Submacular Hemorrhages Show No Significant Seasonal Variations in a European Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Data Sources and Measurements
2.3. Primary and Secondary Objectives
- ▪ Small: hemorrhage size equals 1–≤4 disc diameters
- ▪ Medium: hemorrhage size equals >4 disc diameters, not extending beyond the vascular arcades
- ▪ Large: hemorrhage extends beyond the temporal vascular arcades, but not past the equator
- ▪ Massive: hemorrhage extends past the equator in at least two quadrants
2.4. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Primary Outcome
3.3. Secondary Outcome
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berrocal, M.H.; Lewis, M.L.; Flynn, H.W. Variations in the Clinical Course of Submacular Hemorrhage. Am. J. Ophthalmol. 1996, 122, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, H.; Williams, D.F.; Thomas, M.A.; Ruby, A.J.; Meredith, T.A.; Boniuk, I.; Grand, M.G. Surgical Management of Submacular Hemorrhage. Arch. Ophthalmol. 1995, 113, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.L.; Bunce, C.; Desai, R.; Hillenkamp, J.; Lee, C.N.; Lois, N.; Peto, T.; Reeves, B.C.; Steel, D.H.; Edwards, R.T.; et al. Vitrectomy, subretinal Tissue plasminogen activator and Intravitreal Gas for submacular haemorrhage secondary to Exudative Age-Related macular degeneration (TIGER): Study protocol for a phase 3, pan-European, two-group, non-commercial, active-control, observer-masked, superiority, randomised controlled surgical trial. Trials 2022, 23, 99. [Google Scholar] [CrossRef]
- Scupola, A.; Coscas, G.; Soubrane, G.; Balestrazzi, E. Natural History of Macular Subretinal Hemorrhage in Age-Related Macular Degeneration. Ophthalmologica 1999, 213, 97–102. [Google Scholar] [CrossRef]
- Gabrielle, P.; Maitrias, S.; Nguyen, V.; Arnold, J.J.; Squirrell, D.; Arnould, L.; Sanchez-Monroy, J.; Viola, F.; O’Toole, L.; Barthelmes, D.; et al. Incidence, risk factors and outcomes of submacular haemorrhage with loss of vision in neovascular age-related macular degeneration in daily clinical practice: Data from the FRB! registry. Acta Ophthalmol. 2022, 100, 1569–1578. [Google Scholar] [CrossRef]
- Stewart, S.; Keates, A.K.; Redfern, A.; McMurray, J.J.V. Seasonal variations in cardiovascular disease. Nat. Rev. Cardiol. 2017, 14, 654–664. [Google Scholar] [CrossRef] [PubMed]
- El Sibai, R.H.; Bachir, R.H.; El Sayed, M.J. Seasonal variation in incidence and outcomes of out of hospital cardiac arrest. Medicine 2021, 100, e25643. [Google Scholar] [CrossRef]
- Tsementzis, S.A.; Kennet, R.P.; Hitchcock, E.R.; Gill, J.S.; Beevers, D.G. Seasonal variation of cerebrovascular diseases. Acta Neurochir. 1991, 111, 80–83. [Google Scholar] [CrossRef]
- Fujii, T.; Arima, H.; Takashima, N.; Kita, Y.; Miyamatsu, N.; Tanaka-Mizuno, S.; Shitara, S.; Urushitani, M.; Miura, K.; Nozaki, K. Seasonal Variation in Incidence of Stroke in a General Population of 1.4 Million Japanese: The Shiga Stroke Registry. Cerebrovasc. Dis. 2021, 51, 75–81. [Google Scholar] [CrossRef]
- Brennan, P.J.; Greenberg, G.; E Miall, W.; Thompson, S.G. Seasonal variation in arterial blood pressure. BMJ 1982, 285, 919–923. [Google Scholar] [CrossRef]
- Iguchi, Y.; Ito, Y.; Kikuchi, M.; Ishikawa, K.; Oshima, H.; Yatsuya, H.; Terasaki, H. Seasonal variations of acute massive submacular haemorrhage associated with age-related macular degeneration. Br. J. Ophthalmol. 2006, 90, 1256–1258. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Takashi, N.; Matsunaga, M.; Ito, Y.; Takeuchi, J.; Terasaki, H.; Yatsuya, H.; Nishiguchi, K.M. Seasonal variation in submacular hemorrhages in retinal macroaneurysms and its disappearance in age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 3589–3596. [Google Scholar] [CrossRef] [PubMed]
- Vision Academy Steering Committee. Vision Academy Viewpoint: Management of Subfoveal Hemorrhage. Available online: https://www.visionacademy.org/resource-zone/resources/all (accessed on 7 January 2023).
- Roger, J.H. A significance test for cyclic trends in incidence data. Biometrika 1977, 64, 152–155. [Google Scholar] [CrossRef]
- Coscas, G.; Yamashiro, K.; Coscas, F.; De Benedetto, U.; Tsujikawa, A.; Miyake, M.; Cheung, C.M.G.; Wong, T.Y.; Yoshimura, N. Comparison of Exudative Age-related Macular Degeneration Subtypes in Japanese and French Patients: Multicenter Diagnosis with Multimodal Imaging. Am. J. Ophthalmol. 2014, 158, 309–318.e2. [Google Scholar] [CrossRef] [PubMed]
- Ciardella, A.P.; Donsoff, I.M.; Huang, S.J.; Costa, D.L.; A Yannuzzi, L. Polypoidal choroidal vasculopathy. Surv. Ophthalmol. 2004, 49, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Uyama, M.; Wada, M.; Nagai, Y.; Matsubara, T.; Matsunaga, H.; Fukushima, I.; Takahashi, K.; Matsumura, M. Polypoidal choroidal vasculopathy: Natural history. Am. J. Ophthalmol. 2002, 133, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.C.; Cho, J.; Park, K.H.; Woo, S.J. Massive submacular haemorrhage in polypoidal choroidal vasculopathy versus typical neovascular age-related macular degeneration. Acta Ophthalmol. 2020, 99, 706–714. [Google Scholar] [CrossRef]
- Yuzawa, M.; Mori, R.; Kawamura, A. The origins of polypoidal choroidal vasculopathy. Br. J. Ophthalmol. 2005, 89, 602–607. [Google Scholar] [CrossRef]
- Kim, J.-B.; Nirwan, R.S.; Kuriyan, A.E. Polypoidal Choroidal Vasculopathy. Curr. Ophthalmol. Rep. 2017, 5, 176–186. [Google Scholar] [CrossRef]
- Weber, C.; Bertelsmann, M.; Kiy, Z.; Stasik, I.; Holz, F.G.; Liegl, R. Antiplatelet and anticoagulant therapy in patients with submacular hemorrhage caused by neovascular age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 261, 1413–1421. [Google Scholar] [CrossRef]
- Kuhli-Hattenbach, C.; Fischer, I.B.; Schalnus, R.; Hattenbach, L.-O. Subretinal Hemorrhages Associated with Age-Related Macular Degeneration in Patients Receiving Anticoagulation or Antiplatelet Therapy. Am. J. Ophthalmol. 2010, 149, 316–321.e1. [Google Scholar] [CrossRef] [PubMed]
- Tilanus, M.A.D.; Vaandrager, W.; Cuypers, M.H.M.; Verbeek, A.M.; Hoyng, C.B. Relationship between anticoagulant medication and massive intraocular hemorrhage in age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2000, 238, 482–485. [Google Scholar] [CrossRef] [PubMed]

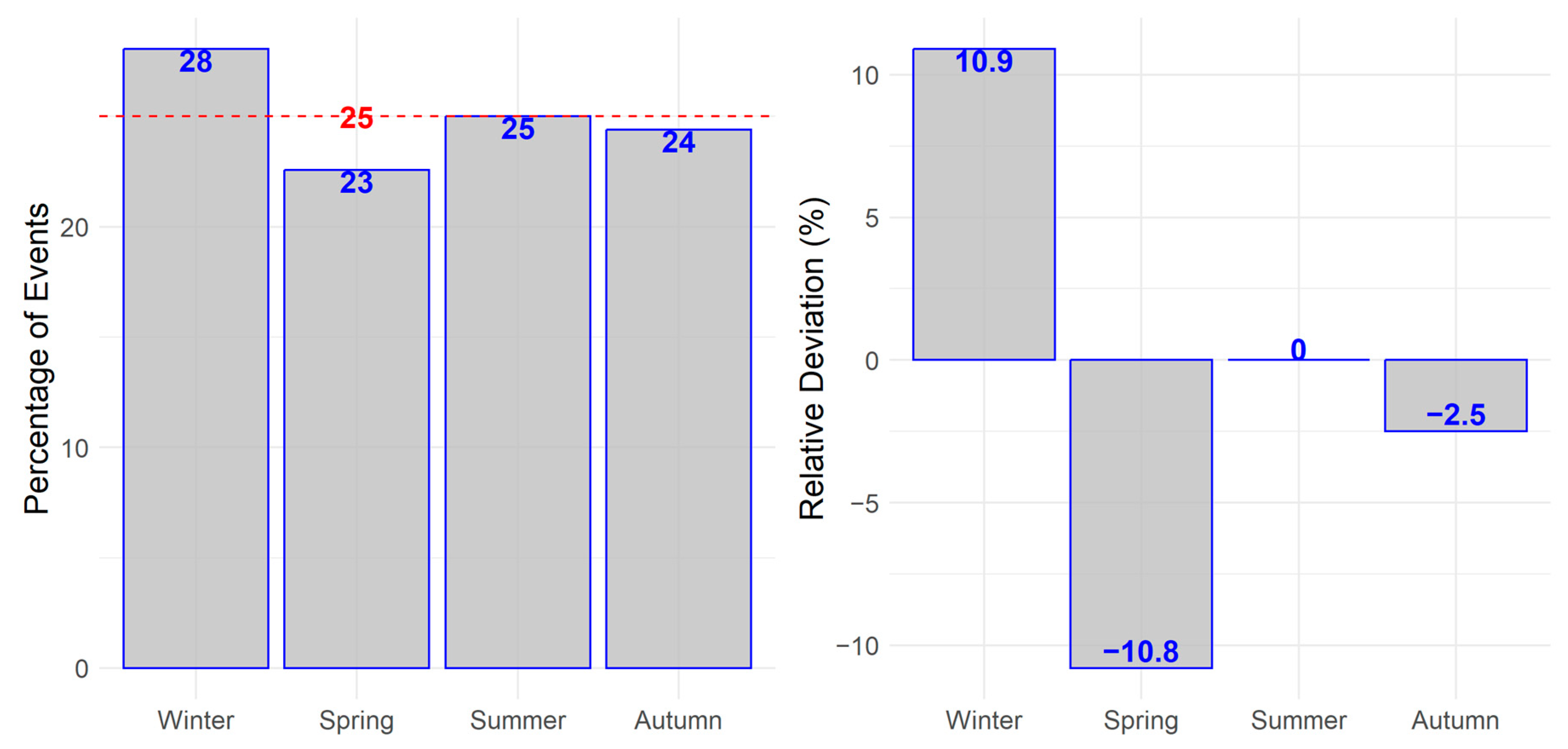
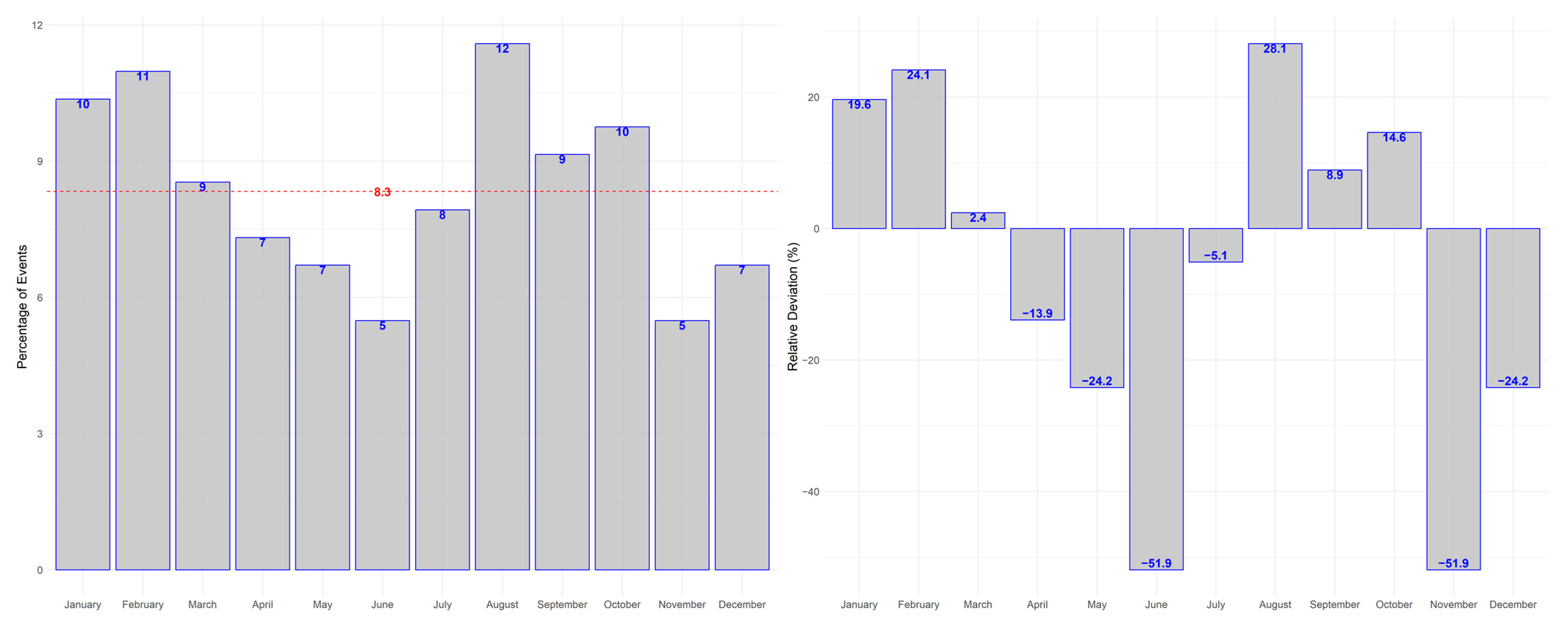
| Parameter | Value |
|---|---|
| no. of patients | 164 |
| median age at onset of hemorrhage (25th and 75th quantile) | 81.3 (75.4; 85.9) |
| gender (f; m) | 115; 49 |
| laterality (OD; OS) | 81; 83 |
| no. of patients taking AC/AP medication | 100 |
| no. of patients with arterial hypertension | 102 |
| cause of macular hemorrhage | |
| nAMD | 147 |
| macroaneursym | 7 |
| myopic CNV | 5 |
| secondary CNV due to macular teleangiectasia | 2 |
| secondary CNV due to chorioretinits | 1 |
| cause unknown | 2 |
| Group 1 | Group 2 | ||
|---|---|---|---|
| 101 (62%) | 63 (38%) | ||
| Small | Medium | Large | Massive |
| 59 (36%) | 42 (26%) | 50 (30%) | 13 (8%) |
| Group 1 n (%) | Group 2 n (%) | p Values | |
|---|---|---|---|
| seasons | 0.19 * | ||
| winter | 23 (14.02%) | 23 (14.02%) | |
| spring | 27 (16.46%) | 10 (6.10%) | |
| summer | 25 (15.24%) | 16 (9.76%) | |
| autumn | 26 (15.85%) | 14 (8.54%) | |
| arterial hypertension | 0.34 * | ||
| yes | 58 (41.13%) | 44 (31.21%) | |
| no | 26 (18.44%) | 13 (9.22%) | |
| AC/AP medication | 0.03 * | ||
| yes | 55 (33.54%) | 45 (27.44%) | |
| no | 46 (28.05%) | 18 (10.98%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Storp, J.J.; Diener, R.; Eter, N.; Bormann, E.; Treder, M. Submacular Hemorrhages Show No Significant Seasonal Variations in a European Cohort. J. Clin. Med. 2023, 12, 3622. https://doi.org/10.3390/jcm12113622
Storp JJ, Diener R, Eter N, Bormann E, Treder M. Submacular Hemorrhages Show No Significant Seasonal Variations in a European Cohort. Journal of Clinical Medicine. 2023; 12(11):3622. https://doi.org/10.3390/jcm12113622
Chicago/Turabian StyleStorp, Jens Julian, Raphael Diener, Nicole Eter, Eike Bormann, and Maximilian Treder. 2023. "Submacular Hemorrhages Show No Significant Seasonal Variations in a European Cohort" Journal of Clinical Medicine 12, no. 11: 3622. https://doi.org/10.3390/jcm12113622
APA StyleStorp, J. J., Diener, R., Eter, N., Bormann, E., & Treder, M. (2023). Submacular Hemorrhages Show No Significant Seasonal Variations in a European Cohort. Journal of Clinical Medicine, 12(11), 3622. https://doi.org/10.3390/jcm12113622






