Clustering Analysis Identified Three Long COVID Phenotypes and Their Association with General Health Status and Working Ability
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Study Population Characteristics
3.2. Cluster Analysis of Three Combined Sub-Population
3.3. Characteristic of the Phenotypes
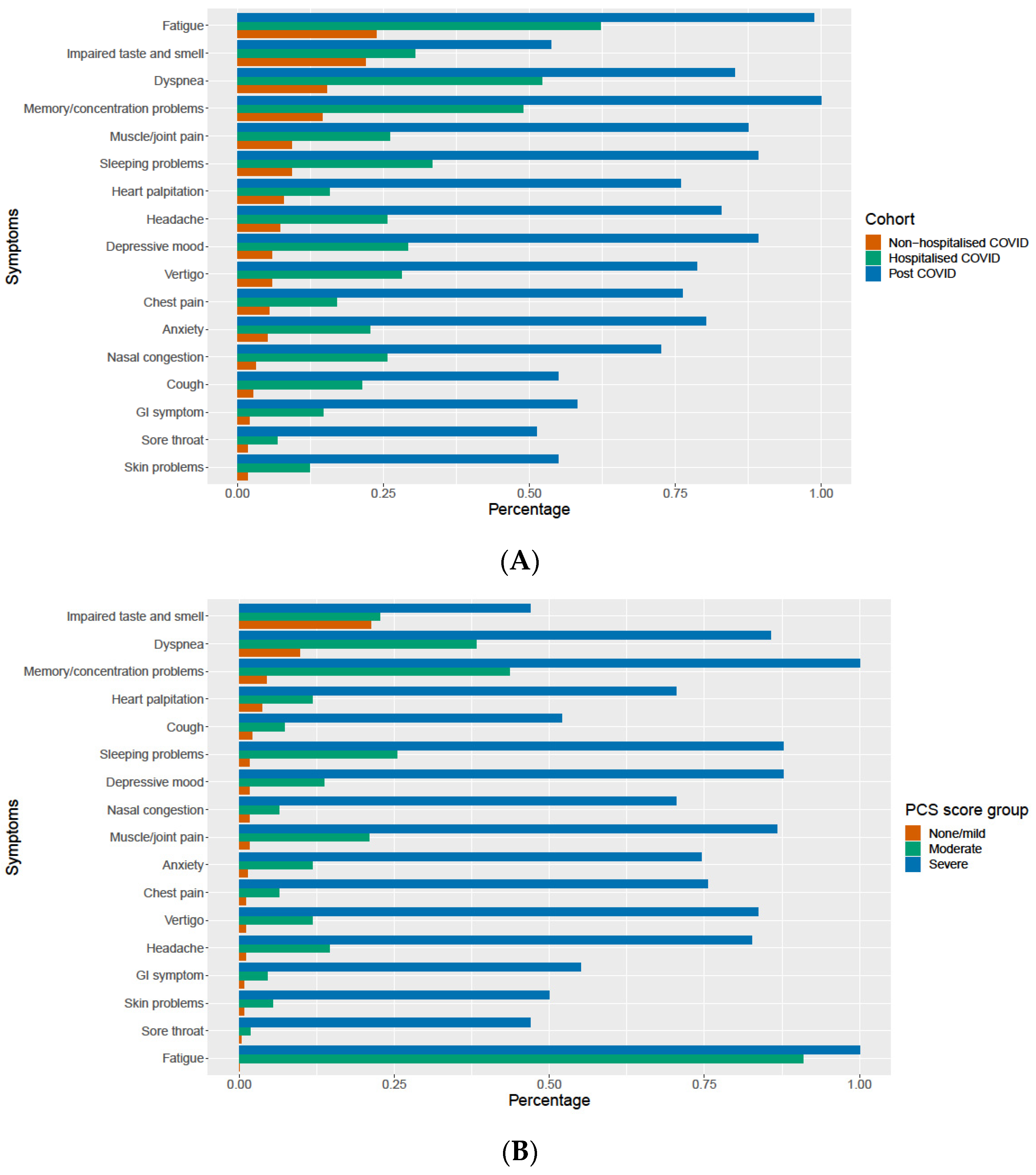
3.4. The Relevance of Persistent Symptoms
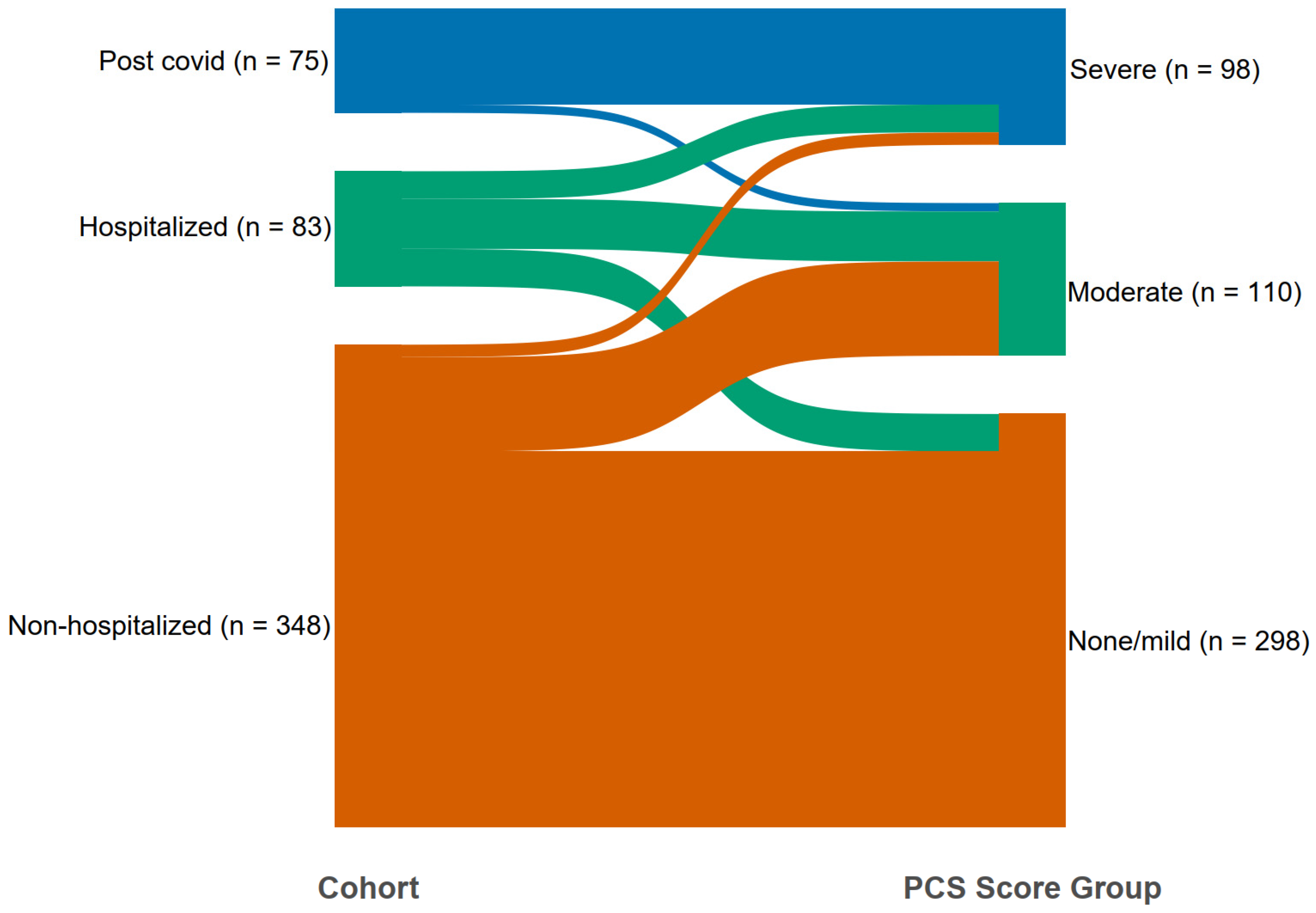
3.5. Cluster Analysis in the Test Population
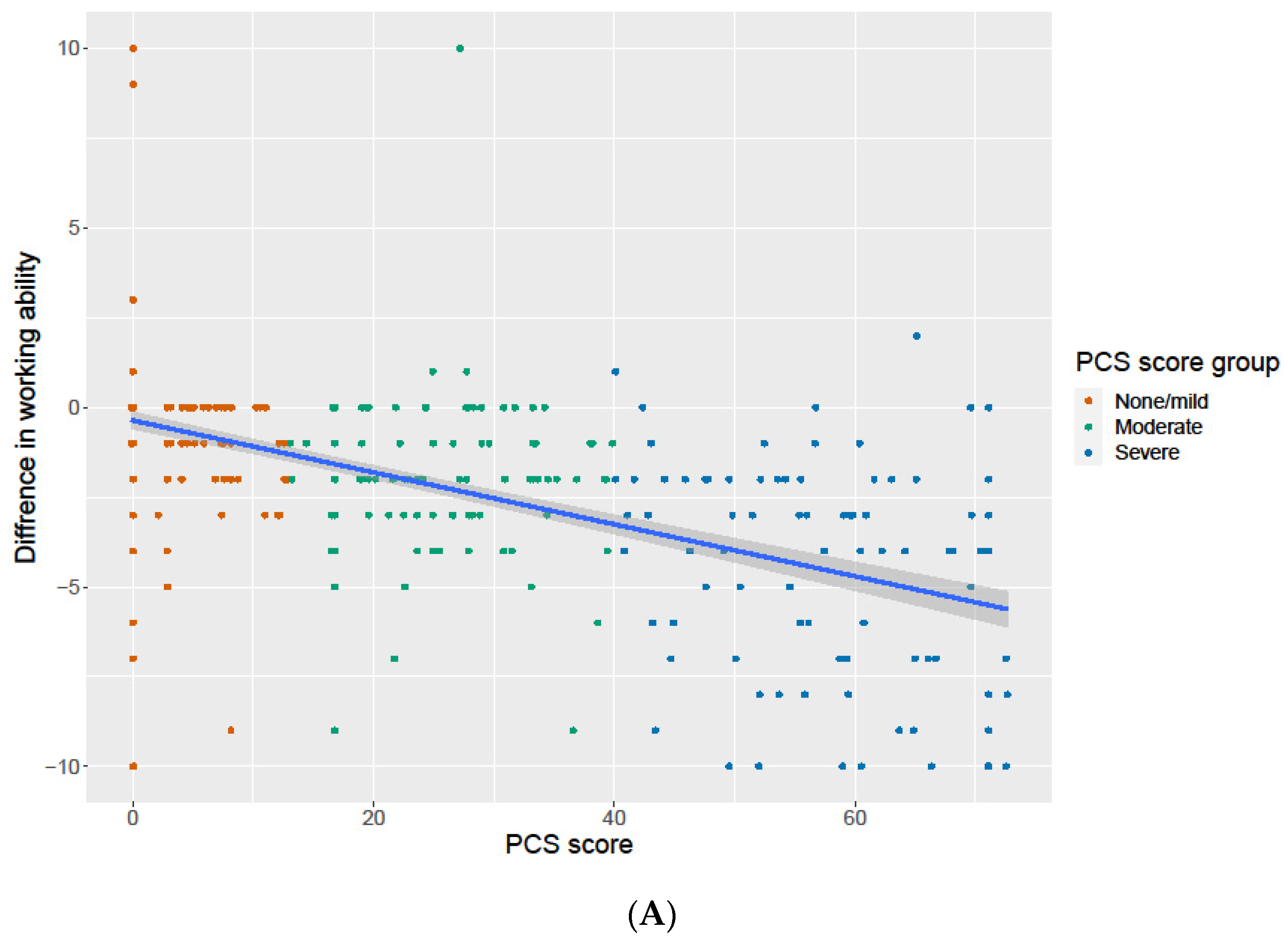
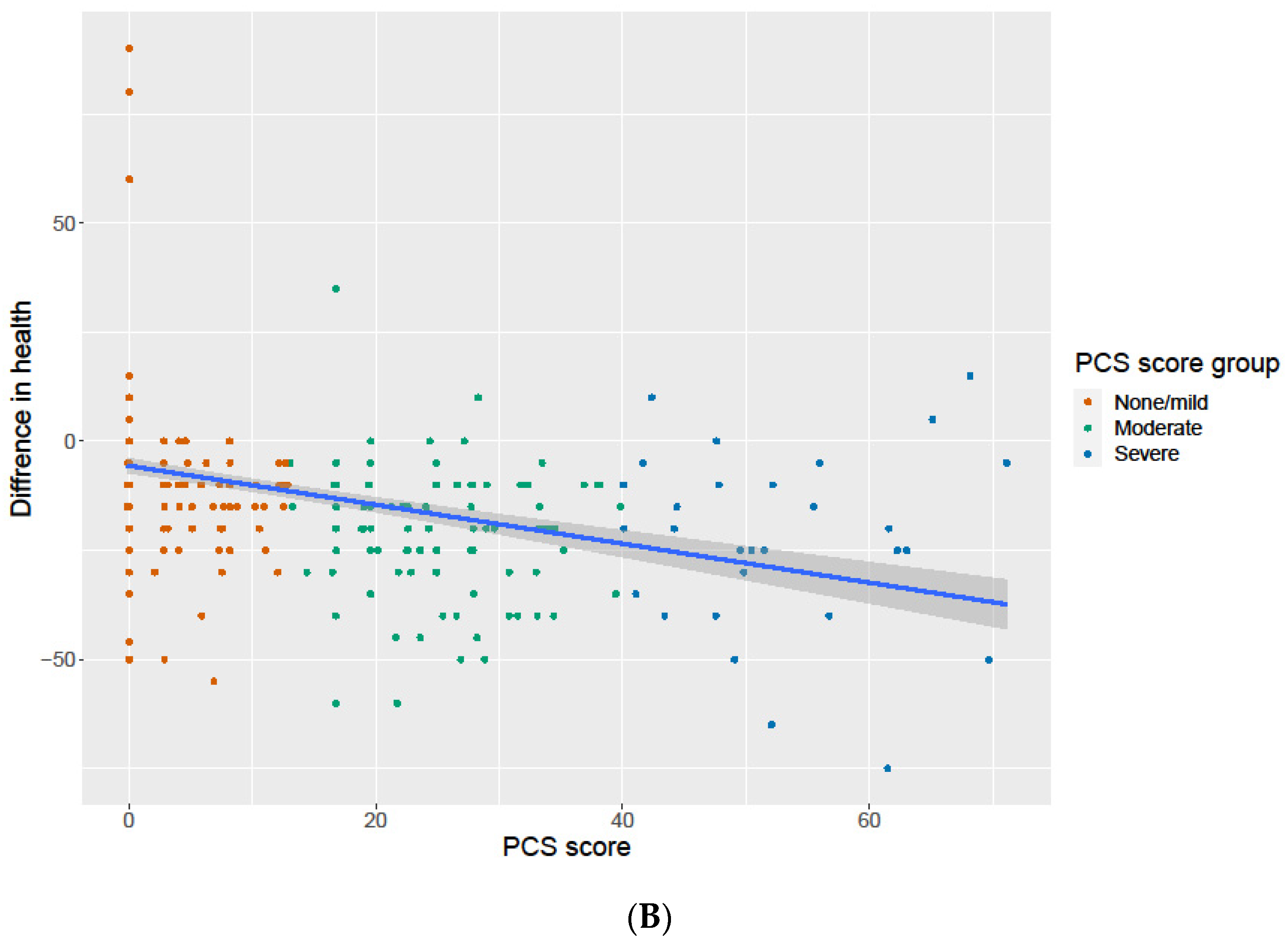
3.6. Association of the PCS Score with Change in General Health and Work Ability
3.7. The Predictors of the PCS Score Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. The Patients Questionnaire


References
- Thompson, E.J.; Williams, D.M.; Walker, A.J.; Mitchell, R.E.; Niedzwiedz, C.L.; Yang, T.C.; Huggins, C.F.; Kwong, A.S.F.; Silverwood, R.J.; Di Gessa, G.; et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat. Commun. 2022, 13, 3528. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Post COVID-19 Condition. 2022. Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (accessed on 10 May 2023).
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2021, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.A.; McAuley, H.; Harrison, E.M.; Shikotra, A.; Singapuri, A.; Sereno, M.; Elneima, O.; Docherty, A.B.; Lone, N.I.; Leavy, O.C.; et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): A UK multicentre, prospective cohort study. Lancet Respir. Med. 2021, 9, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’Em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. Eclinicalmedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Fernández-De-Las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Florencio, L.L.; Cuadrado, M.L.; Plaza-Manzano, G.; Navarro-Santana, M. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: A systematic review and meta-analysis. Eur. J. Intern. Med. 2021, 92, 55–70. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. At Least 17 Million People in the WHO European Region Experienced Long COVID in the FIRST Two Years of the Pandemic; Millions May Have to Live with It for Years to Come 2022. Available online: https://www.who.int/europe/news/item/13-09-2022-at-least-17-million-people-in-the-who-european-region-experienced-long-covid-in-the-first-two-years-of-the-pandemic--millions-may-have-to-live-with-it-for-years-to-come (accessed on 10 May 2023).
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing TK, P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk Factors Associated With Post-COVID-19 Condition: A Systematic Review and Meta-analysis. JAMA Intern Med. 2023. [Google Scholar] [CrossRef]
- Fischer, A.; Zhang, L.; Elbéji, A.; Wilmes, P.; Oustric, P.; Staub, T.; Nazarov, P.V.; Ollert, M.; Fagherazzi, G. Long COVID Symptomatology After 12 Months and Its Impact on Quality of Life According to Initial Coronavirus Disease 2019 Disease Severity. Open Forum Infect. Dis. 2022, 9, ofac397. [Google Scholar] [CrossRef]
- Zhao, Y.; Le Shi, L.; Jiang, Z.; Na Zeng, N.; Mei, H.; Lu, Y.; Yang, J.; Jin, F.; Ni, S.; Wu, S.; et al. The phenotype and prediction of long-term physical, mental and cognitive COVID-19 sequelae 20 months after recovery, a community-based cohort study in China. Mol. Psychiatry 2023, 1–9. [Google Scholar] [CrossRef]
- Fischer, A.; Badier, N.; Zhang, L.; Elbéji, A.; Wilmes, P.; Oustric, P.; Benoy, C.; Ollert, M.; Fagherazzi, G. Long COVID Classification: Findings from a Clustering Analysis in the Predi-COVID Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 16018. [Google Scholar] [CrossRef]
- Kenny, G.; McCann, K.; O’brien, C.; Savinelli, S.; Tinago, W.; Yousif, O.; Lambert, J.S.; O’broin, C.; Feeney, E.R.; De Barra, E.; et al. Identification of Distinct Long COVID Clinical Phenotypes Through Cluster Analysis of Self-Reported Symptoms. Open Forum Infect. Dis. 2022, 9, ofac060. [Google Scholar] [CrossRef]
- Bahmer, T.; Borzikowsky, C.; Lieb, W.; Horn, A.; Krist, L.; Fricke, J.; Scheibenbogen, C.; Rabe, K.F.; Maetzler, W.; Maetzler, C.; et al. Severity, predictors and clinical correlates of Post-COVID syndrome (PCS) in Germany: A prospective, multi-centre, population-based cohort study. Eclinicalmedicine 2022, 51, 101549. [Google Scholar] [CrossRef]
- Frontera, J.A.; Thorpe, L.E.; Simon, N.M.; de Havenon, A.; Yaghi, S.; Sabadia, S.B.; Yang, D.; Lewis, A.; Melmed, K.; Balcer, L.J.; et al. Post-acute sequelae of COVID-19 symptom phenotypes and therapeutic strategies: A prospective, observational study. PLoS ONE 2022, 17, e0275274. [Google Scholar] [CrossRef]
- Kisiel, M.A.; Janols, H.; Nordqvist, T.; Bergquist, J.; Hagfeldt, S.; Malinovschi, A.; Svartengren, M. Predictors of post-COVID-19 and the impact of persistent symptoms in non-hospitalized patients 12 months after COVID-19, with a focus on work ability. Upsala J. Med. Sci. 2022, 127. [Google Scholar] [CrossRef]
- Kisiel, M.A.; Nordqvist, T.; Westman, G.; Svartengren, M.; Malinovschi, A.; Janols, H. Patterns and predictors of sick leave among Swedish non-hospitalized healthcare and residential care workers with COVID-19 during the early phase of the pandemic. PLoS ONE 2021, 16, e0260652. [Google Scholar] [CrossRef]
- Lyon, J.A.; Garcia-Milian, R.; Norton, H.F.; Tennant, M.R. The Use of Research Electronic Data Capture (REDCap) Software to Create a Database of Librarian-Mediated Literature Searches. Med. Ref. Serv. Q. 2014, 33, 241–252. [Google Scholar] [CrossRef]
- WHO/ISARIC, COVID-19 Case Record form. Global COVID-19 Clonical Platform. Novel Coronavirus COVID-19 Rapid Version. 2020. Available online: https://apps.who.int/iris/handle/10665/331768 (accessed on 21 May 2023).
- Sun, X.M.D.F.; Puzniak, L.; Coetzer, H.; Zamparo, J.M.; Tabak, Y.P.; Cappeller, J.C. Assessment of Retrospective Collection of EQ-5D-5L in US Patients with COVID-19 in 2023. Available online: https://www.medrxiv.org/content/10.1101/2023.01.18.23284602v1.full.pdf (accessed on 21 May 2023).
- R Core Team R: A Language and Environment for Statistical Computing. V. R Foundation for Statistical Computing, Austria. 2023. Available online: https://www.R-project.org/ (accessed on 21 May 2023).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Hand, D.J.; Till, R.J. A Simple Generalisation of the Area Under the ROC Curve for Multiple Class Classification Problems. Mach. Learn. 2001, 45, 171–186. [Google Scholar] [CrossRef]
- Filbin, M.R.; Mehta, A.; Schneider, A.M.; Kays, K.R.; Guess, J.R.; Gentili, M.; Fenyves, B.G.; Charland, N.C.; Gonye, A.L.; Gushterova, I.; et al. Longitudinal proteomic analysis of severe COVID-19 reveals survival-associated signatures, tissue-specific cell death, and cell-cell interactions. Cell Rep. Med. 2021, 2, 100287. [Google Scholar] [CrossRef]
- Goldhaber, N.H.; Kohn, J.N.; Ogan, W.S.; Sitapati, A.; Longhurst, C.A.; Wang, A.; Lee, S.; Hong, S.; Horton, L.E. Deep Dive into the Long Haul: Analysis of Symptom Clusters and Risk Factors for Post-Acute Sequelae of COVID-19 to Inform Clinical Care. Int. J. Environ. Res. Public Health 2022, 19, 16841. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Martín-Guerrero, J.D.; Florencio, L.L.; Navarro-Pardo, E.; Rodríguez-Jiménez, J.; Torres-Macho, J.; Pellicer-Valero, O.J. Clustering analysis reveals different profiles associating long-term post-COVID symptoms, COVID-19 symptoms at hospital admission and previous medical co-morbidities in previously hospitalized COVID-19 survivors. Infection 2022, 51, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, H.R.; Gulea, C.; Koteci, A.; Kallis, C.; Morgan, A.D.; Iwundu, C.; Weeks, M.; Gupta, R.; Quint, J.K. GP consultation rates for sequelae after acute COVID-19 in patients managed in the community or hospital in the UK: Population based study. BMJ 2021, 375, e065834. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.B.; von Feilitzen, G.S.; Andersson, M.E.; Sikora, P.; Lindh, M.; Nordén, R.; Nilsson, S.; Sigström, R. Self-reported symptom severity, general health, and impairment in post-acute phases of COVID-19: Retrospective cohort study of Swedish public employees. Sci. Rep. 2022, 12, 19818. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Giszas, B.; Trommer, S.; Schüßler, N.; Rodewald, A.; Besteher, B.; Bleidorn, J.; Dickmann, P.; Finke, K.; Katzer, K.; Lehmann-Pohl, K.; et al. Post-COVID-19 condition is not only a question of persistent symptoms: Structured screening including health-related quality of life reveals two separate clusters of post-COVID. Infection 2022, 51, 365–377. [Google Scholar] [CrossRef]
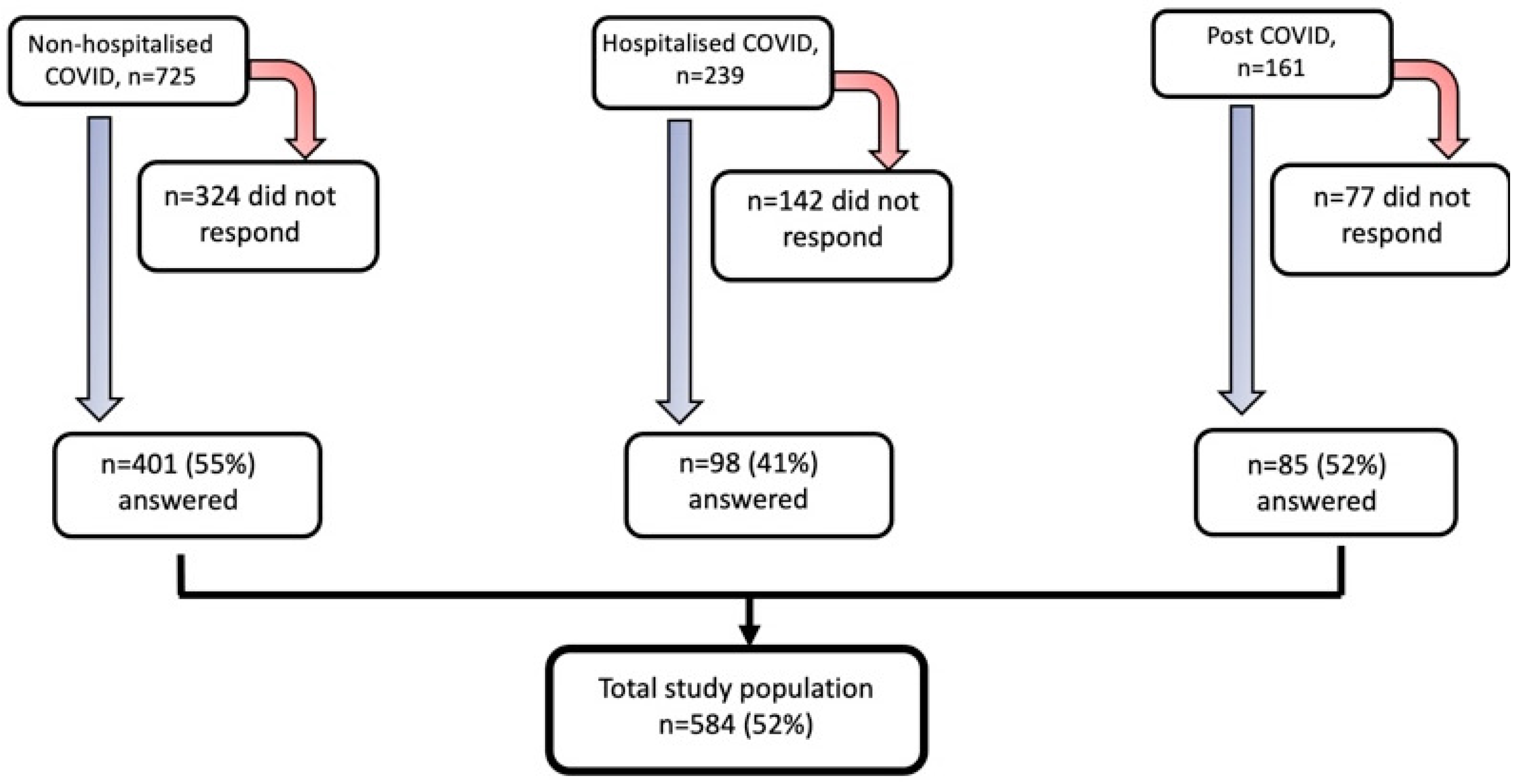
| Non-Hospitalized COVID (n = 401) | Hospitalized COVID (n = 98) | Post-COVID (n = 85) | p-Values, + | |
|---|---|---|---|---|
| Sociodemographic and lifestyle | ||||
| Age, mean (SD) | 44.2 (13.8) | 58.5 (10.1) | 50.0 (11.9) | <0.001 |
| Female, n (%) | 298 (74.3) | 43 (44.3) | 55 (64.7) | <0.001 |
| Country of birth, n (%) Sweden | 319 (79.8) | 74 (78.7) | 74 (87.1) | 0.263 |
| Education level, n (%) Up to secondary school Vocational education University | 141 (35.3) 37 (9.3) 221 (55.4) | 50 (51.5) 18 (18.6) 29 (29.9) | 35 (42.7) 13 (15.9) 34 (41.4) | <0.001 |
| Working status, n (%) Working Parental leave Looking for a job Retired Sick leave Student | 350 (87.3) 5 (1.2) 2 (0.5) 11 (2.7) 18 (4.5) 6 (1.5) | 70 (71.4) 0 (0) 2 (2.0) 18 (18.4) 5 (5.1) 1 (1.0) | 52 (61.9) 0 (0) 2 (2.4) 5 (6.0) 22 (26.2) 3 (3.5) | <0.001 |
| Marital status, n (%) Married Partner Divorced or separated Widower/er Single | 134 (33.5) 139 (34.7) 75 (18.7) 15 (3.8) 37 (9.3) | 58 (59.8) 18 (18.6) 14 (14.4) 3 (3.1) 4 (4.1) | 41 (48.2) 21(24.7) 6 (7.1) 0 (0) 17 (20.0) | <0.001 |
| Smoking, n (%) Never smoked Ex-smoker Current smoker | 303 (76.1) 82 (20.6) 13 (3.3) | 55 (58.5) 36 (38.3) 3 (3.2) | 2 (2.4) 28 (32.9) 55 (64.7) | <0.001 |
| Snuff, n (%) | 316 (81.0) | 19 (20.4) | 13 (15.5) | <0.001 |
| Pre-existing comorbidities, n (%) and BMI | ||||
| BMI, mean (SD) | 25.7(4.7) | 30.1 (6.3) | 28.4 (6.1) | <0.001 |
| Hypertension | 50 (13.7) | 41 (42.3) | 27 (31.8) | <0.001 |
| Heart disease | 14 (3.9) | 7 (7.2) | 6 (7.1) | 0.265 |
| Hypo/hyperthyroidism | 33 (9.1) | 4 (4.1) | 6 (7.1) | 0.245 |
| Diabetes | 14 (3.9) | 12 (12.2) | 4 (4.7) | 0.006 |
| Lung disease | 45 (12.4) | 25 (25.5) | 24 (28.2) | <0.001 |
| Liver disease | 1 (0.3) | 0 (0.0) | 1 (1.2) | 0.382 |
| Cancer | 21 (5.8) | 11 (11.3) | 3 (3.5) | 0.078 |
| Immunosuppressive treatment | 16 (4.5) | 4 (4.1) | 4 (4.7) | 0.971 |
| Depression/Anxiety | 93 (25.3) | 22 (22.4) | 34 (40.0) | 0.012 |
| Chronic pain | 18 (5.0) | 9 (9.3) | 23 (27.1) | <0.001 |
| Other measurements | ||||
| Symptom severity at onset, median (IQR) | 3 (2, 3) | 4 (3, 4) | 4 (3, 4) | <0.001 |
| Hospitalized, n (%) | 0 | 98 (100) | 21 (25.0) | <0.001 |
| Laboratory-confirmed COVID-19 infection n (%) | 401 (100) | 98 (100) | 56 (67.5) | <0.001 |
| Number of months from infection onset, median | 12 | 12 | 22 (IQR:18, 27) | <0.001 |
| Mean number of remaining symptoms, mean (SD) | 1.3 (2.1) | 4.3 (4.5) | 12.3 (3.7) | <0.001 |
| Health status COVID-19, median (IQR) | 90 (85, 95) | 90 (10, 95) | Missing variable | 0.045 |
| Health status today, Median (IQR) | 80 (70, 90) | 70 (55, 85) | 40 (20, 60) | <0.001 |
| Difference of health status COVID-19 and today, Median (IQR) | −5 (−15, 0) | −10 (−25, −5) | Missing variable | <0.001 |
| Working ability COVID-19, median (IQR) | 10 (9, 10) | 10 (7, 10) | 10 (9, 10) | 0.306 |
| Work ability today, Median (IQR) | 9 (8, 10) | 8 (4, 9) | 4 (1, 6) | <0.001 |
| Difference working ability COVID-19 and today, median (IQR) | 0 (−1.3, 0) | −1 (−2, 0) | −5 (−8, −3) | <0.001 |
| No | Symptom Complex | Cluster I Center (n = 299) | Cluster II Center (n = 120) | Cluster III Center (n = 87) | Regression Coefficient | PCS * Score Weight |
|---|---|---|---|---|---|---|
| 2 | Fatigue | 0 | 0.933 | 0.989 | 16.758 | 16.8 |
| 15 | Memory and concentration problems | 0.043 | 0.492 | 1.000 | 8.144 | 8.1 |
| 4 | Sore throat | 0.003 | 0.017 | 0.529 | 7.425 | 7.4 |
| 3 | Muscles and joints pain | 0.020 | 0.217 | 0.931 | 5.849 | 5.8 |
| 1 | Cough | 0.020 | 0.075 | 0.575 | 4.706 | 4.7 |
| 11 | Heart palpitation | 0.037 | 0.133 | 0.759 | 4.500 | 4.5 |
| 7 | Vertigo | 0.013 | 0.15 | 0.874 | 4.031 | 4.0 |
| 6 | Headache | 0.010 | 0.192 | 0.851 | 4.013 | 4.0 |
| 14 | Depressive mood | 0.017 | 0.175 | 0.920 | 4.003 | 4.0 |
| 10 | Chest pain | 0.013 | 0.083 | 0.805 | 3.127 | 3.1 |
| 12 | GI symptoms | 0.007 | 0.05 | 0.609 | 2.934 | 2.9 |
| 5 | Dyspnea | 0.097 | 0.408 | 0.885 | 2.825 | 2.9 |
| 16 | Sleep problems | 0.017 | 0.308 | 0.885 | 2.250 | 2.3 |
| 13 | Anxiety mood | 0.013 | 0.15 | 0.782 | 2.086 | 2 |
| 17 | Impaired taste and smell | 0.214 | 0.208 | 0.517 | 0.046 | 0 |
| 9 | Nasal symptoms | 0.017 | 0.083 | 0.759 | −0.150 | 0 |
| 8 | Skin problems | 0.007 | 0.058 | 0.552 | −1.448 | −1.5 |
| Characteristics | None/Mild PCS * Score ≤ 13 (n = 298) | Moderate PCS * Score > 13 and ≤40 (n = 110) | Severe PCS * Score > 40 (n = 98) | p-Value Unadjusted, + | p-Value Adjusted, + |
|---|---|---|---|---|---|
| Sociodemographic and lifestyle | |||||
| Age, mean (SD) | 44.2 (13.9) | 50.3 (13.6) | 49.8 (12.2) | <0.001 | 0.002 |
| Female, n (%) | 211 (71.0) | 71 (64.5) | 65 (66.3) | 0.387 | 0.397 |
| Country of birth n (%) Sweden | 246 (84.0) | 86 (78.2) | 81 (82.7) | 0.397 | 0.397 |
| Education level, n (%) Up to Gymnasium Two years Three years | 102 (34.5) 27 (9.1) 167 (56.4) | 45 (41.3) 14 (12.8) 50 (45.9) | 45 (46.4) 15 (15.5) 37 (38.1) | 0.022 | 0.028 |
| Working status, n (%) Working Parental leave Looking for a job Retired Sick leave Student | 266 (91.1) 4 (1.4) 2 (0.7) 10 (3.4) 8 (2.7) 2 (0.7) | 91 (85.8) 0 (0) 1 (0.9) 6 (5.7) 6 (5.7) 2 (1.9) | 61 (63.6) 0 (0) 1 (1.0) 10 (10.4) 21 (21.9) 3 (3.1) | <0.001 | <0.001 |
| Marital status, n (%) Married Sambo Divorced Widower Single | 95 (32.1) 110 (37.2) 57 (19.3) 14 (4.7) 20 (6.7) | 53 (48.2) 30 (27.3) 18 (16.3) 2 (1.8) 7 (6.4) | 42 (42.9) 25 (25.5) 10 (10.2) 1 (1.0) 20 (20.4) | <0.001 | 0.002 |
| Smoking, n (%) Never smoked Ex-smoker Current smoker | 224 (76.5) 58 (19.8) 11 (3.7) | 61 (56.5) 40 (37.0) 7 (6.5) | 20 (20.4) 32 (32.7) 46 (46.9) | <0.001 | 0.002 |
| Snuff, n (%) | 218 (75.2) | 66 (61.1) | 20 (20.8) | <0.001 | 0.002 |
| Pre-existing comorbidities, n (%) and BMI | |||||
| BMI, mean (SD) | 25.5 (4.2) | 27.9 (5.4) | 29.2 (7.1) | <0.001 | 0.002 |
| Hypertension | 39 (13.1) | 24 (21.8) | 32 (32.7) | <0.001 | 0.002 |
| Heart disease | 7 (2.3) | 6 (5.5) | 8 (8.2) | 0.032 | 0.051 |
| Hypo/hyperthyroidism | 19 (6.4) | 11 (10.6) | 6 (6.1) | 0.411 | 0.452 |
| Diabetes | 7 (2.3) | 8 (7.7) | 8 (8.2) | 0.017 | 0.031 |
| Lung disease | 29 (9.7) | 19 (18.6) | 31 (31.6) | <0.001 | 0.002 |
| Liver disease | 1 (0.3) | 0 (0) | 1 (1.0) | 0.489 | 0.538 |
| Cancer | 17 (5.7) | 8 (7.7) | 3 (3.1) | 0.407 | 0.452 |
| Immunosuppressive treatment | 8 (2.7) | 8 (7.7) | 6 (6.2) | 0.081 | 0.112 |
| Depression/Anxiety | 59 (19.8) | 28 (25.4) | 39 (40.2) | <0.001 | 0.002 |
| Chronic pain | 9 (3.0) | 10 (9.2) | 24 (24.5) | <0.001 | 0.002 |
| Other measurements | |||||
| Symptom severity at COVID-19 onset, median (IQR) | 3 (2, 3) | 3 (3, 4) | 4 (3, 4) | <0.001 | <0.001 |
| Hospitalized, n (%) | 27 (9.1) | 37 (33.6) | 35 (36.5) | <0.001 | <0.001 |
| Laboratory-confirmed COVID-19, n (%) | 298 (100) | 109 (99.1) | 71 (74.0) | <0.001 | <0.001 |
| Number of months from infection onset, mean (SD) | 12.0 (0.0) | 12.6 (2.5) | 19.3 (6.7) | <0.001 | <0.001 |
| Mean number of remaining symptoms, mean (SD) | 0.5 (0.9) | 3.4 (1.8) | 12.5 (3.0) | <0.001 | <0.001 |
| Health status COVID-19, median, (IQR) | 90 (85, 100) | 90 (85, 100) | 85 (70, 95) | 0.029 | 0.033 |
| Health status today, median (IQR) | 85 (75, 95) | 70 (55, 80) | 45 (30, 60) | <0.001 | <0.001 |
| Difference health status COVID-19 and today, median (IQR) | 0 (10, 0) | −20 (−26.3, −10) | −20 (−35, −10) | <0.001 | <0.001 |
| Work ability COVID-19, median (IQR) | 10 (9, 10) | 10 (9, 10) | 10 (9, 10) | 0.401 | 0.401 |
| Work ability today, median (IQR) | 9 (8, 10) | 8 (6, 9) | 4 (1, 7) | <0.001 | <0.001 |
| Difference working ability COVID-19 and today, median (IQR) | 0 (−1, 0) | −2 (−3, −1) | −4 (−7, −2) | <0.001 | <0.001 |
| Predictor Variable | Level | Regression Coefficient | Odds Ratio | p-Value, + | |||
|---|---|---|---|---|---|---|---|
| Estimate | SD | 95% CI | Estimate | 95% CI | |||
| Sex | Female | 0.356 | 0.226 | (−0.083, 0.805) | 1.427 | (0.920, 2.237) | 0.116 |
| BMI | Scale | 0.060 | 0.020 | (0.021, 0.099) | 1.062 | (1.022, 1.104) | 0.003 |
| Smoking | Have smoked | 1.258 | 0.216 | (0.837, 1.684) | 3.519 | (2.309, 5.387) | <0.001 |
| Snuff | Yes | −1.108 | 0.219 | (−1.540, −0.679) | 0.330 | (0.214 0.507) | <0.001 |
| Heart disease | Yes | 0.910 | 0.469 | (−0.013, 1.840) | 2.484 | (0.987, 6.294) | 0.052 |
| Lung disease | Yes | 0.468 | 0.273 | (−0.071, 1.002) | 1.596 | (0.932, 2.725) | 0.087 |
| Depression/anxiety | Yes | 0.408 | 0.234 | (−0.054, 0.865) | 1.503 | (0.947, 2.375) | 0.081 |
| Diabetes | Yes | 0.972 | 0.464 | (0.062, 1.899) | 2.644 | (1.064, 6.612) | 0.036 |
| Chronic pain | Yes | 0.722 | 0.360 | (0.022, 1.437) | 2.059 | (1.022, 4.207) | 0.045 |
| Symptom severity at onset | 1–5 scale | 0.690 | 0.110 | (0.478, 0.911) | 1.995 | (1.612, 2.487) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisiel, M.A.; Lee, S.; Malmquist, S.; Rykatkin, O.; Holgert, S.; Janols, H.; Janson, C.; Zhou, X. Clustering Analysis Identified Three Long COVID Phenotypes and Their Association with General Health Status and Working Ability. J. Clin. Med. 2023, 12, 3617. https://doi.org/10.3390/jcm12113617
Kisiel MA, Lee S, Malmquist S, Rykatkin O, Holgert S, Janols H, Janson C, Zhou X. Clustering Analysis Identified Three Long COVID Phenotypes and Their Association with General Health Status and Working Ability. Journal of Clinical Medicine. 2023; 12(11):3617. https://doi.org/10.3390/jcm12113617
Chicago/Turabian StyleKisiel, Marta A., Seika Lee, Sara Malmquist, Oliver Rykatkin, Sebastian Holgert, Helena Janols, Christer Janson, and Xingwu Zhou. 2023. "Clustering Analysis Identified Three Long COVID Phenotypes and Their Association with General Health Status and Working Ability" Journal of Clinical Medicine 12, no. 11: 3617. https://doi.org/10.3390/jcm12113617
APA StyleKisiel, M. A., Lee, S., Malmquist, S., Rykatkin, O., Holgert, S., Janols, H., Janson, C., & Zhou, X. (2023). Clustering Analysis Identified Three Long COVID Phenotypes and Their Association with General Health Status and Working Ability. Journal of Clinical Medicine, 12(11), 3617. https://doi.org/10.3390/jcm12113617







