34, 2534–2546. [Google Scholar] [CrossRef] [PubMed]
- Herden, U.; Fischer, L.; Sterneck, M.; Grabhorn, E.; Nashan, B. Long-term follow-up after full-split liver transplantation and its applicability in the recent transplant era. Clin. Transplant. 2018, 32, e13205. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.M.; Hung, H.C.; Lee, J.C.; Wu, T.H.; Wang, Y.C.; Cheng, C.H.; Lee, C.F.; Wu, T.J.; Chou, H.S.; Lee, W.C. A review of split liver transplantation with full right/left hemi-liver grafts for 2 adult recipients. Medicine 2021, 100, e27369. [Google Scholar] [CrossRef] [PubMed]
- Lozanovski, V.J.; Probst, P.; Ramouz, A.; Arefidoust, A.; Ghamarnejad, O.; Aminizadeh, E.; Khajeh, E.; Mehrabi, A. Considering extended right lobe grafts as major extended donor criteria in liver transplantation is justified. Transpl. Int. 2021, 34, 622–639. [Google Scholar] [CrossRef]
- Park, G.C.; Hwang, S.; Song, G.W.; Jung, D.H.; Ha, T.Y.; Ahn, C.S.; Moon, D.B.; Kim, K.H.; Yoon, Y.I.; Kang, W.H.; et al. Prognosis of split liver transplantation compared with whole liver transplantation in adult patients: Single-center results under the Korean MELD score-based allocation policy. J. Korean Med. Sci. 2020, 35, e304. [Google Scholar] [CrossRef]
- Kong, L.; Lv, T.; Jiang, L.; Yang, J.; Yang, J. Outcomes of hemi-versus whole liver transplantation in patients from mainland china with high model for end-stage liver disease scores: A matched analysis. BMC Surg. 2020, 20, 290. [Google Scholar] [CrossRef]
- Perkins, J.D.; Dick, A.A.; Healey, P.J.; Montenovo, M.I.; Biggins, S.W.; Sibulesky, L.; Reyes, J.D. New evidence supporting increased use of split liver transplantation. Transplantation 2020, 104, 299–307. [Google Scholar] [CrossRef]
- Cauley, R.P.; Vakili, K.; Fullington, N.; Potanos, K.; Graham, D.A.; Finkelstein, J.A.; Kim, H.B. Deceased-donor split-liver transplantation in adult recipients: Is the learning curve over? J. Am. Coll. Surg. 2013, 217, 672–684.e1. [Google Scholar] [CrossRef]
- Moussaoui, D.; Toso, C.; Nowacka, A.; McLin, V.A.; Bednarkiewicz, M.; Andres, A.; Berney, T.; Majno, P.; Wildhaber, B.E. Early complications after liver transplantation in children and adults: Are split grafts equal to each other and equal to whole livers? Pediatr. Transpl. 2017, 21, e12908. [Google Scholar] [CrossRef]
- Doyle, M.M.; Maynard, E.; Lin, Y.; Vachharajani, N.; Shenoy, S.; Anderson, C.; Earl, M.; Lowell, J.A.; Chapman, W.C. Outcomes with split liver transplantation are equivalent to those with whole organ transplantation. J. Am. Coll. Surg. 2013, 217, 102–112; discussion 113–114. [Google Scholar] [CrossRef]
- Yagi, S.; Singhal, A.; Jung, D.H.; Hashimoto, K. Living-donor liver transplantation: Right versus left. Int. J. Surg. 2020, 82, 128–133. [Google Scholar] [CrossRef]
- Zambelli, M.; Andorno, E.; De Carlis, L.; Rossi, G.; Cillo, U.; De Feo, T.; Carobbio, A.; Giacomoni, A.; Bottino, G.; Colledan, M. Full-right-full-left split liver transplantation: The retrospective analysis of an early multicenter experience including graft sharing. Am. J. Transplant. 2012, 12, 2198–2210. [Google Scholar] [CrossRef]
- Gavriilidis, P.; Roberts, K.J.; Azoulay, D. Right lobe split liver graft versus whole liver transplantation: A systematic review by updated traditional and cumulative meta-analysis. Dig. Liver Dis. 2018, 50, 1274–1282. [Google Scholar] [CrossRef]
- Nemes, B.; Gámán, G.; Polak, W.G.; Gelley, F.; Hara, T.; Ono, S.; Baimakhanov, Z.; Piros, L.; Eguchi, S. Extended-criteria donors in liver transplantation Part II: Reviewing the impact of extended-criteria donors on the complications and outcomes of liver transplantation. Expert. Rev. Gastroenterol. Hepatol. 2016, 10, 841–859. [Google Scholar] [CrossRef]
- Zimmerman, A.; Flahive, J.M.; Hertl, M.; Cosimi, A.B.; Saidi, R.F. Outcomes of full-right-full-left split liver transplantation in adults in the USA: A propensity-score matched analysis. Int. J. Organ. Transplant. Med. 2016, 7, 69–76. [Google Scholar]
- Giacomoni, A.; Lauterio, A.; Donadon, M.; De Gasperi, A.; Belli, L.; Slim, A. Should we still offer split-liver transplantation for two adult recipients? A retrospective study of our experience. Liver Transplant. 2008, 14, 999–1006. [Google Scholar] [CrossRef]
- Valente, R.; Andorno, E.; Santori, G.; De Feo, T.M.; Ghirelli, R.; Valente, U.; Project, S.I.T.F. Split liver network: A collaborative internet-based scenario to expand the organ pool. Transplant. Proc. 2007, 39, 1923–1926. [Google Scholar] [CrossRef]
- Ferla, F.; Lauterio, A.; Di Sandro, S.; Mangoni, I.; Poli, C.; Concone, G.; Cusumano, C.; Giacomoni, A.; Andorno, E.; Luciano, L.D.C. Split-liver full-left full-right: Proposal for an operative protocol. Transplant. Proc. 2014, 46, 2279–2282. [Google Scholar] [CrossRef]
- Hackl, C.; Schmidt, K.M.; Süsal, C.; Döhler, B.; Zidek, M.; Schlitt, H.J. Split liver transplantation: Current developments. World J. Gastroenterol. 2018, 24, 5312–5321. [Google Scholar] [CrossRef]
- Lee, W.C.; Chan, K.M.; Chou, H.S.; Wu, T.J.; Lee, C.F.; Soong, R.S.; Wu, T.H.; Lee, C.S. Feasibility of split liver transplantation for 2 adults in the model of end-stage liver disease era. Ann. Surg. 2013, 258, 306–311. [Google Scholar] [CrossRef]
- Hashimoto, K.; Quintini, C.; Aucejo, F.N.; Fujiki, M.; Diago, T.; Watson, M.J.; Kelly, D.M.; Winans, C.G.; Eghtesad, B.; Fung, J.J.; et al. Split liver transplantation using Hemiliver graft in the MELD era: A single center experience in the United States. Am. J. Transplant. 2014, 14, 2072–2080. [Google Scholar] [CrossRef] [PubMed]
- Nadalin, S.; Schaffer, R.; Fruehauf, N. Split-liver transplantation in the high-MELD adult patient: Are we being too cautious? Transpl. Int. 2009, 22, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Aseni, P.; De Feo, T.M.; De Carlis, L.; Valente, U.; Colledan, M.; Cillo, U.; Rossi, G.; Mazzaferro, V.; Donataccio, M.; De Fazio, N.; et al. A prospective policy development to increase split-liver transplantation for 2 adult recipients: Results of a 12-year multicenter collaborative study. Ann. Surg. 2014, 259, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.S.; Jacques, A.; McCaughan, G.; Crawford, M.; Liu, K.; Pulitano, C. Addressing the challenges of split liver transplantation through technical advances. A systematic review. Transplant. Rev. 2021, 35, 100627. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Miller, C.M.; Quintini, C.; Aucejo, F.N.; Hirose, K.; Uso, T.D.; Trenti, L.; Kelly, D.M.; Winans, C.G.; Vogt, D.P.; et al. Is impaired hepatic arterial buffer response a risk factor for biliary anastomotic stricture in liver transplant recipients? Surgery 2010, 148, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Vagefi, P.A.; Parekh, J.; Ascher, N.L.; Roberts, J.P.; Freise, C.E. Ex vivo split-liver transplantation: The true right/left split. HPB 2014, 16, 267–274. [Google Scholar] [CrossRef]
- Chan, K.M.; Cheng, C.H.; Wu, T.H.; Wu, T.J.; Chou, H.S.; Lee, C.S.; Lee, W.C. Clinical strategy for the reconstruction of middle hepatic vein tributaries in right liver living donor liver transplantation. World J. Surg. 2014, 38, 2927–2933. [Google Scholar] [CrossRef]
- Lee, S.G.; Park, K.M.; Hwang, S.; Kim, K.H.; Choi, D.N.; Joo, S.H.; Anh, C.S.; Nah, Y.W.; Jeon, J.Y.; Park, S.H.; et al. Modified right liver graft from a living donor to prevent congestion. Transplantation 2002, 74, 54–59. [Google Scholar] [CrossRef]
- Guo, H.J.; Wang, K.; Chen, K.C.; Liu, Z.K.; Al-Ameri, A.; Shen, Y.; Xu, X.; Zheng, S.S. Middle hepatic vein reconstruction in adult right lobe living donor liver transplantation improves recipient survival. Hepatobiliary Pancreat. Dis. Int. 2019, 18, 125–131. [Google Scholar] [CrossRef]
- Fan, S.T.; Lo, C.M.; Liu, C.L.; Wang, W.X.; Wong, J. Safety and necessity of including the middle hepatic vein in the right lobe graft in adult-to-adult live donor liver transplantation. Ann. Surg. 2003, 238, 137–148. [Google Scholar] [CrossRef]
- Broering, D.C.; Bok, P.; Mueller, L.; Wilms, C.; Rogiers, X. Splitting of the middle hepatic vein in full-right full-left splitting of the liver. Liver Transplant. 2005, 11, 350–352. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).