Risk Stratification in Transthyretin Cardiac Amyloidosis: The Added Value of Lung Spirometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Context, Design and Population
2.2. Diagnosis of ATTR Amyloidosis
2.3. Pulmonary Function Testing (PFT)
2.4. Cardiopulmonary Exercise Testing (CPET)
2.5. Follow-Up and Endpoints
2.6. Statistical Analysis
3. Results
3.1. Clinical and Biological Phenotype
3.2. Cardiopulmonary Functional Phenotype
3.3. Risk Profiles
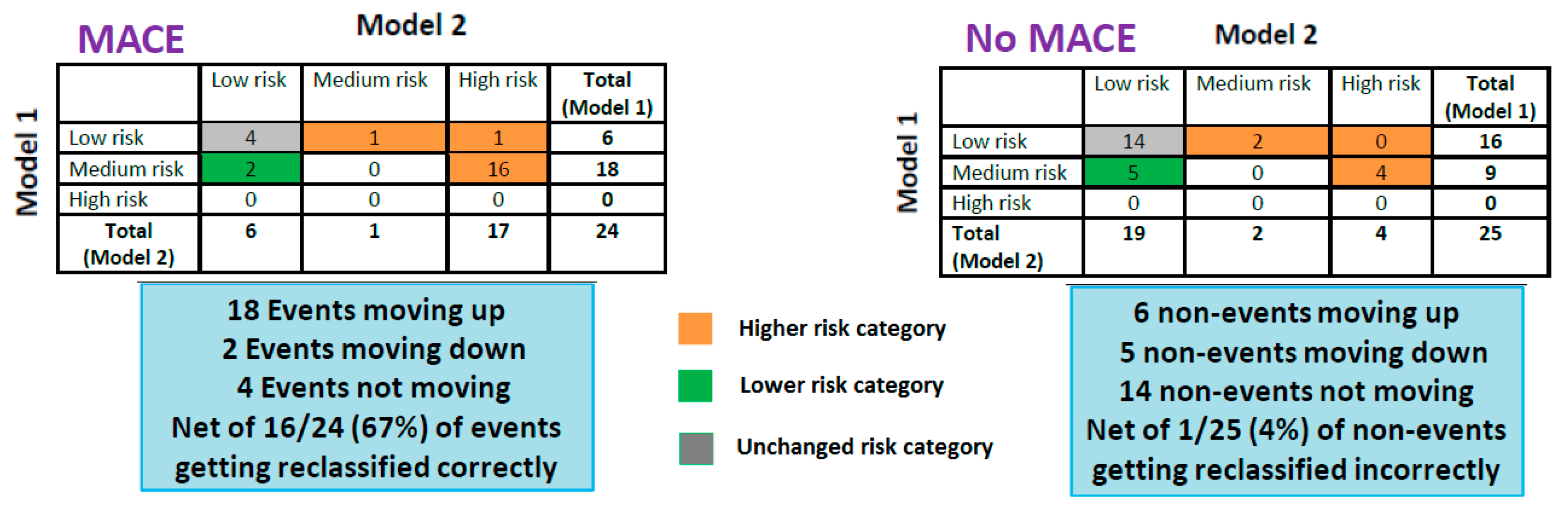
3.4. Prognostic Value of Impaired pVO2 and FVC
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AL | light-chain amyloidosis |
| AT | anaerobic threshold |
| ATTR-CA | transthyretin cardiac amyloidosis |
| ATTRwt | non-mutated transthyretin |
| ATTRv | variant transthyretin |
| AUC | area under the ROC curve |
| BF | breathing frequency |
| BMI | body mass index |
| bpm | beat per minute |
| BF | breathing frequency |
| CI | confidence interval |
| CPET | Cardio Pulmonary Exercise testing |
| DLCO | diffusing capacity for carbon monoxide |
| ECG | Electrocardiogram |
| E/Ea | peak of pulsed Doppler E wave/average peak of annulus tissue Doppler imaging E’ waves |
| eGFR | estimated glomerular filtration rate |
| ECG | electrocardiogram |
| ERS | European Respiratory Society |
| FEV1 | forced expiratory volume in the first second |
| FVC | forced vital capacity |
| GLI | global lung function initiative |
| HR | heart rate OR hazards ratio (resulting from Cox regression analysis) |
| hs | high sensitivity |
| IQR | interquartile range |
| IDI | integrated discrimination improvement |
| IRB | institutional review board |
| IVS | interventricular septum thickness |
| LA | left atrium |
| LVEF | left ventricular ejection fraction |
| LVEDV | left ventricle end diastolic volume |
| LVMI | Left Ventricle Mass Index |
| MACE | composite of all-cause death or heart-failure-related hospitalization |
| MVV | maximal voluntary ventilation |
| NRI | net reclassification improvement |
| NT-proBNP | N-terminal pro-B-type Natriuretic Peptide |
| NYHA | New York Heart Association (NYHA) classification |
| O2 pulse | peak oxygen pulse |
| PAP | pulmonary arterial pressure |
| PFT | Pulmonary Function Testing |
| pVO2 | peak aerobic capacity |
| RER | respiratory exchange ratio |
| ROC | receiver operating characteristic |
| TAPSE | tricuspid annular systolic excursion |
| TDE | E deceleration time |
| TLC | Total Lung Capacity |
| VE | minute ventilation |
| VCO2 | pulmonary carbon dioxide output |
| VO2 | oxygen uptake |
| Vt | tidal volume |
References
- Gertz, M.A.; Dispenzieri, A. Systemic Amyloidosis Recognition, Prognosis, and Therapy: A Systematic Review. JAMA 2020, 324, 79–89. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Bengel, F.; Brito, D.; Damy, T.; Duca, F.; Dorbala, S.; Nativi-Nicolau, J.; Obici, L.; Rapezzi, C.; Sekijima, Y.; et al. Expert consensus on the monitoring of transthyretin amyloid cardiomyopathy. Eur. J. Heart Fail. 2021, 23, 895–905. [Google Scholar] [CrossRef]
- Castiglione, V.; Franzini, M.; Aimo, A.; Carecci, A.; Lombardi, C.M.; Passino, C.; Rapezzi, C.; Emdin, M.; Vergaro, G. Use of biomarkers to diagnose and manage cardiac amyloidosis. Eur. J. Heart Fail. 2021, 23, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Chacko, L.; Karia, N.; Venneri, L.; Bandera, F.; Passo, B.D.; Buonamici, L.; Lazari, J.; Ioannou, A.; Porcari, A.; Patel, R.; et al. Progression of echocardiographic parameters and prognosis in transthyretin cardiac amyloidosis. Eur. J. Heart Fail. 2022, 24, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Nicol, M.; Deney, A.; Lairez, O.; Vergaro, G.; Emdin, M.; Carecci, A.; Inamo, J.; Montfort, A.; Neviere, R.; Damy, T.; et al. Prognostic value of cardiopulmonary exercise testing in cardiac amyloidosis. Eur. J. Heart Fail. 2021, 23, 231–239. [Google Scholar] [CrossRef]
- Hein, S.; Siepen, F.A.D.; Bauer, R.; Katus, H.A.; Kristen, A.V. Peak V’O2 is an independent predictor of survival in patients with cardiac amyloidosis. Amyloid 2018, 25, 167–173. [Google Scholar] [CrossRef]
- Yunis, A.; Doros, G.; Luptak, I.; Connors, L.; Sam, F. Use of Ventilatory Efficiency Slope as a Marker for Increased Mortality in Wild-Type Transthyretin Cardiac Amyloidosis. Am. J. Cardiol. 2019, 124, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Eggleston, R.H.; Hartman, T.E.; Walkoff, L.A.; Yi, E.S.; Ryu, J.H.; Baqir, M. Clinical, radiologic, and pathologic features and outcomes of pulmonary transthyretin amyloidosis. Respir. Med. 2022, 194, 106761. [Google Scholar] [CrossRef]
- Banydeen, R.; Vergaro, G.; Deney, A.; Monfort, A.; Emdin, M.; Lairez, O.; Giguet, A.G.; Inamo, J.; Neviere, R. Restrictive spirometry pattern and abnormal cardiopulmonary response to exercise in transthyretin cardiac amyloidosis. Eur. Respir. J. 2022, 59, 2102838. [Google Scholar] [CrossRef]
- Silverii, M.V.; Argirò, A.; Baldasseroni, S.; Fumagalli, C.; Zampieri, M.; Guerrieri, L.; Bartolini, S.; Mazzoni, C.; Burgisser, C.; Tomberli, A.; et al. Prognostic value of cardiopulmonary exercise testing in patients with transthyretin cardiac amyloidosis. Intern. Emerg. Med. 2023, 18, 585–593. [Google Scholar] [CrossRef]
- Kawakami, R.; Nakada, Y.; Hashimoto, Y.; Ueda, T.; Nakagawa, H.; Nishida, T.; Onoue, K.; Soeda, T.; Watanabe, M.; Saito, Y. Prevalence and Prognostic Significance of Pulmonary Function Test Abnormalities in Hospitalized Patients with Acute Decompensated Heart Failure with Preserved and Reduced Ejection Fraction. Circ. J. 2021, 85, 1426–1434. [Google Scholar] [CrossRef]
- Olson, T.P.; Beck, K.C.; Johnson, B.D. Pulmonary function changes associated with cardiomegaly in chronic heart failure. J. Card. Fail. 2007, 13, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Van Iterson, E.H.; Cho, L.; Tonelli, A.; Finet, J.E.; Laffin, L.J. All-cause mortality predicted by peak oxygen uptake differs depending on spirometry pattern in patients with heart failure and reduced ejection fraction. ESC Heart Fail. 2021, 8, 2731–2740. [Google Scholar] [CrossRef]
- Dorbala, S.; Ando, Y.; Bokhari, S.; Dispenzieri, A.; Falk, R.H.; Ferrari, V.A.; Fontana, M.; Gheysens, O.; Gillmore, J.D.; Glaudemans, A.W.J.M.; et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI Expert Consensus Recommendations for Multimodality Imaging in Cardiac Amyloidosis: Part 2 of 2-Diagnostic Criteria and Appropriate Utilization. Circ. Cardiovasc. Imaging 2021, 14, e000030. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Damy, T.; FontAna, M.; Hutchinson, M.; Lachmann, H.J.; Martinez-Naharro, A.; Quarta, C.C.; Rezk, T.; Whelan, C.J.; Gonzalez-Lopez, E.; et al. A new staging system for cardiac transthyretin amyloidosis. Eur. Heart J. 2018, 39, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Guerra, S.; Sherrill, D.L.; Venker, C.; Ceccato, C.M.; Halonen, M.; Martinez, F.D. Morbidity and mortality associated with the restrictive spirometric pattern: A longitudinal study. Thorax 2010, 65, 499–504. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- American Thoracic Society, American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef]
- Kennedy, K.F.; Pencina, M.J. A SAS® Macro to Compute Added Predictive Ability of New Markers Predicting a Dichotomous Outcome. 2010. Available online: https://analytics.ncsu.edu/sesug/2010/SDA07.Kennedy.pdf (accessed on 31 August 2022).
- Guo, C. Evaluating Predictive Accuracy of Survival Models with PROC PHREG. 2022. Available online: https://www.lexjansen.com/phuse/2021/as/PAP_AS04.pdf (accessed on 31 August 2022).
- Roxane, D.; Lotte, J. Evaluating Predictive Accuracy of Survival Models with PROC PHREG. SAS. Available online: https://support.sas.com/resources/papers/proceedings17/SAS0462-2017.pdf (accessed on 31 August 2022).
- Khoor, A.; Colby, T.V. Amyloidosis of the lung. Arch. Pathol. Lab. Med. 2017, 141, 247–254. [Google Scholar] [CrossRef]
- Milani, P.; Basset, M.; Russo, F.; Foli, A.; Palladini, G.; Merlini, G. The lung in amyloidosis. Eur. Respir. Rev. 2017, 26, 170046. [Google Scholar] [CrossRef] [PubMed]
- Ussavarungsi, K.; Yi, E.S.; Maleszewski, J.J.; Kurtin, P.J.; Dasari, S.; Theis, J.D.; Dispenzieri, A.; Ryu, J.H. Clinical relevance of pulmonary amyloidosis: An analysis of 76 autopsy derived cases. Eur. Respir. J. 2017, 49, 1602313. [Google Scholar] [CrossRef] [PubMed]
- Revelo, A.E.; Magaspi, C.; Maguire, G.; Aronow, W.S. Hereditary amyloidosis with recurrent lung infiltrates. Am. J. Case Rep. 2016, 17, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Abe, R.; Katoh, N.; Takahashi, Y.; Takasone, K.; Yoshinaga, T.; Yazaki, M.; Kametani, F.; Sekijima, Y. Distribution of amyloidosis subtypes based on tissue biopsy site—Consecutive analysis of 729 patients at a single amyloidosis center in Japan. Pathol. Int. 2021, 71, 70–79. [Google Scholar] [CrossRef]
- Dubé, B.P.; Agostoni, P.; Laveneziana, P. Exertional dyspnoea in chronic heart failure: The role of the lung and respiratory mechanical factors. Eur. Respir. Rev. 2016, 25, 317–332. [Google Scholar] [CrossRef]
- Joho, S.; Ushijima, R.; Akabane, T.; Hirai, T.; Inoue, H. Restrictive lung function is related to sympathetic hyperactivity in patients with heart failure. J. Card. Fail. 2017, 23, 96–103. [Google Scholar] [CrossRef]
- Rapezzi, C.; Quarta, C.C.; Obici, L.; Perfetto, F.; Longhi, S.; Salvi, F.; Biagini, E.; Lorenzini, M.; Grigioni, F.; Leone, O.; et al. Disease profile and differential diagnosis of hereditary transthyretin-related amyloidosis with exclusively cardiac phenotype: An Italian perspective. Eur. Heart J. 2013, 34, 520–528. [Google Scholar] [CrossRef]


| All Patients (n = 82) | No MACE (n = 51) | MACE (n = 31) | p-Value | |
|---|---|---|---|---|
| Age, years | 70 ± 11 | 71 ± 11 | 68 ± 10 | 0.258 |
| Sex (Male gender) | 73 (89) | 45 (88) | 28 (90) | 1.000 |
| BMI, kg/m2 | 26 ± 4 | 26 ± 5 | 25 ± 4 | 0.497 |
| Type 2 Diabetes | 15 (18) | 10 (20) | 5 (16) | 0.693 |
| Hypertension | 37 (45) | 19 (37) | 18 (58) | 0.066 |
| Carpal tunnel | 46 (56) | 30 (59) | 16 (52) | 0.524 |
| NYHA III/IV n = 77 | 52 (68) | 29 (62) | 23 (77) | 0.171 |
| Non-sinusal rhythm | 27 (33) | 13 (25) | 14 (45) | 0.066 |
| ECG/Echocardiography | ||||
| QS wave n = 72 | 19 (26) | 10 (23) | 9 (31) | 0.463 |
| Low QRS voltage n = 78 | 11 (14) | 6 (12) | 5 (17) | 0.738 |
| IVS thickness, mm n = 79 | 17 ± 4 | 16 ± 3 | 19 ± 3 | <0.001 |
| LVEDV, mL n = 61 | 101 ± 40 | 101 ± 43 | 100 ± 36 | 0.478 |
| LVMI, g/m2 n = 74 | 170 ± 52 | 154 ±51 | 194 ± 45 | 0.002 |
| LVEF, % n = 80 | 49 ± 15 | 51 ± 15 | 46 ± 15 | 0.106 |
| Cardiac index, L/min/m2 n = 71 | 2.0 ± 0.5 | 2.1 ± 0.5 | 1.9 ± 0.4 | 0.245 |
| LA diameter, mm n = 41 | 52 ± 11 | 52 ± 11 | 53 ± 10 | 0.448 |
| E/Ea n = 47 | 2 ± 2 | 2 ± 2 | 2 ± 1 | 0.479 |
| E/e’ ratio n = 72 | 16 ± 7 | 14 ± 6 | 18 ± 8 | 0.095 |
| TDE, msec n = 56 | 156 ± 41 | 154 ± 43 | 157 ± 38 | 0.419 |
| Systolic PAP, mmHg n = 30 | 38 ± 12 | 37 ± 12 | 40 ± 11 | 0.595 |
| TAPSE, mm n = 28 | 18 ± 4 | 19 ± 4 | 18 ± 6 | 1.000 |
| Biological parameters | ||||
| eGFR, mL/min/1.72 m2 n = 78 | 69 [9–169] | 73 [11–169] | 55 [9–162] | 0.103 |
| Cardiactroponin T, ng/L n = 60 | 73 [7–566] | 58 [7–376] | 109 [22–566] | 0.030 |
| NT-proBNP, ng/L n = 53 | 2684 [50–15,770] | 1736 [50–5766] | 3442 [1154–15,770] | 0.004 |
| ATTR Biomarker staging II + III vs. I n = 53 * | 30 (57) vs. 23 (43) | 12 (41) vs. 17 (59) | 18 (75) vs. 6 (25) | 0.014 |
| All Patients (n = 82) | No MACE (n = 51) | MACE (n = 31) | p-Value | |
|---|---|---|---|---|
| FEV1, % predicted * | 76.6 ± 17.0 | 80.8 ± 15.2 | 69.8 ± 17.8 | 0.038 |
| FVC, % predicted * | 77.8 ± 17.4 | 82.0 ± 15.5 | 70.8 ± 18.4 | 0.113 |
| FVC < 70 % predicted † | 25 (30.5) | 9 (17.7) | 16 (51.6) | 0.001 |
| FEV1/FVC, % n = 80 | 77.7 ± 9.4 | 77.4 ± 10.2 | 78.2 ± 8.0 | 1.000 |
| TLC, % predicted * n = 41 | 75.8 ± 16.3 | 75.1 ± 17.4 | 78.8 ± 10.8 | 0.939 |
| DLCO, % n = 40 | 78.0 ± 17.6 | 76.3 ± 19.0 | 81.5 ± 14.6 | 0.732 |
| Peak workload, watts n = 53 | 75.8 ± 30.3 | 80.1 ± 32.1 | 66.8 ± 24.2 | 0.164 |
| Peak workload, % n = 49 | 56.4 ± 19.2 | 59.4 ± 18.7 | 50.3 ± 19.3 | 0.087 |
| Peak VO2, mL.kg−1.min−1 n = 74 | 15.0 ± 3.8 | 16.1 ± 3.7 | 13.3 ± 3.3 | 0.001 |
| Predicted peak VO2, % n = 76 | 60.5 ± 18.9 | 67.0 ± 17.6 | 51.0 ± 16.6 | <0.001 |
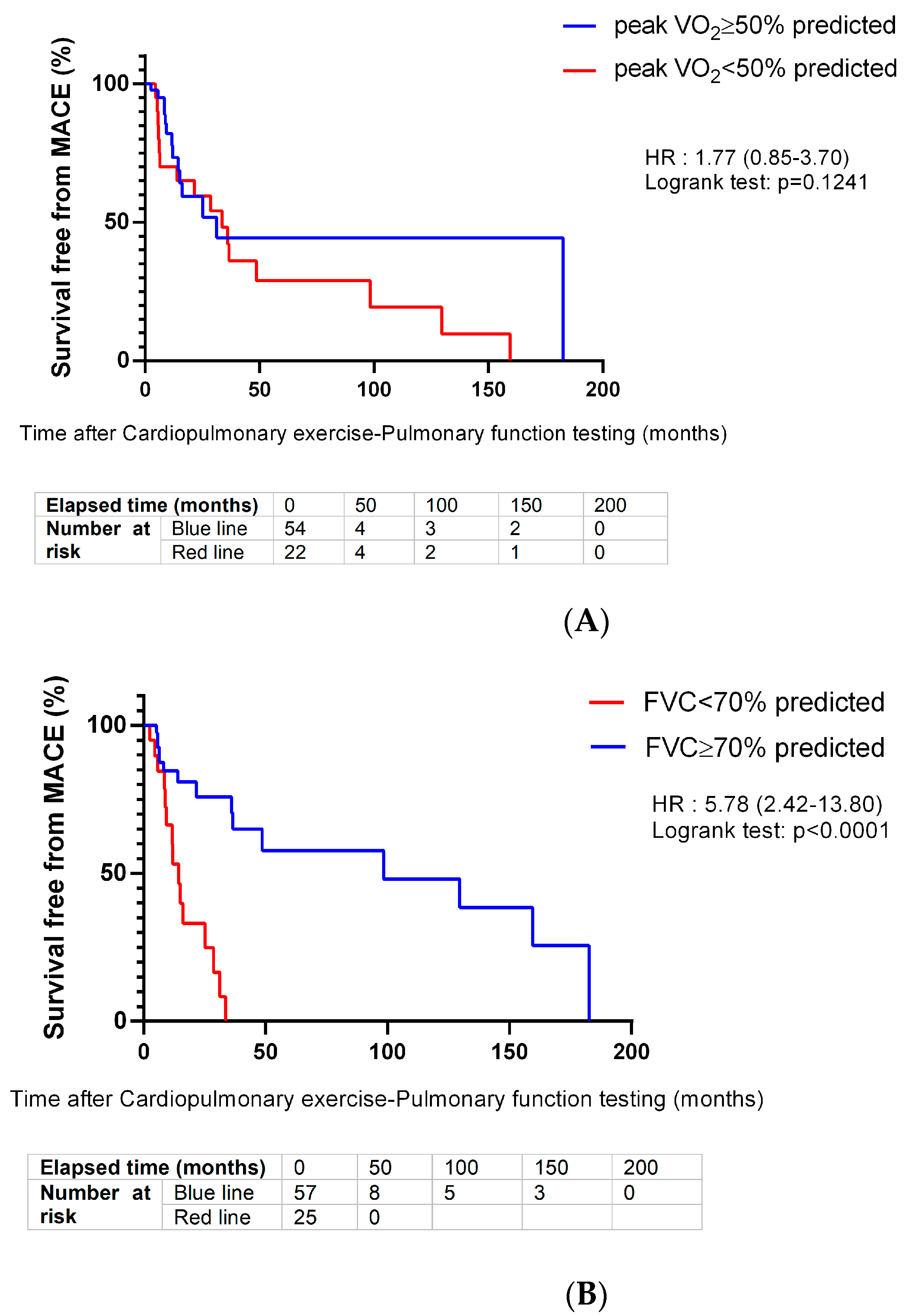
| Peak VO2 < 50% predicted † n = 76 | 22 (29.0) | 5 (11.1) | 17 (54.8) | <0.001 |
| ATVO2, mL.kg−1.min−1 n = 48 | 11.1 ± 3.2 | 11.6 ± 3.5 | 10.1 ± 2.4 | 0.069 |
| Peak VO2/watt slope, mL.watt−1 n = 53 | 10.2 ± 2.4 | 9.6 ± 1.9 | 11.5 ± 3.0 | 0.333 |
| Peak RER n = 75 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.2 | 0.561 |
| Peak VE/VO2 n = 67 | 45.1 ± 10.1 | 43.1 ± 9.7 | 47.7 ± 10.2 | 0.019 |
| Peak VE/VCO2 n = 67 | 38.5 ± 6.6 | 37.4 ± 6.2 | 40.1 ± 7.0 | 0.277 |
| Peak BF, min−1 n = 67 | 37.6 ± 16.8 | 36.2 ± 9.9 | 39.6 ± 23.4 | 0.552 |
| Peak Vt/FVC, % n = 65 | 51.6 ± 14.3 | 51.7 ± 13.4 | 51.4 ± 15.8 | 0.394 |
| Ventilatory reserve, % n = 51 | 31.5 ± 18.4 | 34.3 ± 17.6 | 25.8 ± 19.1 | 0.170 |
| VE VCO2 slope n = 62 | 39.2 ± 7.8 | 37.7 ± 7.9 | 42.6 ± 6.6 | 0.003 |
| Peak O2 pulse, % n = 51 | 72.9 ± 19.1 | 74.1 ± 20.3 | 70.5 ± 16.8 | 0.170 |
| Systolic pressure at rest, mmHg n = 68 | 128.4 ± 23.6 | 129.7 ± 18.4 | 126.7 ± 29.4 | 0.465 |
| Peak systolic pressure, mmHg n = 73 | 156.2 ± 38.5 | 162.4 ± 34.8 | 147.4 ± 42.2 | 0.187 |
| Diastolic pressure at rest, mmHg n = 68 | 80.3 ± 14.8 | 80.6 ± 12.7 | 79.8 ± 17.5 | 0.687 |
| Peak diastolic pressure, mmHg n = 73 | 86.1 ± 22.4 | 87.2 ± 21.6 | 84.5 ± 23.9 | 0.187 |
| Heart rate at rest, bpmn = 75 | 79.9 ± 13.4 | 80.2 ± 14.2 | 79.6 ± 12.3 | 0.793 |
| Peak heart rate, bpmn = 75 | 122.2 ± 21.3 | 123.5 ± 22.6 | 120.4 ± 19.4 | 0.703 |
| Peak heart rate, % maximal pred. n = 75 | 81.1 ± 13.4 | 82.2 ± 13.2 | 79.4 ± 13.8 | 0.925 |
| Heart rate reserve used, % n = 75 | 61.5 ± 26.1 | 64.2 ± 25.8 | 57.6 ± 26.4 | 0.708 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age at CPET-PFT, years | 0.98 (0.94–1.02) | 0.32 | ||
| Male gender | 1.24 (0.29–5.38) | 0.77 | ||
| BMI kg/m2 | 0.97 (0.87–1.07) | 0.50 | ||
| NYHA III/IV | 2.04 (0.73–5.71) | 0.18 | ||
| Non-sinusal rhythm, % | 2.41 (0.93–6.21) | 0.07 | ||
| Carpal tunnel, % | 0.75 (0.30–1.83) | 0.52 | ||
| Hypertension, % | 2.33 (0.94–5.80) | 0.07 | ||
| Diabetes, % | 0.79 (0.24–2.57) | 0.69 | ||
| eGFR, mL/min/1.72 m2 | 0.98 (0.97–1.00) | 0.02 ‡ | ||
| Cardiac troponin T, ng/L | 1.006 (1.002–1.011) | 0.01 ‡ | ||
| NT-proBNP, ng/L | 1.001 (1.000–1.001) | <0.01 ‡ | ||
| ATTR Biomarker staging: Stage II + III vs. Stage I * | 4.25 (1.30–13.87) | 0.02 ‡ | ||
| IVS thickness, mm | 1.26 (1.09–1.46) | <0.01 ‡ | 1.23 (1.00–1.50) | 0.05 |
| LVEF, % | 0.98 (0.95–1.01) | 0.15 | ||
| Systolic PAP, mmHg | 1.02 (0.95–1.09) | 0.58 | ||
| FEV1, % predicted † | <0.01 ‡ | |||
| <70 | 4.38 (1.60–11.98) | |||
| ≥70 | reference | |||
| FVC, % predicted † | <0.01 ‡ | 16.17 (3.47–75.48) | <0.01 | |
| <70 | 4.98 (1.82–13.63) | |||
| ≥70 | reference | |||
| Peak VO2, % predicted † | <0.01 ‡ | 18.27 (3.73–89.48) | <0.01 | |
| <50 | 9.71 (3.02–31.24) | |||
| ≥50 | reference | |||
| Ventilatory reserve, % | 0.97 (0.94–1.01) | 0.12 | ||
| VE VCO2 slope | 1.09 (1.01–1.18) | 0.03 ‡ | ||
| Peak O2 pulse, % | 0.99 (0.96–1.02) | 0.53 | ||
| Peak heart rate, % maximal predicted | 0.99 (0.95–1.02) | 0.39 | ||
| Heart rate reserve used, % | 0.99 (0.97–1.01) | 0.28 | ||
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age, years | 1.04 (1.00–1.08) | 0.07 ‡ | ||
| Male gender | 1.37 (0.32–5.89) | 0.67 | ||
| BMI kg/m2 | 0.92 (0.83–1.02) | 0.09 ‡ | ||
| NYHA III/IV | 0.74 (0.31–1.76) | 0.49 | ||
| Non-sinusal rhythm,% | 1.52 (0.74–3.13) | 0.25 | ||
| Hypertension, % | 0.67 (0.31–1.44) | 0.31 | ||
| Type 2 diabetes, % | 2.34 (0.87–6.35) | 0.09 ‡ | ||
| eGFR, mL/min/1.72 m2 | 1.00 (0.99–1.01) | 0.78 | ||
| Cardiactroponin T, ng/L | 1.00 (1.00–1.01) | 0.06 ‡ | ||
| NT-proBNP, ng/L | 1.00 (1.00–1.00) | 0.02 ‡ | ||
| ATTR Biomarker staging: Stage II + III vs. Stage I * | 1.66 (0.65–4.24) | 0.29 | ||
| IVS thickness, mm | 1.04 (0.94–1.16) | 0.42 | ||
| LVEF, % | 0.99 (0.97–1.02) | 0.55 | ||
| Systolic PAP, mmHg | 0.99 (0.94–1.05) | 0.79 | ||
| FEV1, % predicted † | 0.01 ‡ | |||
| <70 | 2.58 (1.20–5.54) | |||
| ≥70 | reference | |||
| FVC, % predicted † | <0.01 ‡ | 7.01 (2.92–16.82) | <0.01 | |
| <70 | 5.78 (2.42–13.80) | |||
| ≥70 | reference | |||
| PeakVO2, % predicted † | 0.13 ‡ | 2.48 (1.15–5.35) | 0.02 | |
| <50 | 1.77 (0.85–3.70) | |||
| ≥50 | reference | |||
| Ventilatory reserve, % | 0.99 (0.95–1.02) | 0.37 | ||
| VE VCO2 slope | 1.05 (1.00–1.11) | 0.05 ‡ | ||
| Peak O2 pulse, % | 0.99 (0.96–1.01) | 0.32 | ||
| Peak heart rate, % maximal predicted | 1.00 (0.97–1.03) | 0.82 | ||
| Heart rate reserve used, % | 1.00 (0.99–1.01) | 0.95 | ||
| Harrell’s C-Index | Integrated AUC | |
|---|---|---|
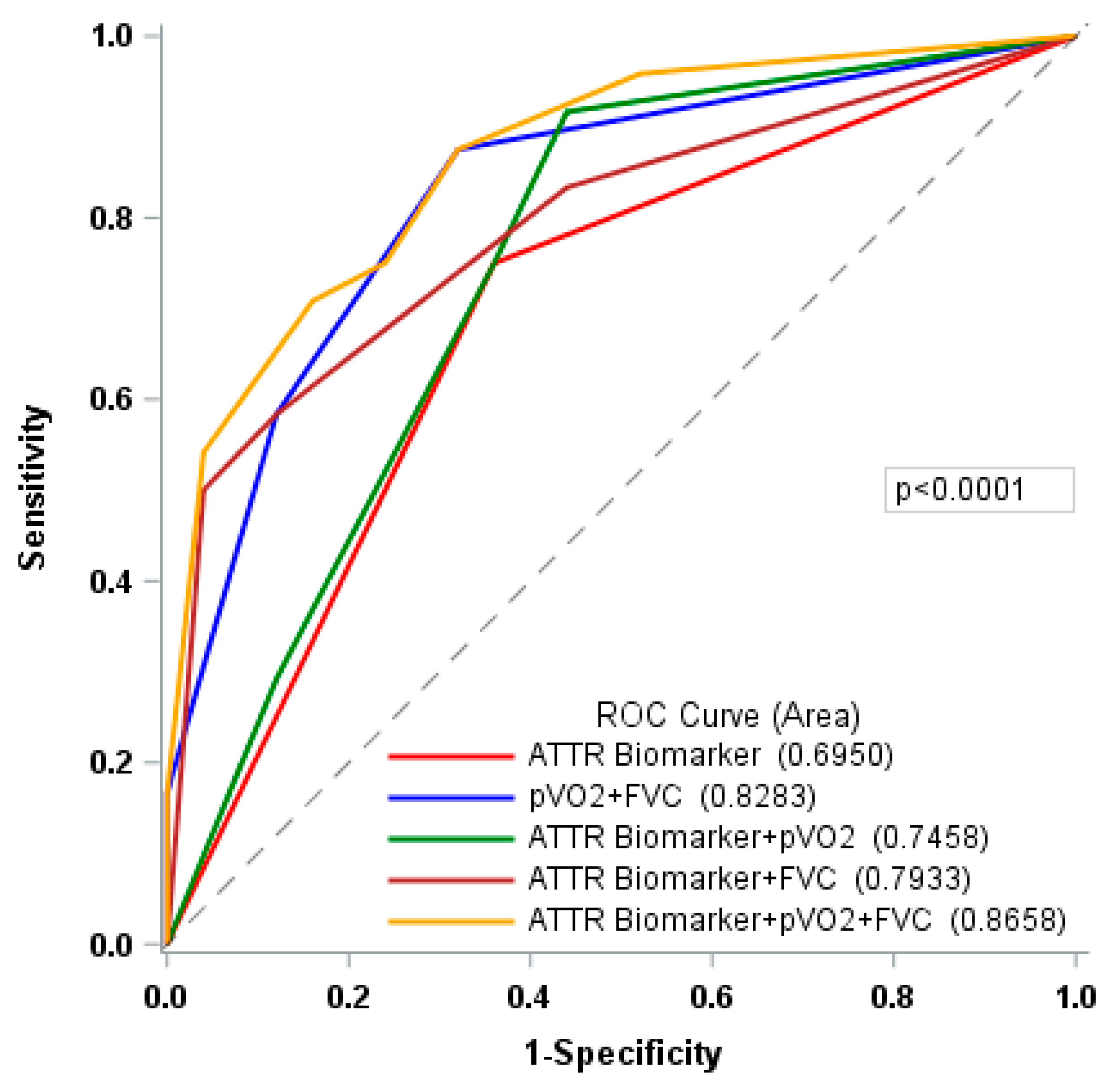
| ATTR Biomarker | 0.5675 | 0.4341 |
| pVO2 + FVC | 0.7228 | 0.6769 |
| ATTR Biomarker + pVO2 | 0.6127 | 0.4775 |
| ATTR Biomarker + FVC | 0.6393 | 0.5265 |
| ATTR Biomarker + pVO2 + FVC | 0.7039 | 0.5770 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banydeen, R.; Eggleston, R.; Deney, A.; Monfort, A.; Ryu, J.H.; Vergaro, G.; Castiglione, V.; Lairez, O.; Emdin, M.; Inamo, J.; et al. Risk Stratification in Transthyretin Cardiac Amyloidosis: The Added Value of Lung Spirometry. J. Clin. Med. 2023, 12, 3684. https://doi.org/10.3390/jcm12113684
Banydeen R, Eggleston R, Deney A, Monfort A, Ryu JH, Vergaro G, Castiglione V, Lairez O, Emdin M, Inamo J, et al. Risk Stratification in Transthyretin Cardiac Amyloidosis: The Added Value of Lung Spirometry. Journal of Clinical Medicine. 2023; 12(11):3684. https://doi.org/10.3390/jcm12113684
Chicago/Turabian StyleBanydeen, Rishika, Reid Eggleston, Antoine Deney, Astrid Monfort, Jay H. Ryu, Giuseppe Vergaro, Vincenzo Castiglione, Olivier Lairez, Michele Emdin, Jocelyn Inamo, and et al. 2023. "Risk Stratification in Transthyretin Cardiac Amyloidosis: The Added Value of Lung Spirometry" Journal of Clinical Medicine 12, no. 11: 3684. https://doi.org/10.3390/jcm12113684
APA StyleBanydeen, R., Eggleston, R., Deney, A., Monfort, A., Ryu, J. H., Vergaro, G., Castiglione, V., Lairez, O., Emdin, M., Inamo, J., Baqir, M., & Neviere, R. (2023). Risk Stratification in Transthyretin Cardiac Amyloidosis: The Added Value of Lung Spirometry. Journal of Clinical Medicine, 12(11), 3684. https://doi.org/10.3390/jcm12113684







