A Retrospective Study of the Functional Outcomes in Patients with Proximal Humeral Bone Defect after Shoulder Fusion or Prosthetic Replacement
Abstract
1. Introduction
2. Patients and Methods
2.1. Clinical Series
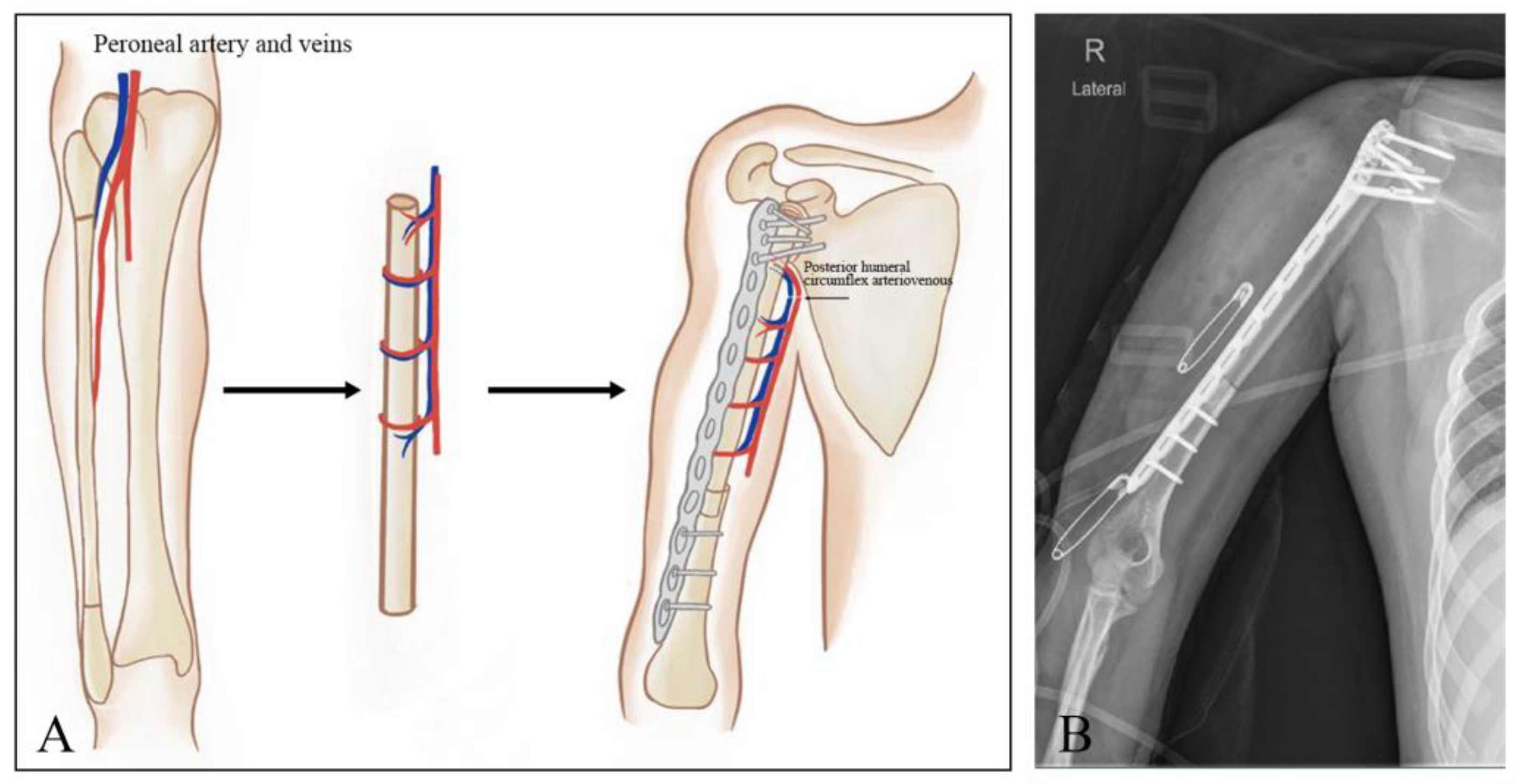
2.2. Resection and Reconstructive Procedures
2.3. Tumor Resection
2.4. Prosthetic Replacement
2.5. Shoulder Arthrodesis
2.6. Postoperative Care
2.7. Functional Assessment
3. Results
3.1. Summary of Demographic Data
3.2. Oncological Evaluation
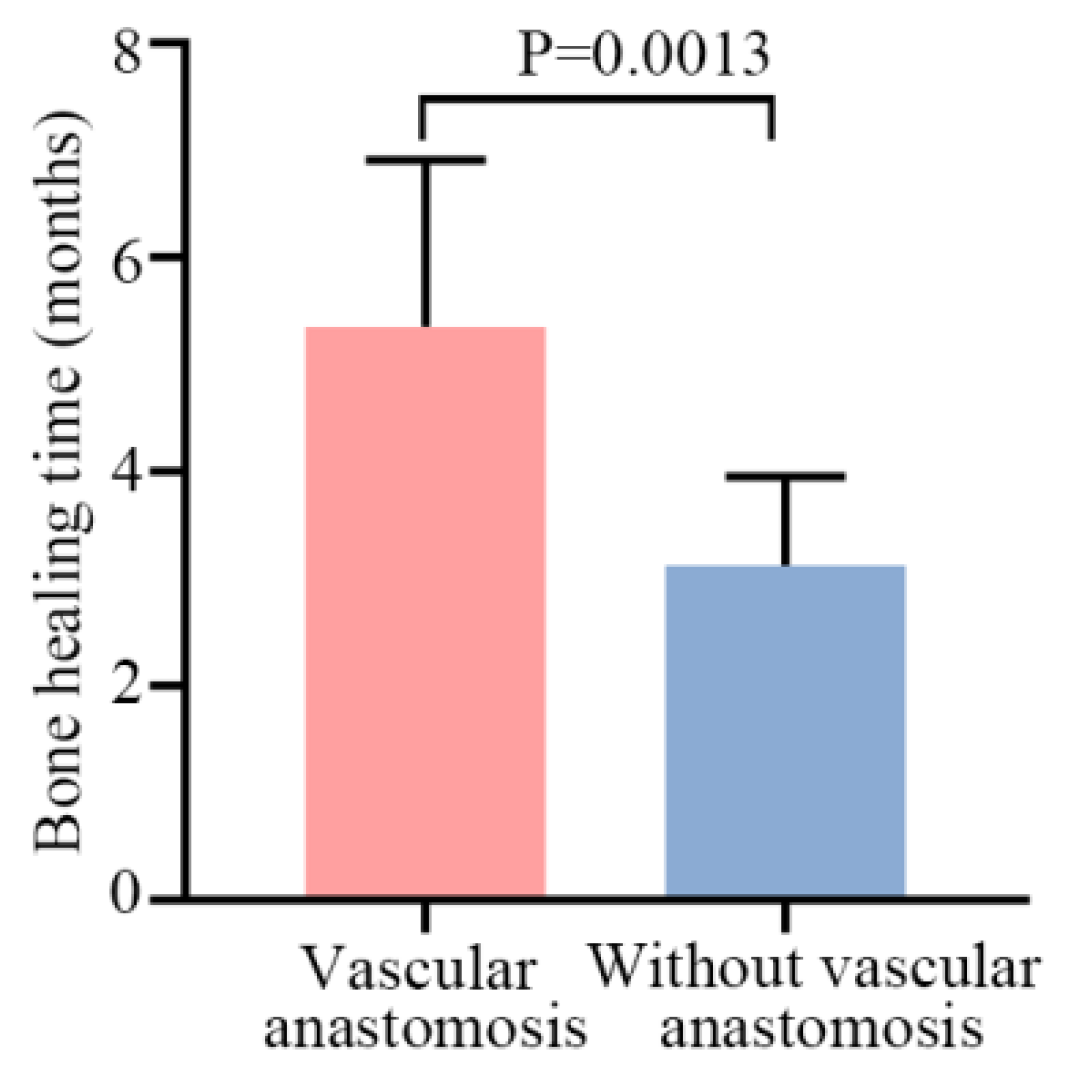
3.3. Arthrodesis Union and Healing
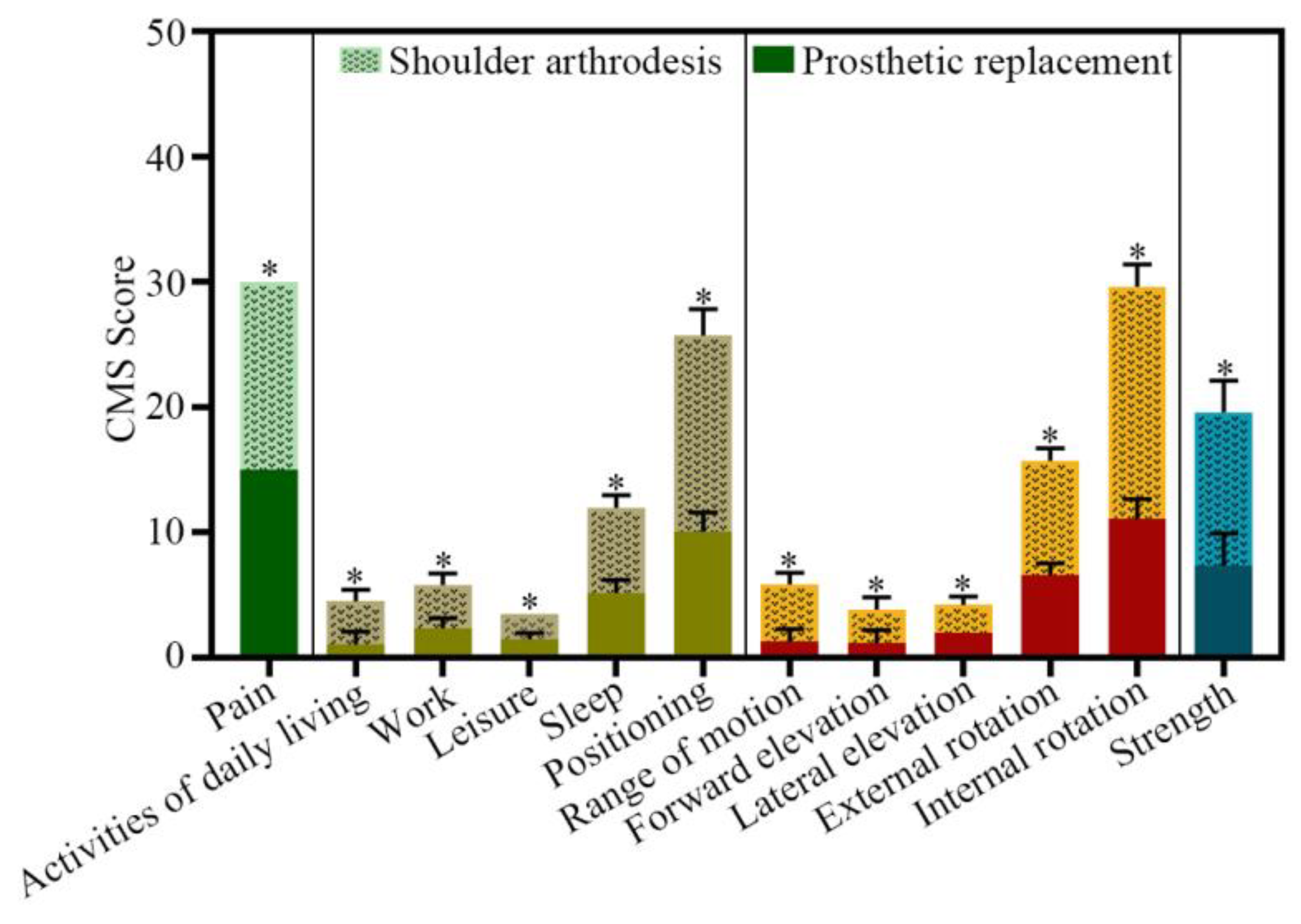
3.4. Clinical Outcome
4. Complications
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Review Statement
References
- Ottaviani, G.; Jaffe, N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009, 152, 3–13. [Google Scholar] [CrossRef]
- Katzer, A.; Meenen, N.M.; Grabbe, F.; Rueger, J. Surgery of skeletal metastases. Arch. Orthop. Trauma Surg. 2002, 122, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.; O’Connor, M.I.; Padgett, D.J.; Kaufman, K.; Sim, F.H. Arthrodesis of the shoulder after tumor resection. Clin. Orthop. Relat. Res. 2005, 436, 202–207. [Google Scholar] [CrossRef]
- Bilgin, S.S. Reconstruction of proximal humeral defects with shoulder arthrodesis using free vascularized fibular graft. J. Bone Jt. Surg. 2012, 94, e94. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, X.-C.; Xu, M.; Zheng, K.; Hu, Y.-C.; Wang, F.; Lun, D.-X. Intercalary prosthetic reconstruction for pathologic diaphyseal humeral fractures due to metastatic tumors: Outcomes and improvements. J. Shoulder Elb. Surg. 2018, 27, 2013–2020. [Google Scholar] [CrossRef]
- Wang, J.; Shen, J.; Dickinson, I.C. Functional outcome of arthrodesis with a vascularized fibular graft and a rotational latissimus dorsi flap after proximal humerus sarcoma resection. Ann. Surg. Oncol. 2011, 18, 1852–1859. [Google Scholar] [CrossRef]
- Mimata, Y.; Nishida, J.; Sato, K.; Suzuki, Y.; Doita, M. Glenohumeral arthrodesis for malignant tumor of the shoulder girdle. J. Shoulder Elb. Surg. 2015, 24, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Houdek, M.T.; Wagner, E.R.; Bishop, A.T.; Shin, A.Y.; Rose, P.S.; Sim, F.H.; Moran, S.L. Complications and Long-Term Outcomes of Free Fibula Reconstruction following Resection of a Malignant Tumor in the Extremities. Plast. Reconstr. Surg. 2017, 139, 510e–519e. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xiao, X.; Li, M.; Chen, G.; Huang, M.; Ji, C.; Wang, Z.; Li, J. Use of Vascularized Fibular Epiphyseal Transfer with Massive Bone Allograft for Proximal Humeral Reconstruction in Children with Bone Sarcoma. Ann. Surg. Oncol. 2021, 28, 7834–7841. [Google Scholar] [CrossRef]
- Gebert, C.; Hillmann, A.; Schwappach, A.; Hoffmann, C.; Hardes, J.; Kleinheinz, J.; Gosheger, G. Free vascularized fibular grafting for reconstruction after tumor resection in the upper extremity. J. Surg. Oncol. 2006, 94, 114–127. [Google Scholar] [CrossRef]
- Enneking, W.F.; Dunham, W.; Gebhardt, M.C.; Malawar, M.; Pritchard, D.J. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin. Orthop. Relat. Res. 1993, 286, 241–246. [Google Scholar] [CrossRef]
- Jeys, L.M.; Thorne, C.J.; Parry, M.; Gaston, C.L.L.; Sumathi, V.P.; Grimer, R.J. A Novel System for the Surgical Staging of Primary High-grade Osteosarcoma: The Birmingham Classification. Clin. Orthop. Relat. Res. 2017, 475, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Malawer, M.M.; Meller, I.; Dunham, W.K. A New surgical classification system for shoulder-girdle resections. Analysis of 38 patients. Clin. Orthop. Relat. Res. 1991, 267, 33–44. [Google Scholar] [CrossRef]
- El-Sherbiny, M. Reconstruction of the proximal humerus after wide resection of tumors: Comparison of three reconstructive options. J. Egypt. Natl. Cancer Inst. 2008, 20, 369–378. [Google Scholar]
- Nota, S.; Teunis, T.; Kortlever, J.; Ferrone, M.; Ready, J.; Gebhardt, M.; Raskin, K.; Hornicek, F.; Schwab, J.; Calderon, S.L. Functional Outcomes and Complications After Oncologic Reconstruction of the Proximal Humerus. J. Am. Acad. Orthop. Surg. 2018, 26, 403–409. [Google Scholar] [CrossRef]
- Rödl, R.W.; Gosheger, G.; Gebert, C.; Lindner, N.; Ozaki, T.; Winkelmann, W. Reconstruction of the proximal humerus after wide resection of tumours. J. Bone Jt. Surg. 2002, 84, 1004–1008. [Google Scholar] [CrossRef]
- Tagliero, A.J.; Bukowski, B.R.; Rose, P.S.; Morrey, M.E.; Elhassan, B.T.; Barlow, J.D.; Wagner, E.R.; Sanchez-Sotelo, J.; Houdek, M.T. High incidence of complications associated with shoulder girdle reconstruction utilizing a Stryker proximal humerus cap endoprosthesis following Tikhoff-Linberg resections. Int. Orthop. 2020, 44, 2449–2455. [Google Scholar] [CrossRef]
- Górecki, M.; Czarnecki, P. The influence of shoulder arthrodesis on the function of the upper limb in adult patients after a brachial plexus injury: A systematic literature review with elements of meta-analysis. EFORT Open Rev. 2021, 6, 797–807. [Google Scholar] [CrossRef]
- Taylor, G.I.; Miller, G.D.H.; Ham, F.J. The free vascularized bone graft. A clinical extension of microvascular techniques. Plast. Reconstr. Surg. 1975, 55, 533–544. [Google Scholar] [CrossRef]
- Malizos, K.N.; Zalavras, C.G.; Soucacos, P.N.; Beris, A.E.; Urbaniak, J.R. Free vascularized fibular grafts for reconstruction of skeletal defects. J. Am. Acad. Orthop. Surg. 2004, 12, 360–369. [Google Scholar] [CrossRef]
- Xu, L.; Wen, L.; Qiao, J.; Zhu, Z.; Qiu, Y.; Xiong, J.; Mao, H.; Wang, S. Clinical Outcome of Free Vascularized Fibula Graft in the Surgical Treatment of Extremity Osteosarcoma. Orthop. Surg. 2020, 12, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Zilber, S. Shoulder Arthroplasty: Historical Considerations. Open Orthop. J. 2017, 11, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- van de Sande, M.A.J.; Dijkstra, P.D.S.; Taminiau, A.H.M. Proximal humerus reconstruction after tumour resection: Biological versus endoprosthetic reconstruction. Int. Orthop. 2011, 35, 1375–1380. [Google Scholar] [CrossRef]
- Black, A.W.; Szabo, R.M.; Titelman, R.M. Treatment of malignant tumors of the proximal humerus with allograft-prosthesis composite reconstruction. J. Shoulder Elb. Surg. 2007, 16, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Thai, D.; Choong, P. Reconstructions of the shoulder following tumour resection. J. Orthop. Surg. 2007, 15, 201–206. [Google Scholar] [CrossRef]
- D’arienzo, A.; Ipponi, E.; Ruinato, A.D.; De Franco, S.; Colangeli, S.; Andreani, L.; Capanna, R. Proximal Humerus Reconstruction after Tumor Resection: An Overview of Surgical Management. Adv. Orthop. 2021, 2021, 5559377. [Google Scholar] [CrossRef] [PubMed]
- Trovarelli, G.; Cappellari, A.; Angelini, A.; Pala, E.; Ruggieri, P. What Is the Survival and Function of Modular Reverse Total Shoulder Prostheses in Patients Undergoing Tumor Resections in Whom an Innervated Deltoid Muscle Can Be Preserved? Clin. Orthop. Relat. Res. 2019, 477, 2495–2507. [Google Scholar] [CrossRef]


| Types | Shoulder Arthrodesis Using FVFG | Prosthetic Replacement |
|---|---|---|
| Gender | ||
| Male | 3 | 16 |
| Female | 19 | 11 |
| Age (years) | ||
| <20 | 9 | 2 |
| ≥20 | 13 | 25 |
| Average | 28.3 | 49.9 |
| Histological diagnosis | ||
| Osteosarcoma | 14 | 6 |
| Chondrosarcomas | 3 | 6 |
| Metastases | 2 | 10 |
| Others | 3 | 5 |
| Tumor necrosis rate (%) | ||
| <90 | 16 | 20 |
| ≥90 | 6 | 7 |
| AJCC/TNM stage | ||
| I/IIA | 6 | 8 |
| IIB/III/IV | 16 | 19 |
| 5-year survival rate (%) | 81.8% | 63.0% |
| Case | Gender/Age | Histological Diagnosis | MSTS Score % | Vascular Anastomosis | Bone Healing Time (Months) | Length of Humeral Defect (cm) | Length of Fibula Bone (cm) | Number of Proximal/Distal Screws for Internal Fixation | Outcomes | Follow-Up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/19 | osteosarcoma | 90.0 | N | 5 | 12 | 16 | 4/4 | DF | 65 |
| 2 | F/14 | osteosarcoma | 90.0 | Y | 3 | 10 | 14 | 7/5 | DF | 60 |
| 3 | F/28 | osteosarcoma | 83.3 | N | 4 | 14 | 18 | 5/5 | DF | 40 |
| 4 | F/38 | hemangiosarcoma | 86.7 | N | 6 | 8 | 12 | 6/5 | DF | 38 |
| 5 | F/11 | osteosarcoma | 86.7 | Y | 3 | 17 | 21 | 5/3 | DF | 32 |
| 6 | M/14 | osteosarcoma | / | Y | 3 | 15 | 19 | 7/3 | Death | 35 |
| 7 | F/22 | osteosarcoma | 90.0 | N | 4 | 8 | 12 | 6/4 | DF | 51 |
| 8 | F/23 | intermediate fibrohistiocytoma | 86.7 | N | 4 | 8 | 12 | 5/3 | DF | 72 |
| 9 | F/50 | chondrosarcoma | 73.3 | N | 8 | 8 | 12 | 5/4 | DF | 48 |
| 10 | M/14 | osteosarcoma | 80.0 | Y | 5 | 13 | 17 | 5/5 | DF | 30 |
| 11 | F/33 | giant cell tumor | 76.7 | N | 5 | 8 | 12 | 7/4 | DF | 68 |
| 12 | F/17 | osteosarcoma | 80.0 | Y | 3 | 12 | 16 | 4/3 | DF | 87 |
| 13 | F/49 | metastases (lung cancer) | 76.7 | N | 7 | 10 | 14 | 3/3 | DF | 75 |
| 14 | F/63 | osteosarcoma | / | N | 8 | 8 | 12 | 6/3 | Death | 52 |
| 15 | F/9 | osteosarcoma | / | Y | 3 | 12 | 16 | 4/4 | Death | 48 |
| 16 | F/32 | osteosarcoma | 83.3 | N | 4 | 9 | 13 | 6/3 | DF | 81 |
| 17 | F/42 | chondrosarcoma | 73.3 | N | 5 | 8 | 12 | 5/3 | DF | 76 |
| 18 | F/14 | osteosarcoma | 76.7 | Y | 2 | 10 | 14 | 5/5 | DF | 72 |
| 19 | F/73 | metastases (lung cancer) | / | N | 7 | 18 | 22 | 6/3 | Death | 47 |
| 20 | F/28 | chondrosarcoma | 76.7 | N | 4 | 8 | 12 | 8/6 | DF | 61 |
| 21 | M/20 | osteosarcoma | 66.7 | N | 4 | 16 | 20 | 5/6 | DF | 12 |
| 22 | F/10 | osteosarcoma | 80.0 | Y | 3 | 11 | 15 | 5/3 | DF | 75 |
| Average | 28.30 | / | 80.9 | / | 4.5 | 11 | 15 | 5/4 | - | 55.68 |
| Case | Gender/Age | Histological Diagnosis | MSTS Score % | Length of Humeral Defect (cm) | Outcomes | Follow-Up (Months) |
|---|---|---|---|---|---|---|
| 1 | M/42 | chondrosarcoma | - | 14 | Death | 29 |
| 2 | M/22 | osteosarcoma | - | 16 | Death | 23 |
| 3 | F/49 | metastases (kidney cancer) | - | 14 | Death | 38 |
| 4 | M/55 | malignant fibrohistiocytoma | 60.0 | 16 | DF | 124 |
| 5 | M/32 | giant cell tumor | 60.0 | 14 | DF | 117 |
| 6 | M/60 | osteosarcoma | - | 12 | Death | 38 |
| 7 | F/18 | osteosarcoma | 70.0 | 14 | DF | 113 |
| 8 | M/40 | chondrosarcoma | 60.0 | 16 | DF | 111 |
| 9 | M/40 | metastases (liver cancer) | - | 14 | Death | 29 |
| 10 | F/25 | osteofibrous dysplasia | 66.7 | 18 | DF | 102 |
| 11 | M/58 | chondrosarcoma | - | 14 | Death | 27 |
| 12 | M/60 | metastases (liver cancer) | - | 26 | Death | 37 |
| 13 | M/58 | chondroma | 63.3 | 8 | DF | 85 |
| 14 | M/66 | chondrosarcoma | 60.0 | 16 | DF | 60 |
| 15 | F/62 | metastases (lung cancer) | - | 28 | Death | 25 |
| 16 | M/54 | osteosarcoma | 56.7 | 16 | DF | 53 |
| 17 | F/67 | chondrosarcoma | 60.0 | 14 | DF | 54 |
| 18 | F/68 | metastases (lung cancer) | - | 14 | Death | 28 |
| 19 | F/50 | undifferentiated pleomorphic sarcoma | 53.3 | 26 | DF | 45 |
| 20 | M/59 | metastases (kidney cancer) | - | 14 | Death | 30 |
| 21 | F/65 | chondrosarcoma | 46.7 | 24 | DF | 44 |
| 22 | F/65 | metastases (thyroid cancer) | 53.3 | 14 | DF | 38 |
| 23 | M/21 | osteosarcoma | 56.7 | 18 | DF | 24 |
| 24 | F/8 | osteosarcoma | 53.3 | 22 | DF | 23 |
| 25 | M/62 | metastases (kidney cancer) | 53.3 | 12 | DF | 23 |
| 26 | F/63 | metastases (lung cancer) | 50.0 | 10 | DF | 23 |
| 27 | M/80 | metastases (lung cancer) | 53.3 | 14 | DF | 22 |
| Average | 49.90 | - | 57.4 | 16 | - | 50.55 |
| CMS Items | Prosthetic Replacement | Free Vascularized Fibular Graft (FVFG) | ||||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | Min | Max | Mean | SD | |
| Pain | 15 | 15 | 15 | 0 | 15 | 15 | 15 | 0 |
| Activities of daily living | 7 | 12 | 10.06 | 1.52 | 12 | 18 | 15.67 | 2.09 |
| Work | 0 | 2 | 1.06 | 1.03 | 2 | 4 | 3.44 | 0.92 |
| Leisure | 2 | 4 | 2.35 | 0.76 | 2 | 4 | 3.44 | 0.92 |
| Sleep | 1 | 2 | 1.47 | 0.51 | 2 | 2 | 2 | 0 |
| Positioning | 4 | 6 | 5.18 | 1.01 | 6 | 8 | 6.78 | 1.00 |
| Range of motion | 8 | 12 | 11.06 | 0.94 | 16 | 24 | 18.56 | 1.79 |
| Forward elevation | 0 | 2 | 1.29 | 0.99 | 4 | 6 | 4.56 | 0.92 |
| Lateral elevation | 0 | 2 | 1.18 | 1.01 | 2 | 4 | 2.67 | 0.97 |
| External rotation | 2 | 2 | 2 | 0 | 2 | 4 | 2.22 | 0.65 |
| Internal rotation | 4 | 8 | 6.59 | 0.94 | 8 | 10 | 9.11 | 1.02 |
| Strength | 5 | 10 | 7.35 | 2.57 | 10 | 15 | 12.22 | 2.56 |
| Sum of the score | - | - | 61.44 | 4.58 | - | - | 43.47 | 2.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Cheng, D.; Guo, H.; Li, Z.; Fei, X.; Yuan, T.; Yang, Q. A Retrospective Study of the Functional Outcomes in Patients with Proximal Humeral Bone Defect after Shoulder Fusion or Prosthetic Replacement. J. Clin. Med. 2023, 12, 3616. https://doi.org/10.3390/jcm12113616
Pan Z, Cheng D, Guo H, Li Z, Fei X, Yuan T, Yang Q. A Retrospective Study of the Functional Outcomes in Patients with Proximal Humeral Bone Defect after Shoulder Fusion or Prosthetic Replacement. Journal of Clinical Medicine. 2023; 12(11):3616. https://doi.org/10.3390/jcm12113616
Chicago/Turabian StylePan, Zhen, Dongdong Cheng, Hua Guo, Zhaohui Li, Xiang Fei, Ting Yuan, and Qingcheng Yang. 2023. "A Retrospective Study of the Functional Outcomes in Patients with Proximal Humeral Bone Defect after Shoulder Fusion or Prosthetic Replacement" Journal of Clinical Medicine 12, no. 11: 3616. https://doi.org/10.3390/jcm12113616
APA StylePan, Z., Cheng, D., Guo, H., Li, Z., Fei, X., Yuan, T., & Yang, Q. (2023). A Retrospective Study of the Functional Outcomes in Patients with Proximal Humeral Bone Defect after Shoulder Fusion or Prosthetic Replacement. Journal of Clinical Medicine, 12(11), 3616. https://doi.org/10.3390/jcm12113616







