Blood Clots May Compromise Intracranial Pressure Measurement Using Air-Pouch Intracranial Pressure Probes
Abstract
1. Introduction
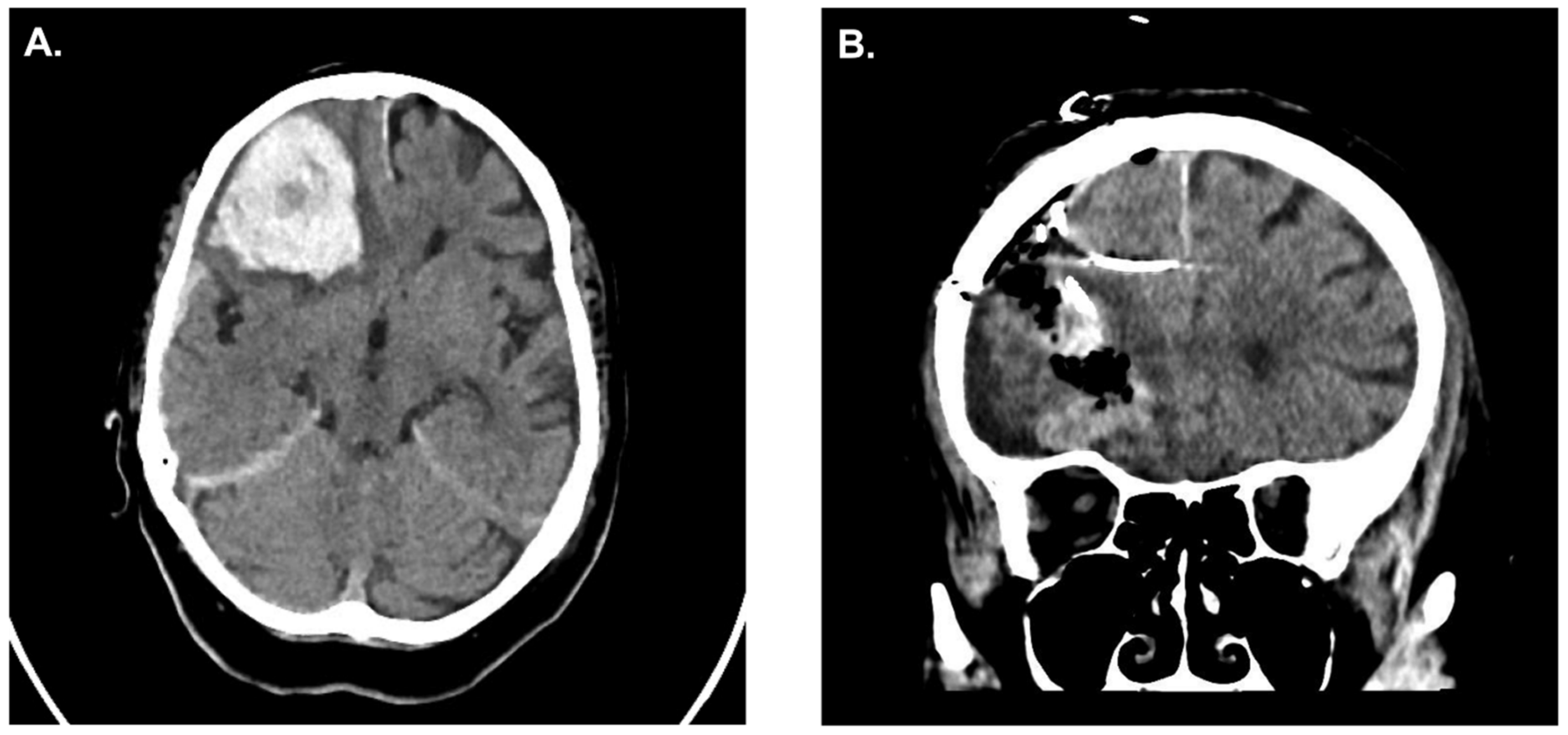
Case Illustration
2. Methods
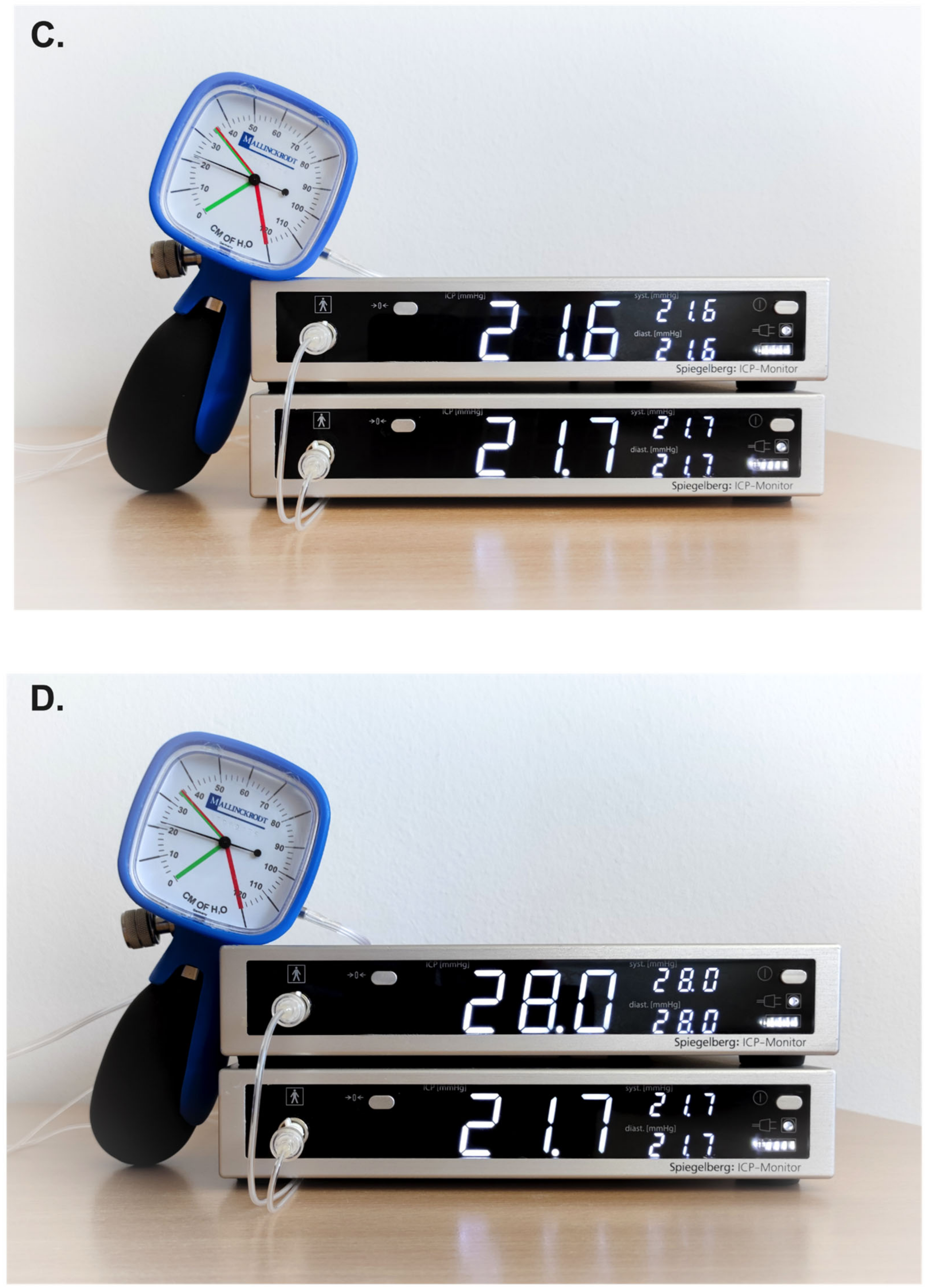
2.1. Experiment Setting
2.2. Study Design
2.3. Translation of Experimental Data (Pilot Clinical Case)
2.4. Statistical Analyses
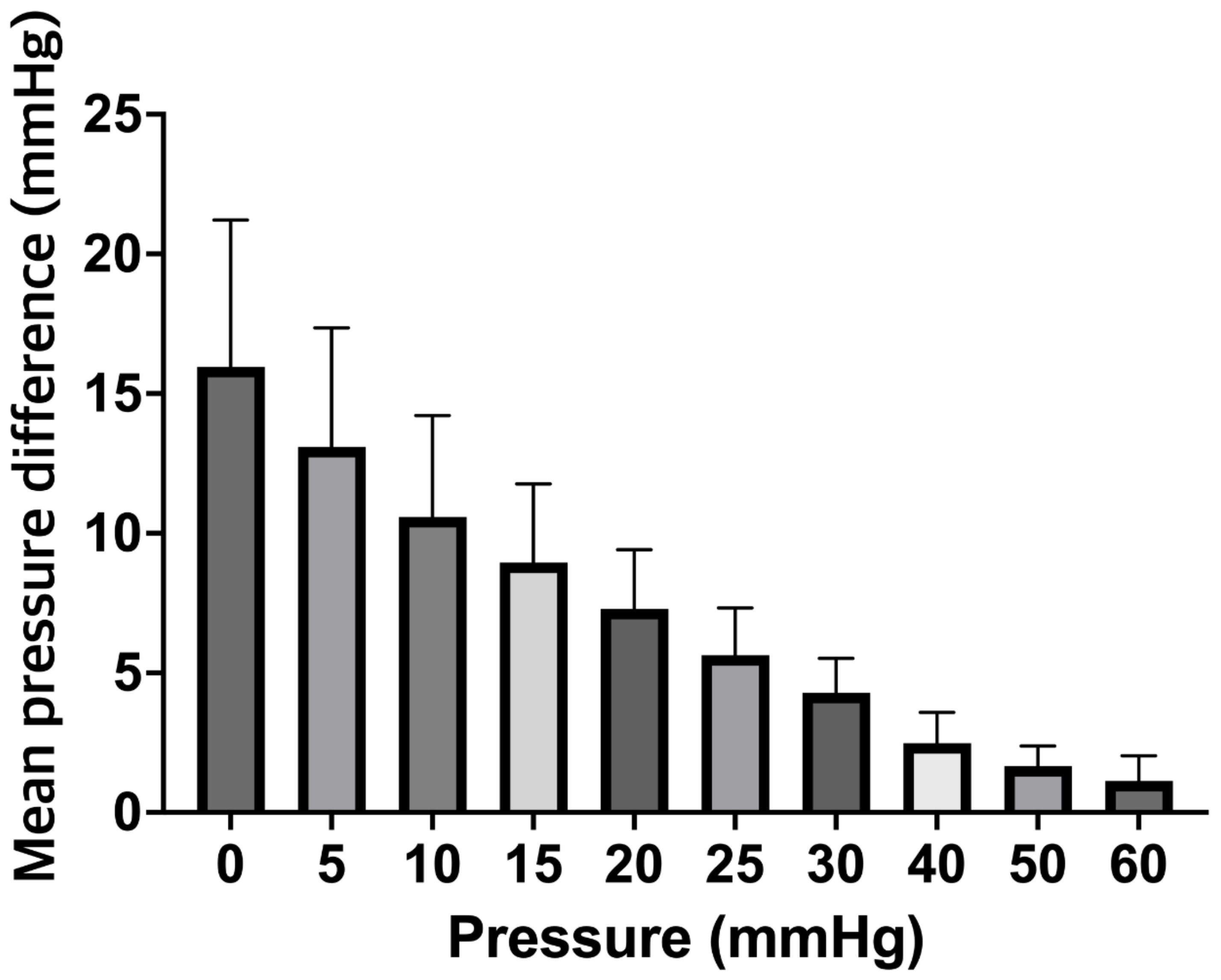
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.J.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef]
- Hawryluk, G.W.J.; Citerio, G.; Hutchinson, P.; Kolias, A.; Meyfroidt, G.; Robba, C.; Stocchetti, N.; Chesnut, R. Intracranial pressure: Current perspectives on physiology and monitoring. Intensive Care Med. 2022, 48, 1471–1481. [Google Scholar] [CrossRef]
- Castaño-Leon, A.M.; Gomez, P.A.; Jimenez-Roldan, L.; Paredes, I.; Munarriz, P.M.; Perez, I.P.; Eiriz Fernandez, C.; García-Pérez, D.; Moreno Gomez, L.M.; Sinovas, O.E.; et al. Intracranial Pressure Monitoring in Patients With Severe Traumatic Brain Injury: Extension of the Recommendations and the Effect on Outcome by Propensity Score Matching. Neurosurgery 2022, 91, 437–449. [Google Scholar] [CrossRef]
- Bales, J.W.; Bonow, R.H.; Buckley, R.T.; Barber, J.; Temkin, N.; Chesnut, R.M. Primary External Ventricular Drainage Catheter Versus Intraparenchymal ICP Monitoring: Outcome Analysis. Neurocrit. Care 2019, 31, 11–21. [Google Scholar] [CrossRef]
- Liu, H.; Wang, W.; Cheng, F.; Yuan, Q.; Yang, J.; Hu, J.; Ren, G. External Ventricular Drains versus Intraparenchymal Intracranial Pressure Monitors in Traumatic Brain Injury: A Prospective Observational Study. World Neurosurg. 2015, 83, 794–800. [Google Scholar] [CrossRef]
- Volovici, V.; Huijben, J.A.; Ercole, A.; Stocchetti, N.; Dirven, C.M.F.; van der Jagt, M.; Steyerberg, E.W.; Lingsma, H.F.; Menon, D.K.; Maas, A.I.R.; et al. Ventricular Drainage Catheters versus Intracranial Parenchymal Catheters for Intracranial Pressure Monitoring-Based Management of Traumatic Brain Injury: A Systematic Review and Meta-Analysis. J. Neurotrauma 2019, 36, 988–995. [Google Scholar] [CrossRef]
- Zacchetti, L.; Magnoni, S.; Di Corte, F.; Zanier, E.R.; Stocchetti, N. Accuracy of intracranial pressure monitoring: Systematic review and meta-analysis. Crit. Care 2015, 19, 420. [Google Scholar] [CrossRef]
- Lang, J.-M.; Beck, J.; Zimmermann, M.; Seifert, V.; Raabe, A. Clinical Evaluation of Intraparenchymal Spiegelberg Pressure Sensor. Neurosurgery 2003, 52, 1455–1459. [Google Scholar] [CrossRef]
- Yau, Y.H.; Piper, I.R.; Clutton, R.E.; Whittle, I.R. Experimental evaluation of the Spiegelberg intracranial pressure and intracranial compliance monitor. Technical note. J. Neurosurg. 2000, 93, 1072–1077. [Google Scholar] [CrossRef]
- Lindblad, C.; Raj, R.; Zeiler, F.A.; Thelin, E.P. Current state of high-fidelity multimodal monitoring in traumatic brain injury. Acta Neurochir. 2022, 164, 3091–3100. [Google Scholar] [CrossRef]
- Talving, P.; Karamanos, E.; Teixeira, P.G.; Skiada, D.; Lam, L.; Belzberg, H.; Inaba, K.; Demetriades, D. Intracranial pressure monitoring in severe head injury: Compliance with Brain Trauma Foundation guidelines and effect on outcomes: A prospective study. J. Neurosurg. 2013, 119, 1248–1254. [Google Scholar] [CrossRef]
- Piper, I.; Barnes, A.; Smith, D.; Dunn, L. The Camino intracranial pressure sensor: Is it optimal technology? An internal audit with a review of current intracranial pressure monitoring technologies. Neurosurgery 2001, 49, 1158–1164; discussion 1164–1165. [Google Scholar] [CrossRef]
- Fernandes, H.M.; Bingham, K.; Chambers, I.R.; Mendelow, A.D. Clinical evaluation of the Codman microsensor intracranial pressure monitoring system. Acta Neurochir. Suppl. 1998, 71, 44–46. [Google Scholar] [CrossRef]
- Morgalla, M.H.; Mettenleiter, H.; Bitzer, M.; Fretschner, R.; Grote, E.H. ICP measurement control: Laboratory test of 7 types of intracranial pressure transducers. J. Med. Eng. Technol. 1999, 23, 144–151. [Google Scholar] [CrossRef]
- Chambers, I.R.; Siddique, M.S.; Banister, K.; Mendelow, A.D. Clinical comparison of the Spiegelberg parenchymal transducer and ventricular fluid pressure. J. Neurol. Neurosurg. Psychiatry 2001, 71, 383–385. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, S.-Y.; Herrmann, S.; Dubinski, D.; Behmanesh, B.; Trnovec, S.; Dinc, N.; Bernstock, J.D.; Freiman, T.M.; Gessler, F.A. Blood Clots May Compromise Intracranial Pressure Measurement Using Air-Pouch Intracranial Pressure Probes. J. Clin. Med. 2023, 12, 3661. https://doi.org/10.3390/jcm12113661
Won S-Y, Herrmann S, Dubinski D, Behmanesh B, Trnovec S, Dinc N, Bernstock JD, Freiman TM, Gessler FA. Blood Clots May Compromise Intracranial Pressure Measurement Using Air-Pouch Intracranial Pressure Probes. Journal of Clinical Medicine. 2023; 12(11):3661. https://doi.org/10.3390/jcm12113661
Chicago/Turabian StyleWon, Sae-Yeon, Sascha Herrmann, Daniel Dubinski, Bedjan Behmanesh, Svorad Trnovec, Nazife Dinc, Joshua D. Bernstock, Thomas M. Freiman, and Florian A. Gessler. 2023. "Blood Clots May Compromise Intracranial Pressure Measurement Using Air-Pouch Intracranial Pressure Probes" Journal of Clinical Medicine 12, no. 11: 3661. https://doi.org/10.3390/jcm12113661
APA StyleWon, S.-Y., Herrmann, S., Dubinski, D., Behmanesh, B., Trnovec, S., Dinc, N., Bernstock, J. D., Freiman, T. M., & Gessler, F. A. (2023). Blood Clots May Compromise Intracranial Pressure Measurement Using Air-Pouch Intracranial Pressure Probes. Journal of Clinical Medicine, 12(11), 3661. https://doi.org/10.3390/jcm12113661






