Sex Dimorphism in Outcome of Trauma Patients Presenting with Severe Shock: A Multicenter Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection and Parameter Outcome
2.3. Statistical Analyses
3. Results
3.1. Patients Characteristics
3.2. Clinical Outcomes
3.3. Multivariable Logistic Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Mortality at 24 h | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. for EXP(B) | ||
| Lower | Upper | |||||||
| Sex | 0.373 | 0.476 | 0.615 | 1 | 0.433 | 1.453 | 0.571 | 3.695 |
| ISS | 0.071 | 0.016 | 18.993 | 1 | 0.000 | 1.074 | 1.040 | 1.108 |
| Age | 0.022 | 0.010 | 5.030 | 1 | 0.025 | 1.023 | 1.003 | 1.043 |
| RTS < 4 | 0.992 | 0.410 | 5.854 | 1 | 0.016 | 2.696 | 1.207 | 6.019 |
| GCS ≤ 8 | 1.369 | 0.544 | 6.339 | 1 | 0.012 | 3.931 | 1.354 | 11.408 |
| Severe comorbidity (≥ASA III) | 1.275 | 0.411 | 9.647 | 1 | 0.002 | 3.580 | 1.601 | 8.007 |
| Constant | −6.262 | 1.026 | 37.244 | 1 | 0.000 | 0.002 | ||
| Mortality Until Hospital Discharge | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. for EXP(B) | ||
| Lower | Upper | |||||||
| Sex | 0.185 | 0.447 | 0.171 | 1 | 0.679 | 1.203 | 0.501 | 2.890 |
| ISS | 0.060 | 0.016 | 13.764 | 1 | 0.000 | 1.061 | 1.028 | 1.095 |
| Age | 0.037 | 0.011 | 12.683 | 1 | 0.000 | 1.038 | 1.017 | 1.060 |
| RTS < 4 | 0.797 | 0.410 | 3.788 | 1 | 0.052 | 2.219 | 0.994 | 4.954 |
| GCS ≤ 8 | 1.427 | 0.476 | 9.003 | 1 | 0.003 | 4.165 | 1.640 | 10.579 |
| Severe comorbidity (≥ASA III) | 1.243 | 0.406 | 9.375 | 1 | 0.002 | 3.467 | 1.564 | 7.686 |
| Constant | −5.667 | 0.959 | 34.947 | 1 | 0.000 | 0.003 | ||
| ICU Admission | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. for EXP(B) | ||
| Lower | Upper | |||||||
| Sex | −0.380 | 0.456 | 0.695 | 1 | 0.404 | 0.684 | 0.280 | 1.671 |
| ISS | −0.012 | 0.014 | 0.729 | 1 | 0.393 | 0.988 | 0.961 | 1.016 |
| Age | −0.022 | 0.010 | 4.916 | 1 | 0.027 | 0.979 | 0.960 | 0.997 |
| RTS < 4 | −0.109 | 0.464 | 0.055 | 1 | 0.815 | 0.897 | 0.361 | 2.229 |
| GCS ≤ 8 | 0.532 | 0.500 | 1.132 | 1 | 0.287 | 1.702 | 0.639 | 4.529 |
| Severe comorbidity (≥ASA III) | −0.354 | 0.424 | 0.699 | 1 | 0.403 | 0.702 | 0.306 | 1.611 |
| Constant | 2.928 | 0.775 | 14.271 | 1 | 0.000 | 18.694 | ||
| Mechanical Ventilation during Admission | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. for EXP(B) | ||
| Lower | Upper | |||||||
| Sex | −0.231 | 0.397 | 0.339 | 1 | 0.560 | 0.793 | 0.364 | 1.729 |
| ISS | −0.003 | 0.012 | 0.061 | 1 | 0.806 | 0.997 | 0.973 | 1.022 |
| Age | −0.017 | 0.008 | 4.331 | 1 | 0.037 | 0.983 | 0.967 | 0.999 |
| RTS < 4 | −0.511 | 0.412 | 1.543 | 1 | 0.214 | 0.600 | 0.268 | 1.344 |
| GCS ≤ 8 | 1.238 | 0.436 | 8.078 | 1 | 0.004 | 3.448 | 1.468 | 8.096 |
| Severe comorbidity (≥ASA III) | −0.094 | 0.371 | 0.064 | 1 | 0.801 | 0.910 | 0.440 | 1.885 |
| Constant | 1.266 | 0.631 | 4.031 | 1 | 0.045 | 3.548 | ||
| Multiple Organ Failure | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. for EXP(B) | ||
| Lower | Upper | |||||||
| Sex | −0.074 | 0.413 | 0.032 | 1 | 0.858 | 0.929 | 0.413 | 2.086 |
| ISS | 0.047 | 0.015 | 9.854 | 1 | 0.002 | 1.048 | 1.018 | 1.079 |
| Age | 0.032 | 0.010 | 11.424 | 1 | 0.001 | 1.033 | 1.014 | 1.052 |
| RTS < 4 | 0.509 | 0.399 | 1.628 | 1 | 0.202 | 1.663 | 0.761 | 3.632 |
| GCS ≤ 8 | 0.838 | 0.420 | 3.982 | 1 | 0.046 | 2.312 | 1.015 | 5.268 |
| Severe comorbidity (≥ASA III) | 0.688 | 0.388 | 3.139 | 1 | 0.076 | 1.989 | 0.930 | 4.257 |
| Constant | −3.646 | 0.776 | 22.056 | 1 | 0.000 | 0.026 | ||
| Acute Kidney Injury | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. for EXP(B) | ||
| Lower | Upper | |||||||
| Sex | −1.693 | 0.765 | 4.902 | 1 | 0.027 | 0.184 | 0.041 | 0.823 |
| ISS | 0.000 | 0.015 | 0.000 | 1 | 0.988 | 1.000 | 0.970 | 1.030 |
| Age | 0.006 | 0.010 | 0.293 | 1 | 0.588 | 1.006 | 0.986 | 1.026 |
| RTS < 4 | −0.354 | 0.476 | 0.555 | 1 | 0.456 | 0.702 | 0.276 | 1.783 |
| GCS ≤ 8 | −0.082 | 0.514 | 0.025 | 1 | 0.874 | 0.922 | 0.336 | 2.526 |
| Severe comorbidity (≥ASA III) | −0.080 | 0.452 | 0.031 | 1 | 0.860 | 0.923 | 0.381 | 2.240 |
| Constant | −1.413 | 0.764 | 3.417 | 1 | 0.065 | 0.244 | ||
| Wound Infection | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. for EXP(B) | ||
| Lower | Upper | |||||||
| Sex | 0.354 | 0.582 | 0.369 | 1 | 0.543 | 1.424 | 0.455 | 4.454 |
| ISS | 0.003 | 0.019 | 0.025 | 1 | 0.873 | 1.003 | 0.966 | 1.041 |
| Age | −0.014 | 0.013 | 1.060 | 1 | 0.303 | 0.986 | 0.961 | 1.013 |
| RTS < 4 | −0.012 | 0.615 | 0.000 | 1 | 0.985 | 0.988 | 0.296 | 3.301 |
| GCS ≤ 8 | −0.605 | 0.627 | 0.929 | 1 | 0.335 | 0.546 | 0.160 | 1.868 |
| Severe comorbidity (≥ASA III) | 0.274 | 0.570 | 0.231 | 1 | 0.631 | 1.315 | 0.431 | 4.017 |
| Constant | −1.528 | 0.935 | 2.675 | 1 | 0.102 | 0.217 | ||
| pRBCs in 24 h ≥ 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. for EXP(B) | ||
| Lower | Upper | |||||||
| Sex | −0.536 | 0.389 | 1.895 | 1 | 0.169 | 0.585 | 0.273 | 1.255 |
| ISS | 0.019 | 0.012 | 2.565 | 1 | 0.109 | 1.019 | 0.996 | 1.044 |
| Age | −0.018 | 0.008 | 4.731 | 1 | 0.030 | 0.983 | 0.967 | 0.998 |
| RTS < 4 | −1.025 | 0.373 | 7.575 | 1 | 0.006 | 0.359 | 0.173 | 0.744 |
| GCS ≤ 8 | −0.360 | 0.420 | 0.736 | 1 | 0.391 | 0.697 | 0.306 | 1.588 |
| Severe comorbidity (≥ASA III) | −0.254 | 0.352 | 0.521 | 1 | 0.470 | 0.776 | 0.389 | 1.546 |
| Constant | 1.477 | 0.627 | 5.555 | 1 | 0.018 | 4.382 | ||
References
- Cannon, J.W. Hemorrhagic Shock. N. Engl. J. Med. 2018, 378, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef] [PubMed]
- Halmin, M.; Chiesa, F.; Vasan, S.K.; Wikman, A.; Norda, R.; Rostgaard, K.; Vesterager Pedersen, O.B.; Erikstrup, C.; Nielsen, K.R.; Titlestad, K.; et al. Epidemiology of Massive Transfusion: A Binational Study from Sweden and Denmark. Crit. Care Med. 2016, 44, 468–477. [Google Scholar] [CrossRef]
- Mitra, B.; Gabbe, B.J.; Kaukonen, K.M.; Olaussen, A.; Cooper, D.J.; Cameron, P.A. Long-term outcomes of patients receiving a massive transfusion after trauma. Shock 2014, 42, 307–312. [Google Scholar] [CrossRef]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef]
- Geeraedts, L.M., Jr.; Kaasjager, H.A.; van Vugt, A.B.; Frolke, J.P. Exsanguination in trauma: A review of diagnostics and treatment options. Injury 2009, 40, 11–20. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.E., Jr.; Barnes, M.W.; Finland, M. Bacteremia at Boston City Hospital: Occurrence and mortality during 12 selected years (1935–1972), with special reference to hospital-acquired cases. J. Infect. Dis. 1975, 132, 316–335. [Google Scholar] [CrossRef]
- George, R.L.; McGwin, G., Jr.; Windham, S.T.; Melton, S.M.; Metzger, J.; Chaudry, I.H.; Rue, L.W., III. Age-related gender differential in outcome after blunt or penetrating trauma. Shock 2003, 19, 28–32. [Google Scholar] [CrossRef]
- Pape, M.G.G.; Zuidema, W.P.; de Lange-Klerk, E.S.; Toor, E.J.; Edwards, M.J.; Verhofstad, M.H.; Tromp, T.N.; van Lieshout, E.M.; Bloemers, F.W.; Geeraedts, L.M.G.; et al. Is there an association between female gender and outcome in severe trauma? A multi-center analysis in the Netherlands. Scand. J. Trauma. Resusc. Emerg. Med. 2019, 27, 16. [Google Scholar] [CrossRef]
- Trentzsch, H.; Lefering, R.; Nienaber, U.; Kraft, R.; Faist, E.; Piltz, S. The role of biological sex in severely traumatized patients on outcomes: A matched-pair analysis. Ann. Surg. 2015, 261, 774–780. [Google Scholar] [CrossRef]
- Yang, K.C.; Zhou, M.J.; Sperry, J.L.; Rong, L.; Zhu, X.G.; Geng, L.; Wu, W.; Zhao, G.; Billiar, T.R.; Feng, Q.M. Significant sex-based outcome differences in severely injured Chinese trauma patients. Shock 2014, 42, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xie, J.; Yang, F.; Chen, J.J.; Li, Z.F.; Yi, C.L.; Gao, W.; Bai, X.J. The influence of sex on outcomes in trauma patients: A meta-analysis. Am. J. Surg. 2015, 210, 911–921. [Google Scholar] [CrossRef]
- Wohltmann, C.D.; Franklin, G.A.; Boaz, P.W.; Luchette, F.A.; Kearney, P.A.; Richardson, J.D.; Spain, D.A. A multicenter evaluation of whether gender dimorphism affects survival after trauma. Am. J. Surg. 2001, 181, 297–300. [Google Scholar] [CrossRef]
- Trentzsch, H.; Nienaber, U.; Behnke, M.; Lefering, R.; Piltz, S. Female sex protects from organ failure and sepsis after major trauma haemorrhage. Injury 2014, 45 (Suppl. S3), S20–S28. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, M.W.; Inthorn, D.; Andress, H.J.; Schildberg, F.W. Incidence and mortality of severe sepsis in surgical intensive care patients: The influence of patient gender on disease process and outcome. Intensive Care Med. 2000, 26, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Mathew, R.O.; Kuo, Y.H.; Asif, A. Risk of severe acute kidney injury in multiple trauma patients: Risk estimation based on a national trauma dataset. Injury 2020, 51, 45–50. [Google Scholar] [CrossRef]
- Haider, A.H.; Crompton, J.G.; Oyetunji, T.; Stevens, K.A.; Efron, D.T.; Kieninger, A.N.; Chang, D.C.; Cornwell, E.E., III; Haut, E.R. Females have fewer complications and lower mortality following trauma than similarly injured males: A risk adjusted analysis of adults in the National Trauma Data Bank. Surgery 2009, 146, 308–315. [Google Scholar] [CrossRef]
- Deitch, E.A.; Livingston, D.H.; Lavery, R.F.; Monaghan, S.F.; Bongu, A.; Machiedo, G.W. Hormonally active women tolerate shock-trauma better than do men: A prospective study of over 4000 trauma patients. Ann. Surg. 2007, 246, 447–453; discussion 53–55. [Google Scholar] [CrossRef]
- Haider, A.H.; Crompton, J.G.; Chang, D.C.; Efron, D.T.; Haut, E.R.; Handly, N.; Cornwell, E.E., III. Evidence of hormonal basis for improved survival among females with trauma-associated shock: An analysis of the National Trauma Data Bank. J. Trauma 2010, 69, 537–540. [Google Scholar] [CrossRef]
- McCrum, M.L.; Leroux, B.; Fang, T.; Bulger, E.; Arbabi, S.; Wade, C.E.; Fox, E.; Holcomb, J.B.; Robinson, B.; PROPPR Study Group. Sex-based differences in transfusion need after severe injury: Findings of the PROPPR study. Surgery 2019, 165, 1122–1127. [Google Scholar] [CrossRef]
- Al Jalbout, N.; Balhara, K.S.; Hamade, B.; Hsieh, Y.H.; Kelen, G.D.; Bayram, J.D. Shock index as a predictor of hospital admission and inpatient mortality in a US national database of emergency departments. Emerg. Med. J. 2019, 36, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonca, A.; Bruining, H.; Reinhart, C.K.; Suter, P.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Allgower, M.; Burri, C. Shock index. Dtsch. Med. Wochenschr. 1967, 92, 1947–1950. [Google Scholar]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
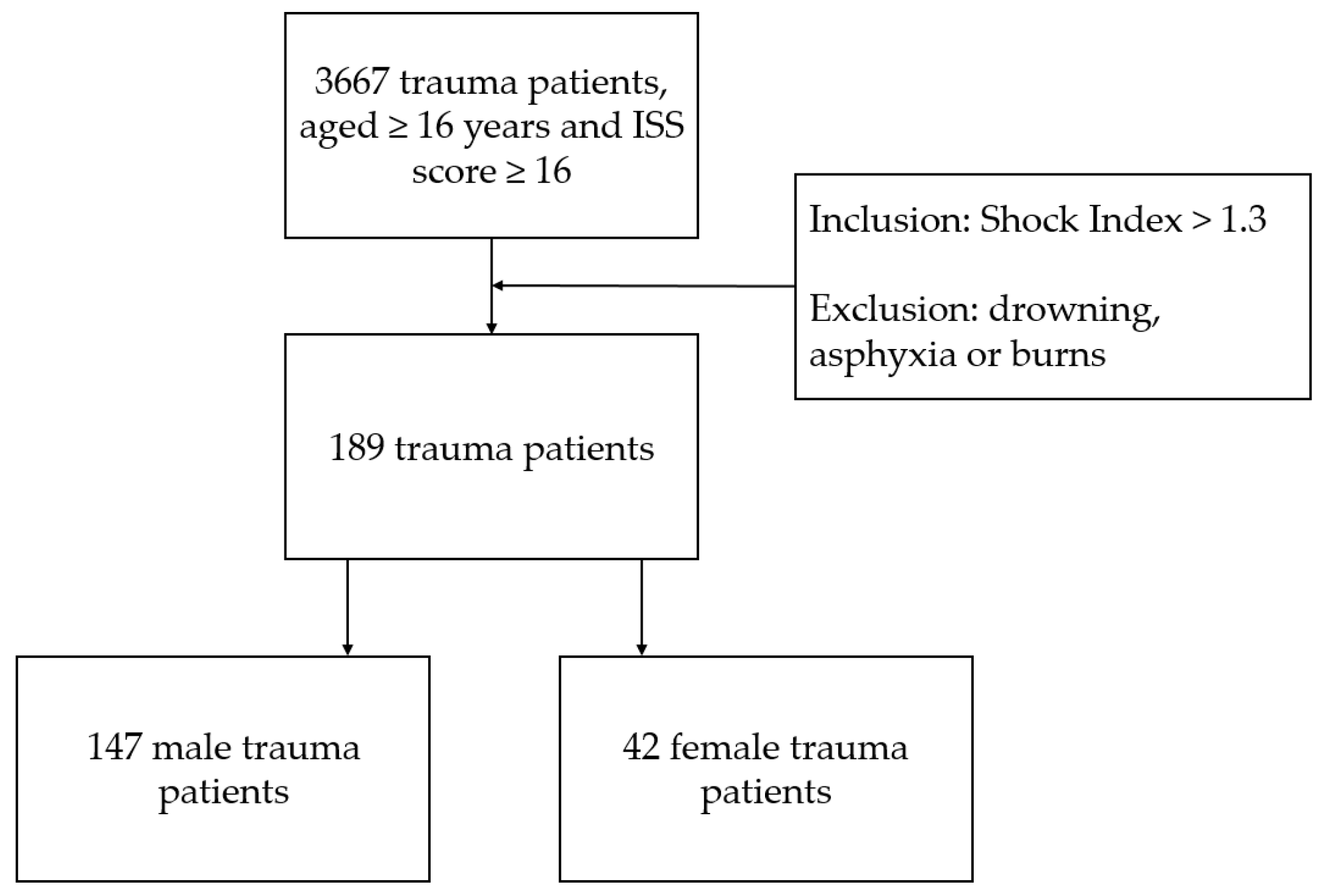
| Total (n = 189) | Male (n = 147) | Female (n = 42) | p-Value | |
|---|---|---|---|---|
| Age | ||||
| Age (years) median (IQR) | 46 (30–62) | 45 (30–60) | 51 (29–65) | 0.107 |
| Age 16–44 years | 88 (46.6%) | 73 (49.7%) | 15 (35.7%) | 0.110 |
| Age 45+ years | 101 (53.4%) | 74 (50.3%) | 27 (64.3%) | 0.110 |
| Injury severity | ||||
| AIS Head ≥ 3 | 96 (50.8%) | 77 (52.4%) | 19 (45.2%) | 0.414 |
| AIS Thorax ≥ 3 | 125 (66.1%) | 98 (66.7%) | 27 (64.3%) | 0.774 |
| AIS Abdomen ≥ 3 | 52 (27.5%) | 38 (25.9%) | 14 (33.3%) | 0.338 |
| AIS Lower extremities ≥ 3 | 73 (38.6%) | 56 (38.1%) | 17 (40.5%) | 0.780 |
| ISS median (IQR) | 34 (25–43) | 34 (25–43) | 29 (22–42) | 0.265 |
| ISS 16–24 | 41 (21.7%) | 27 (18.4%) | 14 (33.3%) | 0.038 |
| ISS 25–50 | 124 (65.6%) | 103 (70.1%) | 21 (50.0%) | 0.016 |
| ISS 51–75 | 23 (12.2%) | 16 (10.9%) | 7 (16.7%) | 0.312 |
| Vital signs, laboratory results and physiologic scoring system | ||||
| RTS median (IQR) | 3.4 (2.6–6.4) | 3.4 (2.6–6.2) | 4.4 (3.2–7.0) | 0.091 |
| RTS < 4 | 85 (45.0%) | 70 (47.6%) | 15 (35.7%) | 0.171 |
| SBP mean ± SD (mmHg) | 77 ± 16.13 | 76 ± 16.67 | 79 ± 13.96 | 0.244 |
| SBP ≤ 89 mmHg | 148 (78.3%) | 116 (78.9%) | 32 (76.2%) | 0.706 |
| Shock Index median (IQR) | 1.6 (1.4–1.8) | 1.6 (1.4–1.9) | 1.5 (1.4–1.8) | 0.236 |
| Base Excess median (IQR) | −9.0 (−15.0—5.0) | −9.0 (−15.4–5.0) | −9.3 (−13.8—4.2) | 0.947 |
| Neurological score | ||||
| GCS on scene median (IQR) | 5 (3–14) | 5 (3–14) | 3 (3–14) | 0.836 |
| GCS at admission median (IQR) | 3 (3–11) | 3 (3–8) | 4 (3–13) | 0.008 |
| GCS ≤ 8 at admission | 127 (67.2%) | 104 (70.7%) | 23 (54.8%) | 0.052 |
| Prehospital | ||||
| Blunt | 165 (87.3%) | 127 (86.4%) | 38 (90.5%) | 0.484 |
| Penetrating | 24 (12.7%) | 20 (13.6%) | 4 (9.5%) | 0.484 |
| Cardiac arrest | 17 (20.2%) | 13 (20.0%) | 4 (21.1%) | 1.000 |
| Prehospital intubation | 125 (67.6%) | 103 (71.0%) | 22 (55.0%) | 0.055 |
| P-HEMS | 90 (47.6%) | 71 (48.3%) | 19 (45.2%) | 0.726 |
| Comorbidity | ||||
| Healthy or mild (ASA I or II) | 125 (66.1%) | 90 (61.2%) | 35 (83.3%) | 0.008 |
| Severe comorbidity (≥ASA III) | 64 (33.9%) | 57 (38.8%) | 7 (16.7%) | 0.008 |
| Total (n = 189) | Male (n = 147) | Female (n = 42) | p-Value | |
|---|---|---|---|---|
| Mortality | ||||
| Mortality at 24 h | 71 (37.6%) | 57 (38.8%) | 14 (33.3%) | 0.521 |
| Mortality until hospital discharge | 94 (49.7%) | 75 (51.0%) | 19 (45.2%) | 0.509 |
| ICU outcomes | ||||
| ICU admission | 154 (81.5%) | 122 (83.0%) | 32 (76.2%) | 0.317 |
| ICU days median (IQR) | 4 (2–12) | 4 (2–12) | 5 (2–12) | 0.642 |
| MV during admission | 130 (68.8%) | 104 (70.7%) | 26 (61.9%) | 0.275 |
| MV duration in days median (IQR) | 3 (1–9) | 3 (1–9) | 2 (1–10) | 0.568 |
| Complications | ||||
| Multiple organ failure | 113 (59.8%) | 90 (61.2%) | 23 (54.8%) | 0.451 |
| Acute kidney injury | 31 (16.4%) | 29 (19.7%) | 2 (4.8%) | 0.021 |
| Wound infection | 18 (9.5%) | 13 (8.8%) | 5 (11.9%) | 0.556 |
| Transfusion | ||||
| pRBCs in 24 h ≥ 1 | 112 (59.3%) | 90 (61.2%) | 22 (54.2%) | 0.304 |
| pRBCs in 24 h median (IQR) | 2 (0–5) | 2 (0–5) | 2 (0–5) | 0.711 |
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Mortality | |||
| Mortality at 24 h | 1.453 | 0.571–3.695 | 0.433 |
| Mortality until hospital discharge | 1.203 | 0.501–2.890 | 0.679 |
| ICU outcomes | |||
| ICU admission | 0.684 | 0.280–1.671 | 0.404 |
| Mechanical ventilation during admission | 0.793 | 0.364–1.729 | 0.560 |
| Complications | |||
| Multiple organ failure | 0.929 | 0.413–2.086 | 0.858 |
| Acute kidney injury | 0.184 | 0.041–0.823 | 0.041 |
| Wound infection | 1.424 | 0.455–4.454 | 0.543 |
| Transfusion | |||
| pRBCs in 24 h ≥ 1 | 0.585 | 0.273–1.255 | 0.169 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Wonderen, S.F.; Pape, M.; Zuidema, W.P.; Edwards, M.J.R.; Verhofstad, M.H.J.; Tromp, T.N.; Van Lieshout, E.M.M.; Bloemers, F.W.; Geeraedts, L.M.G., Jr. Sex Dimorphism in Outcome of Trauma Patients Presenting with Severe Shock: A Multicenter Cohort Study. J. Clin. Med. 2023, 12, 3701. https://doi.org/10.3390/jcm12113701
Van Wonderen SF, Pape M, Zuidema WP, Edwards MJR, Verhofstad MHJ, Tromp TN, Van Lieshout EMM, Bloemers FW, Geeraedts LMG Jr. Sex Dimorphism in Outcome of Trauma Patients Presenting with Severe Shock: A Multicenter Cohort Study. Journal of Clinical Medicine. 2023; 12(11):3701. https://doi.org/10.3390/jcm12113701
Chicago/Turabian StyleVan Wonderen, Stefan F., Merel Pape, Wietse P. Zuidema, Michael J. R. Edwards, Michael H. J. Verhofstad, Tjarda N. Tromp, Esther M. M. Van Lieshout, Frank W. Bloemers, and Leo M. G. Geeraedts, Jr. 2023. "Sex Dimorphism in Outcome of Trauma Patients Presenting with Severe Shock: A Multicenter Cohort Study" Journal of Clinical Medicine 12, no. 11: 3701. https://doi.org/10.3390/jcm12113701
APA StyleVan Wonderen, S. F., Pape, M., Zuidema, W. P., Edwards, M. J. R., Verhofstad, M. H. J., Tromp, T. N., Van Lieshout, E. M. M., Bloemers, F. W., & Geeraedts, L. M. G., Jr. (2023). Sex Dimorphism in Outcome of Trauma Patients Presenting with Severe Shock: A Multicenter Cohort Study. Journal of Clinical Medicine, 12(11), 3701. https://doi.org/10.3390/jcm12113701








