Diagnosis Value of Patient Evaluation Components Applicable in Primary Care Settings for the Diagnosis of Low Back Pain: A Scoping Review of Systematic Reviews
Abstract
1. Introduction
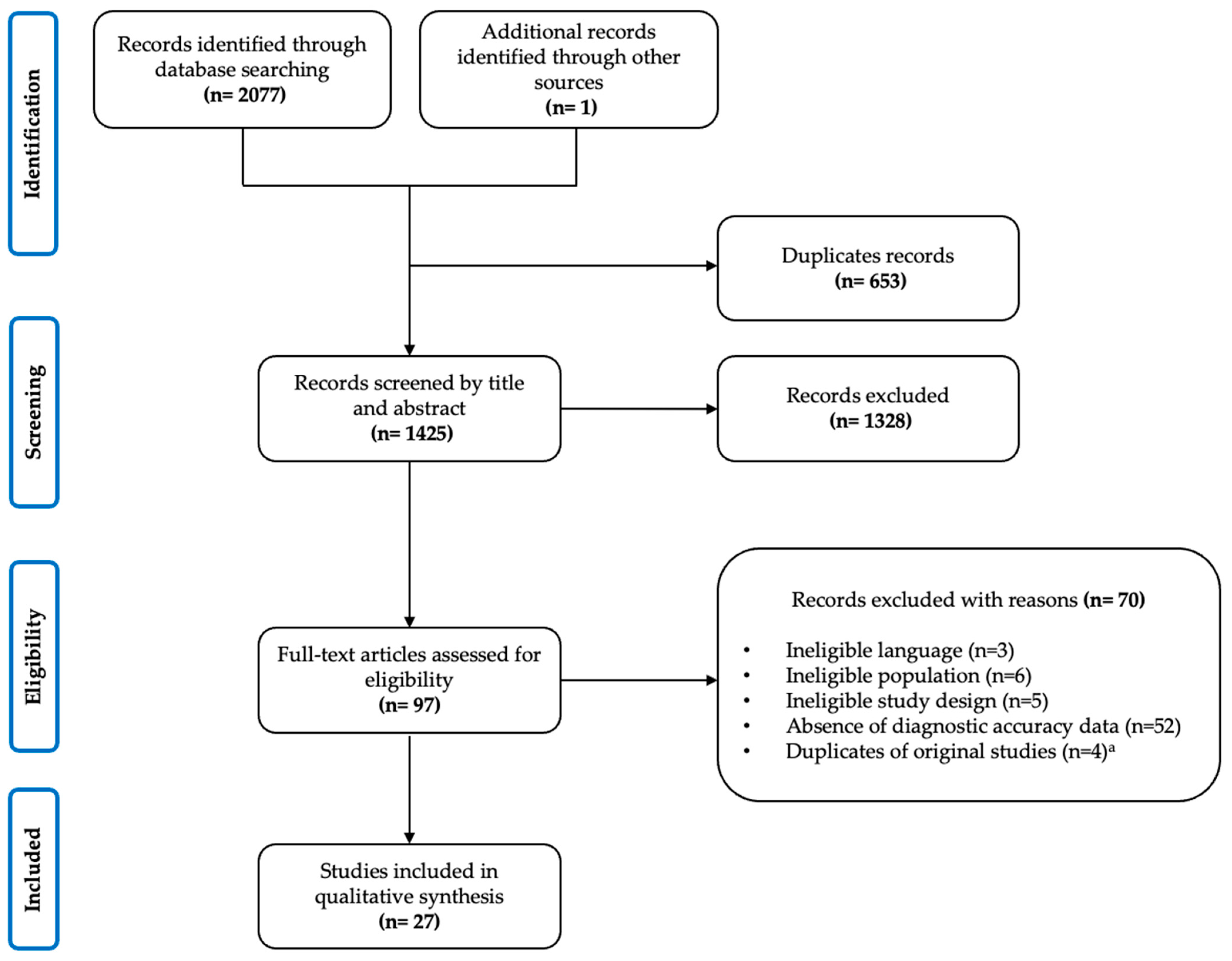
2. Methods
2.1. Study Design
2.2. Search Strategy
2.3. Study Selection
2.3.1. Inclusion Criteria
2.3.2. Screening and Agreement
2.3.3. Data Extraction
2.4. Data synthesis and Analysis
3. Results
3.1. Descriptive Synthesis
3.2. Characteristics of Included Reviews
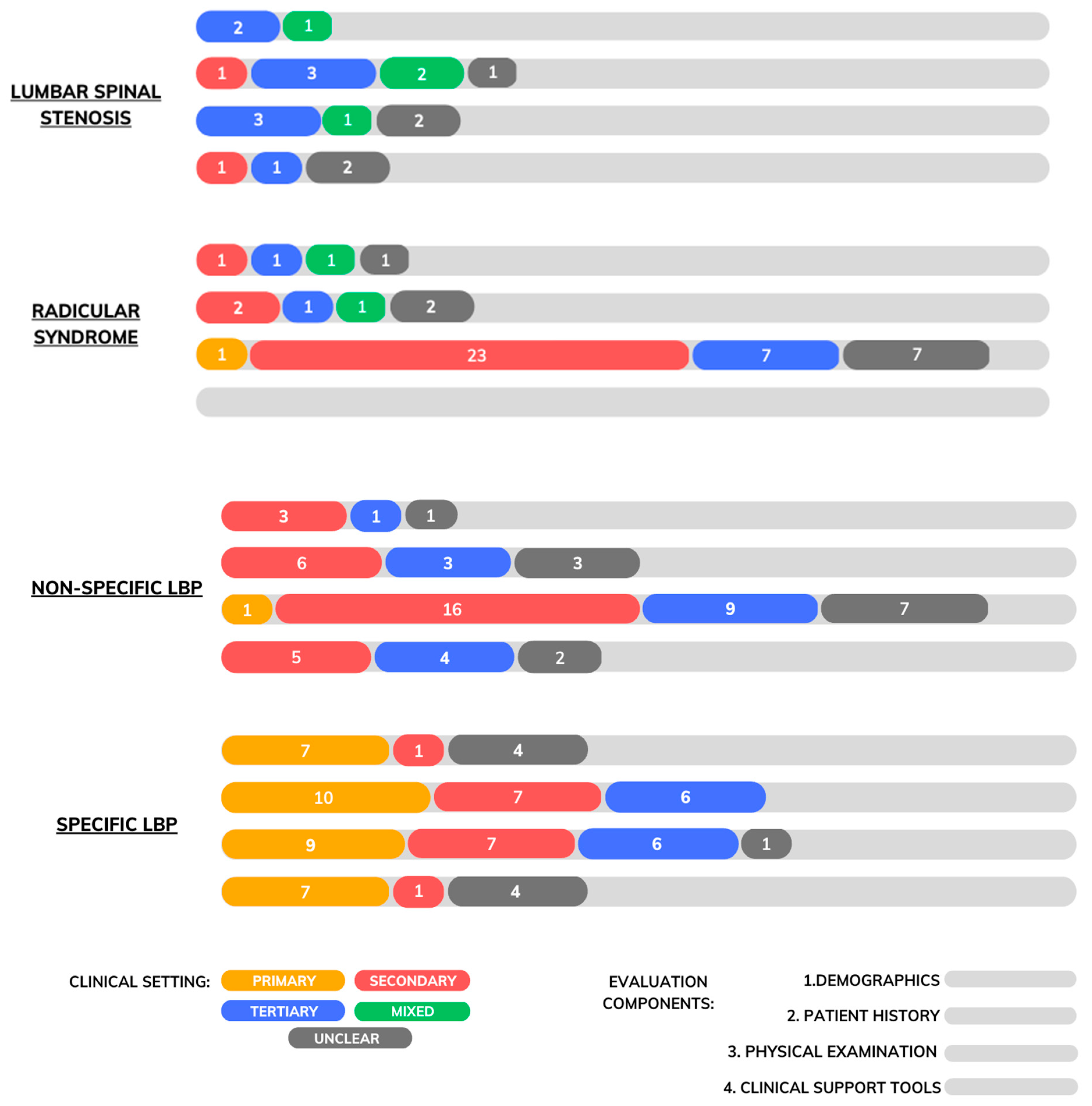
3.3. Lumbar Spinal Stenosis
3.3.1. Demographics
3.3.2. Patient History
3.3.3. Physical Examination
3.3.4. Diagnostic Support Tools
3.4. Radicular Syndrome
3.4.1. Demographics
3.4.2. Patient History
3.4.3. Physical Examination
3.5. Non-Specific Low Back Pain
3.5.1. Demographics
3.5.2. Patient History
3.5.3. Physical Examination
3.5.4. Diagnostic Support Tools
3.6. Specific Low Back Pain
3.6.1. Demographics
- Cauda Equina Syndrome
- Spinal Fracture
- Malignancy
- Any Serious Spinal Pathologies
3.6.2. Patient History
- Cauda Equina Syndrome
- Spinal Fracture
- Malignancy
- Spinal Infection
- Any Serious Spinal Pathologies
3.6.3. Physical Examination
- Cauda Equina Syndrome
- Spinal Fracture
- Malignancy
- Spinal Infection
- Any Serious Spinal Pathologies
3.6.4. Diagnostic Support Tools
- Cauda Equina Syndrome
- Spinal Fracture
- Malignancy
- Spinal Infection
- Inflammatory Back Pain (IBP)
4. Discussion
4.1. Demographics
4.2. Patient History
4.3. Physical Examination
4.4. Clinical Support Tools
4.5. Factors Affecting Interpretation
4.6. Limitations and Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tendances des Dépenses Nationales de Santé-Analyse éclair. Available online: https://www.cihi.ca/fr/tendances-des-depenses-nationales-de-sante-2022-analyse-eclair (accessed on 16 January 2023).
- Rampersaud, Y.R.; Power, J.D.; Perruccio, A.V.; Paterson, J.M.; Veillette, C.; Coyte, P.C.; Badley, E.M.; Mahomed, N.N. Healthcare utilization and costs for spinal conditions in Ontario, Canada—Opportunities for funding high-value care: A retrospective cohort study. Spine J. 2020, 20, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.J.; Côté, P.; Tricco, A.C.; Watson, T.; Rosella, L.C. Effect of back problems on healthcare utilization and costs in Ontario, Canada: A population-based matched cohort study. Pain 2021, 162, 2521–2531. [Google Scholar] [CrossRef] [PubMed]
- Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [CrossRef] [PubMed]
- Buchbinder, R.; van Tulder, M.; Öberg, B.; Costa, L.M.; Woolf, A.; Schoene, M.; Croft, P. Low back pain: A call for action. Lancet 2018, 391, 2384–2388. [Google Scholar] [CrossRef]
- Wong, J.J.; Côté, P.; Sutton, D.A.; Randhawa, K.; Yu, H.; Varatharajan, S.; Goldgrub, R.; Nordin, M.; Gross, D.P.; Shearer, H.M.; et al. Clinical practice guidelines for the noninvasive management of low back pain: A systematic review by the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration. Eur. J. Pain. 2017, 21, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Wilt, T.J.; McLean, R.M.; Forciea, M.A.; Denberg, T.D.; Barry, M.J.; Boyd, C.; Chow, R.D.; Fitterman, N.; Harris, R.P.; et al. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann. Intern. Med. 2017, 166, 514–530. [Google Scholar] [CrossRef] [PubMed]
- Kreiner, D.S.; Shaffer, W.O.; Baisden, J.L.; Gilbert, T.J.; Summers, J.T.; Toton, J.F.; Hwang, S.W.; Mendel, R.C.; Reitman, C.A. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis (update). Spine J. 2013, 13, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Qaseem, A.; Snow, V.; Casey, D.; Cross, J.T., Jr.; Shekelle, P.; Owens, D.K.; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: A joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann. Intern. Med. 2007, 147, 478–491. [Google Scholar] [CrossRef]
- Bussières, A.E.; Stewart, G.; Al-Zoubi, F.; Decina, P.; Descarreaux, M.; Haskett, D.; Hincapié, C.; Pagé, I.; Passmore, S.; Srbely, J.; et al. Spinal Manipulative Therapy and Other Conservative Treatments for Low Back Pain: A Guideline From the Canadian Chiropractic Guideline Initiative. J. Manip. Physiol. Ther. 2018, 41, 265–293. [Google Scholar] [CrossRef]
- Bussières, A.; Cancelliere, C.; Ammendolia, C.; Comer, C.M.; Al Zoubi, F.; Châtillon, C.-E.; Chernish, G.; Cox, J.M.; Gliedt, J.A.; Haskett, D. Non-Surgical Interventions for Lumbar Spinal Stenosis Leading To Neurogenic Claudication: A Clinical Practice Guideline. J. Pain 2021, 22, 1015–1039. [Google Scholar] [CrossRef]
- Bernstein, I.A.; Malik, Q.; Carville, S.; Ward, S. Low back pain and sciatica: Summary of NICE guidance. BMJ 2017, 356, i6748. [Google Scholar] [CrossRef]
- Verhagen, A.P.; Downie, A.; Maher, C.G.; Koes, B.W. Most red flags for malignancy in low back pain guidelines lack empirical support: A systematic review. Pain 2017, 158, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Finucane, L.M.; Downie, A.; Mercer, C.; Greenhalgh, S.M.; Boissonnault, W.G.; Pool-Goudzwaard, A.L.; Beneciuk, J.M.; Leech, R.L.; Selfe, J. International framework for red flags for potential serious spinal pathologies. J. Orthop. Sport. Phys. Ther. 2020, 50, 350–372. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; Maher, C.G.; Hancock, M.J.; McAuley, J.H.; McLachlan, A.J.; Britt, H.; Fahridin, S.; Harrison, C.; Latimer, J. Low back pain and best practice care: A survey of general practice physicians. Arch. Intern. Med. 2010, 170, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Kamper, S.J.; Logan, G.; Copsey, B.; Thompson, J.; Machado, G.C.; Abdel-Shaheed, C.; Williams, C.M.; Maher, C.G.; Hall, A.M. What is usual care for low back pain? A systematic review of health care provided to patients with low back pain in family practice and emergency departments. Pain 2020, 161, 694–702. [Google Scholar] [CrossRef]
- Bishop, P.B.; Wing, P.C. Compliance with clinical practice guidelines in family physicians managing worker’s compensation board patients with acute lower back pain. Spine J. 2003, 3, 442–450. [Google Scholar] [CrossRef]
- Debono, B.; Sabatier, P.; Koudsie, A.; Buffenoir, K.; Hamel, O. Managing spine surgery referrals: The consultation of neurosurgery and its nuances. Neurochirurgie 2017, 63, 267–272. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Peters, M.D.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. JBI Evid. Implement. 2015, 13, 141–146. [Google Scholar] [CrossRef]
- Cook, C.J.; Cook, C.E.; Reiman, M.P.; Joshi, A.B.; Richardson, W.; Garcia, A.N. Systematic review of diagnostic accuracy of patient history, clinical findings, and physical tests in the diagnosis of lumbar spinal stenosis. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2020, 29, 93–112. [Google Scholar] [CrossRef] [PubMed]
- de Schepper, E.I.T.; Overdevest, G.M.; Suri, P.; Peul, W.C.; Oei, E.H.G.; Koes, B.W.; Bierma-Zeinstra, S.M.A.; Luijsterburg, P.A.J. Diagnosis of lumbar spinal stenosis: An updated systematic review of the accuracy of diagnostic tests. Spine 2013, 38, E469–E481. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Heneghan, N.R.; Noblet, T.; Falla, D.; Rushton, A. Diagnostic utility of patient history, clinical examination and screening tool data to identify neuropathic pain in low back related leg pain: A systematic review and narrative synthesis. BMC Musculoskelet. Disord. 2020, 21, 532. [Google Scholar] [CrossRef]
- Tawa, N.; Rhoda, A.; Diener, I. Accuracy of clinical neurological examination in diagnosing lumbo-sacral radiculopathy: A systematic literature review. BMC Musculoskelet. Disord. 2017, 18, 93. [Google Scholar] [CrossRef] [PubMed]
- Al Nezari, N.H.; Schneiders, A.G.; Hendrick, P.A. Neurological examination of the peripheral nervous system to diagnose lumbar spinal disc herniation with suspected radiculopathy: A systematic review and meta-analysis. Spine J. Off. J. N. Am. Spine Soc. 2013, 13, 657–674. [Google Scholar] [CrossRef]
- Scaia, V.; Baxter, D.; Cook, C. The pain provocation-based straight leg raise test for diagnosis of lumbar disc herniation, lumbar radiculopathy, and/or sciatica: A systematic review of clinical utility. J. Back Musculoskelet. Rehabil. 2012, 25, 215–223. [Google Scholar] [CrossRef]
- van der Windt, D.A.; Simons, E.; Riphagen, I.; Ammendolia, C.; Verhagen, A.P.; Laslett, M.; Devillé, W.; Deyo, R.A.; Bouter, L.M.; de Vet, H.C.; et al. Physical examination for lumbar radiculopathy due to disc herniation in patients with low-back pain. Cochrane Database Syst. Rev. 2010, CD007431. [Google Scholar] [CrossRef]
- Devillé, W.L.; van der Windt, D.A.; Dzaferagić, A.; Bezemer, P.D.; Bouter, L.M. The test of Lasègue: Systematic review of the accuracy in diagnosing herniated discs. Spine 2000, 25, 1140–1147. [Google Scholar] [CrossRef]
- Han, C.S.; Hancock, M.J.; Sharma, S.; Sharma, S.; Harris, I.A.; Cohen, S.P.; Magnussen, J.; Maher, C.G.; Traeger, A.C. Low back pain of disc, sacroiliac joint, or facet joint origin: A diagnostic accuracy systematic review. eClinicalMedicine 2023, 59, 101960. [Google Scholar] [CrossRef]
- Nolet, P.S.; Yu, H.; Côté, P.; Meyer, A.-L.; Kristman, V.L.; Sutton, D.; Murnaghan, K.; Lemeunier, N. Reliability and validity of manual palpation for the assessment of patients with low back pain: A systematic and critical review. Chiropr. Man. Ther. 2021, 29, 33. [Google Scholar] [CrossRef]
- Stolz, M.; von Piekartz, H.; Hall, T.; Schindler, A.; Ballenberger, N. Evidence and recommendations for the use of segmental motion testing for patients with LBP—A systematic review. Musculoskelet. Sci. Pract. 2020, 45, 102076. [Google Scholar] [CrossRef] [PubMed]
- Maas, E.T.; Juch, J.N.S.; Ostelo, R.W.J.G.; Groeneweg, J.G.; Kallewaard, J.W.; Koes, B.W.; Verhagen, A.P.; Huygen, F.J.P.M.; Tulder, M.W. Systematic review of patient history and physical examination to diagnose chronic low back pain originating from the facet joints. Eur. J. Pain 2017, 21, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Grødahl, L.H.J.; Fawcett, L.; Nazareth, M.; Smith, R.; Spencer, S.; Heneghan, N.; Rushton, A. Diagnostic utility of patient history and physical examination data to detect spondylolysis and spondylolisthesis in athletes with low back pain: A systematic review. Man. Ther. 2016, 24, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Manni, T.; Bonetti, F.; Hugo Villafañe, J.; Vanti, C. A literature review of clinical tests for lumbar instability in low back pain: Validity and applicability in clinical practice. Chiropr. Man. Ther. 2015, 23, 14. [Google Scholar] [CrossRef]
- Sivayogam, A.; Banerjee, A. Diagnostic performance of clinical tests for sacroiliac joint pain. Phys. Ther. Rev. 2011, 16, 462–467. [Google Scholar] [CrossRef]
- Alqarni, A.M.; Schneiders, A.G.; Hendrick, P.A. Clinical Tests to Diagnose Lumbar Segmental Instability: A Systematic Review. J. Orthop. Sport. Phys. Ther. 2011, 41, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Hancock, M.J.; Maher, C.G.; Latimer, J.; Spindler, M.F.; McAuley, J.H.; Laslett, M.; Bogduk, N. Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2007, 16, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.; Gemmell, H. Accuracy of spinal orthopaedic tests: A systematic review. Chiropr. Osteopat. 2006, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Tabrah, J.; Wilson, N.; Phillips, D.; Böhning, D. Can digital rectal examination be used to detect cauda equina compression in people presenting with acute cauda equina syndrome? A systematic review and meta-analysis of diagnostic test accuracy studies. Musculoskelet. Sci. Pract. 2022, 58, 102523. [Google Scholar] [CrossRef]
- Galliker, G.; Scherer, D.E.; Trippolini, M.A.; Rasmussen-Barr, E.; LoMartire, R.; Wertli, M.M. Low Back Pain in the Emergency Department: Prevalence of Serious Spinal Pathologies and Diagnostic Accuracy of Red Flags. Am. J. Med. 2020, 133, 60. [Google Scholar] [CrossRef]
- Maselli, F.; Palladino, M.; Barbari, V.; Storari, L.; Rossettini, G.; Testa, M. The diagnostic value of Red Flags in thoracolumbar pain: A systematic review. Disabil. Rehabil. 2020, 44, 1190–1206. [Google Scholar] [CrossRef] [PubMed]
- Dionne, N.; Adefolarin, A.; Kunzelman, D.; Trehan, N.; Finucane, L.; Levesque, L.; Walton, D.M.; Sadi, J. What is the diagnostic accuracy of red flags related to cauda equina syndrome (CES), when compared to Magnetic Resonance Imaging (MRI)? A systematic review. Musculoskelet. Sci. Pract. 2019, 42, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; Henschke, N.; Maher, C.G.; van Tulder, M.W.; Koes, B.W.; Macaskill, P.; Irwig, L. Red flags to screen for vertebral fracture in patients presenting with low-back pain. Cochrane Database Syst. Rev. 2013, CD008643. [Google Scholar] [CrossRef] [PubMed]
- Henschke, N.; Maher, C.G.; Ostelo, R.W.; de Vet, H.C.; Macaskill, P.; Irwig, L. Red flags to screen for malignancy in patients with low-back pain. Cochrane Database Syst. Rev. 2013, CD008686. [Google Scholar] [CrossRef] [PubMed]
- Henschke, N.; Maher, C.G.; Refshauge, K.M. A systematic review identifies five “red flags” to screen for vertebral fracture in patients with low back pain. J. Clin. Epidemiol. 2008, 61, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Haskins, R.; Osmotherly, P.G.; Rivett, D.A. Diagnostic Clinical Prediction Rules for Specific Subtypes of Low Back Pain: A Systematic Review. J. Orthop. Sport. Phys. Ther. 2015, 45, 61–76. [Google Scholar] [CrossRef]
- Shultz, S.; Averell, K.; Eickelman, A.; Sanker, H.; Donaldson, M.B. Diagnostic accuracy of self-report and subjective history in the diagnosis of low back pain with non-specific lower extremity symptoms: A systematic review. Man. Ther. 2015, 20, 18–27. [Google Scholar] [CrossRef]
- Stochkendahl, M.J.; Kjaer, P.; Hartvigsen, J.; Kongsted, A.; Aaboe, J.; Andersen, M.; Andersen, M.; Fournier, G.; Højgaard, B.; Jensen, M.B.; et al. National Clinical Guidelines for non-surgical treatment of patients with recent onset low back pain or lumbar radiculopathy. Eur. Spine J. 2018, 27, 60–75. [Google Scholar] [CrossRef]
- Cook, C.; Brown, C.; Michael, K.; Isaacs, R.; Howes, C.; Richardson, W.; Roman, M.; Hegedus, E. The clinical value of a cluster of patient history and observational findings as a diagnostic support tool for lumbar spine stenosis. Physiother. Res. Int. 2011, 16, 170–178. [Google Scholar] [CrossRef]
- Katz, J.N.; Dalgas, M.; Stucki, G.; Katz, N.P.; Bayley, J.; Fossel, A.H.; Chang, L.C.; Lipson, S.J. Degenerative lumbar spinal stenosis. Diagnostic value of the history and physical examination. Arthritis. Rheum. 1995, 38, 1236–1241. [Google Scholar] [CrossRef]
- Konno, S.; Hayashino, Y.; Fukuhara, S.; Kikuchi, S.; Kaneda, K.; Seichi, A.; Chiba, K.; Satomi, K.; Nagata, K.; Kawai, S. Development of a clinical diagnosis support tool to identify patients with lumbar spinal stenosis. Eur. Spine J. 2007, 16, 1951–1957. [Google Scholar] [CrossRef]
- Fritz, J.M.; Erhard, R.E.; Delitto, A.; Welch, W.C.; Nowakowski, P.E. Preliminary results of the use of a two-stage treadmill test as a clinical diagnostic tool in the differential diagnosis of lumbar spinal stenosis. J. Spinal. Disord. 1997, 10, 410–416. [Google Scholar] [CrossRef]
- Sugioka, T.; Hayashino, Y.; Konno, S.; Kikuchi, S.; Fukuhara, S. Predictive value of self-reported patient information for the identification of lumbar spinal stenosis. Fam. Pract. 2008, 25, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.H.; Schmidt-Olsen, S. A new functional test in the diagnostic evaluation of neurogenic intermittent claudication. Clin. Rheumatol. 1989, 8, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, R.; May, S.; Hope, P. The validity of a clinical test for the diagnosis of lumbar spinal stenosis. Man Ther. 2016, 25, 27–34. [Google Scholar] [CrossRef]
- Kato, Y.; Kawakami, T.; Kifune, M.; Kishimoto, T.; Nibu, K.; Oda, H.; Shirasawa, K.; Tominaga, T.; Toyoda, K.; Tsue, K.; et al. Validation study of a clinical diagnosis support tool for lumbar spinal stenosis. J. Orthop. Sci. 2009, 14, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Coster, S.; de Bruijn, S.F.; Tavy, D.L. Diagnostic value of history, physical examination and needle electromyography in diagnosing lumbosacral radiculopathy. J. Neurol. 2010, 257, 332–337. [Google Scholar] [CrossRef]
- Verwoerd, A.J.; Peul, W.C.; Willemsen, S.P.; Koes, B.W.; Vleggeert-Lankamp, C.L.; el Barzouhi, A.; Luijsterburg, P.A.; Verhagen, A.P. Diagnostic accuracy of history taking to assess lumbosacral nerve root compression. Spine J. 2014, 14, 2028–2037. [Google Scholar] [CrossRef]
- Vroomen, P.C.; de Krom, M.C.; Wilmink, J.T.; Kester, A.D.; Knottnerus, J.A. Diagnostic value of history and physical examination in patients suspected of lumbosacral nerve root compression. J. Neurol. Neurosurg. Psychiatry 2002, 72, 630–634. [Google Scholar] [CrossRef]
- Vucetic, N.; de Bri, E.; Svensson, O. Clinical history in lumbar disc herniation. A prospective study in 160 patients. Acta Orthop. Scand. 1997, 68, 116–120. [Google Scholar] [CrossRef]
- Beattie, P.F.; Meyers, S.P.; Stratford, P.; Millard, R.W.; Hollenberg, G.M. Associations between patient report of symptoms and anatomic impairment visible on lumbar magnetic resonance imaging. Spine (Phila Pa 1976) 2000, 25, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Smart, K.M.; Blake, C.; Staines, A.; Thacker, M.; Doody, C. Mechanisms-based classifications of musculoskeletal pain: Part 2 of 3: Symptoms and signs of peripheral neuropathic pain in patients with low back (± leg) pain. Man. Ther. 2012, 17, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Gregg, C.D.; Dean, S.; Schneiders, A.G. Variables associated with active spondylolysis. Phys. Sport 2009, 10, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Laslett, M.; McDonald, B.; Aprill, C.N.; Tropp, H.; Oberg, B. Clinical predictors of screening lumbar zygapophyseal joint blocks: Development of clinical prediction rules. Spine J. 2006, 6, 370–379. [Google Scholar] [CrossRef]
- Manchikanti, L.; Pampati, V.; Fellows, B.; Baha, A.G. The inability of the clinical picture to characterize pain from facet joints. Pain Physician 2000, 3, 158–166. [Google Scholar] [CrossRef]
- Manchikanti, L.; Pampati, V.; Fellows, B.; Bakhit, C.E. Prevalence of lumbar facet joint pain in chronic low back pain. Pain Physician 1999, 2, 59–64. [Google Scholar] [CrossRef]
- Revel, M.; Poiraudeau, S.; Auleley, G.R.; Payan, C.; Denke, A.; Nguyen, M.; Chevrot, A.; Fermanian, J. Capacity of the clinical picture to characterize low back pain relieved by facet joint anesthesia. Proposed criteria to identify patients with painful facet joints. Spine (Phila Pa 1976) 1998, 23, 1972–1976; discussion 1977. [Google Scholar] [CrossRef]
- Depalma, M.J.; Ketchum, J.M.; Trussell, B.S.; Saullo, T.R.; Slipman, C.W. Does the location of low back pain predict its source? Pm R 2011, 3, 33–39. [Google Scholar] [CrossRef]
- Dreyfuss, P.; Michaelsen, M.; Pauza, K.; McLarty, J.; Bogduk, N. The value of medical history and physical examination in diagnosing sacroiliac joint pain. Spine (Phila Pa 1976) 1996, 21, 2594–2602. [Google Scholar] [CrossRef]
- Jung, J.H.; Kim, H.I.; Shin, D.A.; Shin, D.G.; Lee, J.O.; Kim, H.J.; Chung, J.H. Usefulness of pain distribution pattern assessment in decision-making for the patients with lumbar zygapophyseal and sacroiliac joint arthropathy. J. Korean Med. Sci. 2007, 22, 1048–1054. [Google Scholar] [CrossRef]
- Kalpakcioglu, B.; Altinbilek, T.; Senel, K. Determination of spondylolisthesis in low back pain by clinical evaluation. J. Back. Musculoskelet. Rehabil. 2009, 22, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Lewinnek, G.E.; Warfield, C.A. Facet joint degeneration as a cause of low back pain. Clin. Orthop. Relat. Res. 1986, 216–222. [Google Scholar] [CrossRef]
- Revel, M.E.; Listrat, V.M.; Chevalier, X.J.; Dougados, M.; N’Guyen, M.P.; Vallee, C.; Wybier, M.; Gires, F.; Amor, B. Facet joint block for low back pain: Identifying predictors of a good response. Arch. Phys. Med. Rehabil. 1992, 73, 824–828. [Google Scholar] [PubMed]
- Schwarzer, A.C.; Aprill, C.N.; Derby, R.; Fortin, J.; Kine, G.; Bogduk, N. Clinical features of patients with pain stemming from the lumbar zygapophysial joints. Is the lumbar facet syndrome a clinical entity? Spine (Phila Pa 1976) 1994, 19, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Adelmanesh, F.; Jalali, A.; Shirvani, A.; Pakmanesh, K.; Pourafkari, M.; Raissi, G.R.; Shir, Y. The Diagnostic Accuracy of Gluteal Trigger Points to Differentiate Radicular From Nonradicular Low Back Pain. Clin. J. Pain 2016, 32, 666–672. [Google Scholar] [CrossRef]
- Koppenhaver, S.L.; Hebert, J.J.; Kawchuk, G.N.; Childs, J.D.; Teyhen, D.S.; Croy, T.; Fritz, J.M. Criterion validity of manual assessment of spinal stiffness. Man. Ther. 2014, 19, 589–594. [Google Scholar] [CrossRef]
- Walsh, J.; Hall, T. Reliability, validity and diagnostic accuracy of palpation of the sciatic, tibial and common peroneal nerves in the examination of low back related leg pain. Man. Ther. 2009, 14, 623–629. [Google Scholar] [CrossRef]
- Ahn, K.; Jhun, H.J. New physical examination tests for lumbar spondylolisthesis and instability: Low midline sill sign and interspinous gap change during lumbar flexion-extension motion. BMC Musculoskelet. Disord. 2015, 16, 97. [Google Scholar] [CrossRef]
- Collaer, J.W.; McKeough, D.M.; Boissonnault, W.G. Lumbar isthmic spondylolisthesis detection with palpation: Interrater reliability and concurrent criterion-related validity. J. Man. Manip. Ther. 2006, 14, 22–29. [Google Scholar] [CrossRef]
- Laslett, M.; McDonald, B.; Tropp, H.; Aprill, C.N.; Oberg, B. Agreement between diagnoses reached by clinical examination and available reference standards: A prospective study of 216 patients with lumbopelvic pain. BMC Musculoskelet. Disord. 2005, 6, 28. [Google Scholar] [CrossRef]
- Laslett, M.; Oberg, B.; Aprill, C.N.; McDonald, B. Zygapophysial joint blocks in chronic low back pain: A test of Revel’s model as a screening test. BMC Musculoskelet. Disord. 2004, 5, 43. [Google Scholar] [CrossRef]
- Laslett, M.; Young, S.B.; Aprill, C.N.; McDonald, B. Diagnosing painful sacroiliac joints: A validity study of a McKenzie evaluation and sacroiliac provocation tests. Aust. J. Physiother. 2003, 49, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Stanford, G.; Burnham, R.S. Is it useful to repeat sacroiliac joint provocative tests post-block? Pain Med. 2010, 11, 1774–1776. [Google Scholar] [CrossRef]
- van der Wurff, P.; Buijs, E.J.; Groen, G.J. A multitest regimen of pain provocation tests as an aid to reduce unnecessary minimally invasive sacroiliac joint procedures. Arch. Phys. Med. Rehabil. 2006, 87, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Aprill, C.; Laslett, M. Correlation of clinical examination characteristics with three sources of chronic low back pain. Spine J. 2003, 3, 460–465. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, M.A.; Hollingworth, W.; Kinmonth, A.L.; Dixon, A.K. Evidence against the use of lumbar spine radiography for low back pain. Clin. Radiol. 2004, 59, 69–76. [Google Scholar] [CrossRef]
- Deyo, R.A.; Diehl, A.K. Lumbar spine films in primary care: Current use and effects of selective ordering criteria. J. Gen. Intern. Med. 1986, 1, 20–25. [Google Scholar] [CrossRef]
- Enthoven, W.T.; Geuze, J.; Scheele, J.; Bierma-Zeinstra, S.M.; Bueving, H.J.; Bohnen, A.M.; Peul, W.C.; van Tulder, M.W.; Berger, M.Y.; Koes, B.W.; et al. Prevalence and “Red Flags” Regarding Specified Causes of Back Pain in Older Adults Presenting in General Practice. Phys. Ther. 2016, 96, 305–312. [Google Scholar] [CrossRef]
- Henschke, N.; Maher, C.G.; Refshauge, K.M.; Herbert, R.D.; Cumming, R.G.; Bleasel, J.; York, J.; Das, A.; McAuley, J.H. Prevalence of and screening for serious spinal pathology in patients presenting to primary care settings with acute low back pain. Arthritis Rheum 2009, 60, 3072–3080. [Google Scholar] [CrossRef]
- Premkumar, A.; Godfrey, W.; Gottschalk, M.B.; Boden, S.D. Red Flags for Low Back Pain Are Not Always Really Red: A Prospective Evaluation of the Clinical Utility of Commonly Used Screening Questions for Low Back Pain. J. Bone Jt. Surg. Am. 2018, 100, 368–374. [Google Scholar] [CrossRef]
- Roman, M.; Brown, C.; Richardson, W.; Isaacs, R.; Howes, C.; Cook, C. The development of a clinical decision making algorithm for detection of osteoporotic vertebral compression fracture or wedge deformity. J. Man. Manip. Ther. 2010, 18, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Diehl, A.K. Cancer as a cause of back pain: Frequency, clinical presentation, and diagnostic strategies. J. Gen. Intern. Med. 1988, 3, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Frazier, L.M.; Carey, T.S.; Lyles, M.F.; Khayrallah, M.A.; McGaghie, W.C. Selective criteria may increase lumbosacral spine roentgenogram use in acute low-back pain. Arch. Intern. Med. 1989, 149, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.F. Musculoskeletal pain as an indicator of occult malignancy. Yield of bone scintigraphy. Arch. Intern. Med. 1997, 157, 105–109. [Google Scholar] [CrossRef]
- Shaw, B.; Kinsella, R.; Henschke, N.; Walby, A.; Cowan, S. Back pain “red flags”: Which are most predictive of serious pathology in the Emergency Department? Eur. Spine J. 2020, 29, 1870–1878. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Kalsi, P.; Greenough, C.G.; Kuskoor Seetharam, M.P. Reliability of clinical assessment in diagnosing cauda equina syndrome. Br. J. Neurosurg. 2010, 24, 383–386. [Google Scholar] [CrossRef]
- Bell, D.A.; Collie, D.; Statham, P.F. Cauda equina syndrome: What is the correlation between clinical assessment and MRI scanning? Br. J. Neurosurg. 2007, 21, 201–203. [Google Scholar] [CrossRef]
- Domen, P.M.; Hofman, P.A.; van Santbrink, H.; Weber, W.E. Predictive value of clinical characteristics in patients with suspected cauda equina syndrome. Eur. J. Neurol. 2009, 16, 416–419. [Google Scholar] [CrossRef]
- Gooding, B.W.; Higgins, M.A.; Calthorpe, D.A. Does rectal examination have any value in the clinical diagnosis of cauda equina syndrome? Br. J. Neurosurg. 2013, 27, 156–159. [Google Scholar] [CrossRef]
- Rooney, A.; Statham, P.F.; Stone, J. Cauda equina syndrome with normal MR imaging. J. Neurol. 2009, 256, 721–725. [Google Scholar] [CrossRef]
- Tsiang, J.T.; Kinzy, T.G.; Thompson, N.; Tanenbaum, J.E.; Thakore, N.L.; Khalaf, T.; Katzan, I.L. Sensitivity and specificity of patient-entered red flags for lower back pain. Spine J. 2019, 19, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.; Zoltie, N. Radiography for back pain presenting to accident and emergency departments. Arch. Emerg. Med. 1992, 9, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J.F.; Panacek, E.A.; Miller, P.Q.; Lapidis, A.D.; Mower, W.R. Prospective evaluation of criteria for obtaining thoracolumbar radiographs in trauma patients. J. Emerg. Med. 2003, 24, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Patrick, J.D.; Doris, P.E.; Mills, M.L.; Friedman, J.; Johnston, C. Lumbar spine x-rays: A multihospital study. Ann. Emerg. Med. 1983, 12, 84–87. [Google Scholar] [CrossRef]
- Reinus, W.R.; Strome, G.; Zwemer, F.L., Jr. Use of lumbosacral spine radiographs in a level II emergency department. AJR Am. J. Roentgenol. 1998, 170, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Scavone, J.G.; Latshaw, R.F.; Rohrer, G.V. Use of lumbar spine films. Statistical evaluation at a university teaching hospital. Jama 1981, 246, 1105–1108. [Google Scholar] [CrossRef]
- Donner-Banzhoff, N.; Roth, T.; Sönnichsen, A.C.; Luckmann, J.; Leonhardt, C.; Chenot, J.F.; Becker, A.; Keller, S.; Griffiths, F.; Baum, E. Evaluating the accuracy of a simple heuristic to identify serious causes of low back pain. Fam. Pract. 2006, 23, 682–686. [Google Scholar] [CrossRef]
- Davis, D.P.; Salazar, A.; Chan, T.C.; Vilke, G.M. Prospective evaluation of a clinical decision guideline to diagnose spinal epidural abscess in patients who present to the emergency department with spine pain. J. Neurosurg. Spine 2011, 14, 765–770. [Google Scholar] [CrossRef]
- Thiruganasambandamoorthy, V.; Turko, E.; Ansell, D.; Vaidyanathan, A.; Wells, G.A.; Stiell, I.G. Risk factors for serious underlying pathology in adult emergency department nontraumatic low back pain patients. J. Emerg. Med. 2014, 47, 1–11. [Google Scholar] [CrossRef]
- Angus, M.; Berg, A.; Carrasco, R.; Horner, D.; Leach, J.; Siddique, I. The Cauda Scale—Validation for Clinical Practice. Br. J. Neurosurg. 2020, 34, 453–456. [Google Scholar] [CrossRef]
- Raison, N.T.; Alwan, W.; Abbot, A.; Farook, M.; Khaleel, A. The reliability of red flags in spinal cord compression. Arch. Trauma Res. 2014, 3, e17850. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, M.; Nasto, L.; Tsegaye, M.; Grevitt, M. Bladder Scans and Postvoid Residual Volume Measurement Improve Diagnostic Accuracy of Cauda Equina Syndrome. Spine (Phila Pa 1976) 2019, 44, 1303–1308. [Google Scholar] [CrossRef]
- Gestring, M.L.; Gracias, V.H.; Feliciano, M.A.; Reilly, P.M.; Shapiro, M.B.; Johnson, J.W.; Klein, W.; Kauder, D.R.; Schwab, C.W. Evaluation of the lower spine after blunt trauma using abdominal computed tomographic scanning supplemented with lateral scanograms. J. Trauma 2002, 53, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.M.; Joseph, T.; Ellis, A.M. Thoracolumbar fracture in blunt trauma patients: Guidelines for diagnosis and imaging. Injury 2003, 34, 426–433. [Google Scholar] [CrossRef]
- Terregino, C.A.; Ross, S.E.; Lipinski, M.F.; Foreman, J.; Hughes, R. Selective indications for thoracic and lumbar radiography in blunt trauma. Ann. Emerg. Med. 1995, 26, 126–129. [Google Scholar] [CrossRef]
- Khoo, L.A.; Heron, C.; Patel, U.; Given-Wilson, R.; Grundy, A.; Khaw, K.T.; Dundas, D. The diagnostic contribution of the frontal lumbar spine radiograph in community referred low back pain--a prospective study of 1030 patients. Clin. Radiol. 2003, 58, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.P.; Wold, R.M.; Patel, R.J.; Tran, A.J.; Tokhi, R.N.; Chan, T.C.; Vilke, G.M. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J. Emerg. Med. 2004, 26, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Saracbasi, E.; Grifka, J.; Schnitker, J.; Braun, J. Identifying patients with axial spondyloarthritis in primary care: How useful are items indicative of inflammatory back pain? Ann. Rheum. Dis. 2011, 70, 1782–1787. [Google Scholar] [CrossRef]
- Chan, C.C.; Inrig, T.; Molloy, C.B.; Stone, M.A.; Derzko-Dzulynsky, L. Prevalence of inflammatory back pain in a cohort of patients with anterior uveitis. Am. J. Ophthalmol. 2012, 153, 1025–1030.e1. [Google Scholar] [CrossRef]
- Rudwaleit, M.; Metter, A.; Listing, J.; Sieper, J.; Braun, J. Inflammatory back pain in ankylosing spondylitis: A reassessment of the clinical history for application as classification and diagnostic criteria. Arthritis Rheum 2006, 54, 569–578. [Google Scholar] [CrossRef]
- Sieper, J.; van der Heijde, D.; Landewé, R.; Brandt, J.; Burgos-Vagas, R.; Collantes-Estevez, E.; Dijkmans, B.; Dougados, M.; Khan, M.A.; Leirisalo-Repo, M.; et al. New criteria for inflammatory back pain in patients with chronic back pain: A real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann. Rheum. Dis. 2009, 68, 784–788. [Google Scholar] [CrossRef]
- Verhagen, A.P.; Downie, A.; Popal, N.; Maher, C.; Koes, B.W. Red flags presented in current low back pain guidelines: A review. Eur. Spine J. 2016, 25, 2788–2802. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.; Polisena, J.; Husereau, D.; Moulton, K.; Clark, M.; Fiander, M.; Mierzwinski-Urban, M.; Clifford, T.; Hutton, B.; Rabb, D. The effect of English-language restriction on systematic review-based meta-analyses: A systematic review of empirical studies. Int. J. Technol. Assess Health Care 2012, 28, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer-Streit, B.; Klerings, I.; Dobrescu, A.I.; Persad, E.; Stevens, A.; Garritty, C.; Kamel, C.; Affengruber, L.; King, V.J.; Gartlehner, G. Excluding non-English publications from evidence-syntheses did not change conclusions: A meta-epidemiological study. J. Clin. Epidemiol. 2020, 118, 42–54. [Google Scholar] [CrossRef] [PubMed]
| Likelihood Ratios | Interpretation |
|---|---|
| +LR > 10 or −LR < 0.1 | Large |
| +LR 5–10 or −LR 0.1–0.2 | Moderate |
| +LR 2–5 or −LR 0.2–0.5 | Small |
| +LR 1–2 or −LR 0.5–1 | Very small |
| LUMBAR SPINAL STENOSIS | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| First Author | Year of Publication | End of Search | Country | Settings of Data Collection | Study Design(s) | Number of Primary Studies | Number of Participants | Population | Meta-Analysis |
| Cook | 2020 [22] | Nov 2018 | USA | Mixed | Prospective (7); Multicenter cross-sectional (1); Retrospective cohort (1) | n = 9 | N = 36,228 | Adult (>18 yrs) patients with LBP of any duration with a suspicion of LSS | No |
| De Schepper | 2013 [23] | March 2011 | NLD | Mixed | Prospective | n = 15 | N = 2909 | Adult patients with LSS | No |
| RADICULAR SYNDROME (Lumbar radiculopathy, LDH) | |||||||||
| Mistry | 2020 [24] | July 2019 | UK | Mixed | Cross-sectional observational study (11) | n = 11 | N = 3908 | Adult participants with LBLP | No |
| Tawa | 2017 [25] | July 2016 | Kenya | Mixed | Cohort study (11); case control (1) | n = 12 | N = 1026 | Subjects with clinical signs and symptoms consistent with lumbo-sacral radiculopathy. | No |
| Al Nezari | 2013 [26] | March 2011 | NZ | Mixed | Prospective cohort (12); Case-control (2) | n = 14 | N = 7200 | Patients with LBP of any duration with suspicion of radiculopathy caused by a potential LDH | Yes |
| Scaia | 2012 [27] | Dec 2011 | USA | Mixed | Case control, case-based case control, and cohort studies | n = 7 | N = 4311 | Patients with suspected LDH, lumbar radiculopathy or sciatica | No |
| Van der Windt | 2010 [28] | April 2008 | UK | Mixed | Case-control study (3); prospective cohort (11); retrospective cohort (5) | n = 19 | Cohort: median N = 126, range 71-2504 Case control: 38–100 cases | Patients with low-back pain with pain radiating into the leg, who were suspected of having radiculopathy due to LDH | Yes |
| Devillé | 2000 [29] | 1997 | NLD | Mixed | Unclear | n = 15 | NA | Unclear | Yes |
| NON-SPECIFIC LOW BACK PAIN | |||||||||
| Han | 2023 [30] | Jan 2023 | Australia | Mixed (secondary and tertiary care) | NA | n = 62 | Ranged from 15 to 736 | Patients with LBP without serious pathology | Yes |
| Nolet | 2021 [31] | July 2019 | Canada | NA | NA | n = 7 | N = 777 | Adult patients with LBP with or without radiculopathy of any duration | No |
| Stolz | 2020 [32] | Sept 2019 | Germany | Mixed | NA | n = 13 (3 validity studies) | N = 235 | Included at least one group of adult participants that had suffered from LBP | No |
| Maas | 2017 [33] | June 2016 | NLD | Mixed | Cross-sectional (10); case-control (1); Retrospective cohort (1) | n = 12 | N = 1504 | Adult patients, of either gender, suffering from CLBP | No |
| Grodahl | 2016 [34] | Nov 2015 | Norway | Mixed | Prospective (3); Retrospective (2); Cross-sectional (1); Non-experimental (1); Case series (1) | n = 8 | N = 654 | Population with LBP with/without radiculopathy presenting with suspected spondylolysis and/or spondylolisthesis | No |
| Ferrari | 2015 [35] | Dec 2013 | Italy | Mixed | NA | n = 6 | N = 333 | Adult population with sub-acute or chronic LBP | No |
| Sivayogam | 2011 [36] | Feb 2011 | Singapore | NA | NA | n = 6 | N = 409 | Adult patients with non-specific, non-pregnancy related LBP and/or buttock pain, with or without lower- extremity symptoms | No |
| Alqarni | 2011 [37] | March 2010 | NZ | Mixed | Prospective (3); Cross-sectional (1) | n = 4 | N = 351 | Patient with CLBP or with mixed lumbar pathology | No |
| Hancock | 2007 [38] | Feb 2006 | Australia | NA | NA | n = 41 | NA | Patients with low back pain and no known or suspected serious pathology | No |
| Simpson | 2006 [39] | Dec 2005 | UK | NA | NA | n = 11 | NA | Adult patients with LBP | No |
| SPECIFIC LOW BACK PAIN | |||||||||
| Tabrah | 2022 [40] | Oct 2020 | UK | Mixed | Retrospective cohort (5); Prospective cohort (1) | n = 6 | N = 679 | People presenting with acute CES | Yes |
| Galliker | 2020 [41] | Jan 2019 | Switzerland | Mixed | Prospective cohort (3); Retrospective cohort (15); Cross-sectional (3); Mixed (1) | n = 22 (10 on DA of RF) | N = 41,320 | Adult patients presenting with LBP of any duration to an ED | No |
| Maselli | 2020 [42] | June 2020 | Italy | Mixed | Retrospective (19); Prospective (1); Cross-sectional (3); Observational (6); Cohort (1) | n = 40 (21 focused on LBP patients) | N = 49,422 | Patients consulting healthcare professionals for LBP | No |
| Dionne | 2019 [43] | Jan 2018 | Canada | Mixed | Retrospective cohort (6); Prospective cohort (1) | n = 7 | N = 869 | Adults who presented with suspected CES from an insidious onset or herniated disc prolapse | Yes |
| Williams | 2013 [44] | March 2012 | Australia | Mixed (primary and secondary) | Prospective cohort (6); Retrospective cohort (2) | n = 8 | N = 7378 | Patients presenting with LBP or for lumbar spine examination | No |
| Henschke | 2013 [45] | April 2012 | Germany | Mixed | Prospective cohort (6); Retrospective cohort (2) | n = 8 | N = 8905 | Patients with LBP or requiring examination of the lumbar spine | No |
| Henschke | 2008 [46] | Feb 2007 | Australia | Mixed | Prospective (8); Retrospective (4) | n = 12 | N = 7147 | Patients with back pain presenting to the ED | No |
| MIXED | |||||||||
| Haskins | 2015 [47] | July 2013 | Australia | Mixed | Prospective (n = 9); Retrospective (n = 2); NA (n = 4) | n = 15 | NA | Mixed | No |
| Shultz | 2015 [48] | Nov 2013 | USA | Mixed | Prospective (n = 9); Retrospective (n = 1); Cross-sectional (n = 1) | n = 11 | N = 2899 | Patients with low back pain (LBP) and related lower-extremity pain condition | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathieu, J.; Pasquier, M.; Descarreaux, M.; Marchand, A.-A. Diagnosis Value of Patient Evaluation Components Applicable in Primary Care Settings for the Diagnosis of Low Back Pain: A Scoping Review of Systematic Reviews. J. Clin. Med. 2023, 12, 3581. https://doi.org/10.3390/jcm12103581
Mathieu J, Pasquier M, Descarreaux M, Marchand A-A. Diagnosis Value of Patient Evaluation Components Applicable in Primary Care Settings for the Diagnosis of Low Back Pain: A Scoping Review of Systematic Reviews. Journal of Clinical Medicine. 2023; 12(10):3581. https://doi.org/10.3390/jcm12103581
Chicago/Turabian StyleMathieu, Janny, Mégane Pasquier, Martin Descarreaux, and Andrée-Anne Marchand. 2023. "Diagnosis Value of Patient Evaluation Components Applicable in Primary Care Settings for the Diagnosis of Low Back Pain: A Scoping Review of Systematic Reviews" Journal of Clinical Medicine 12, no. 10: 3581. https://doi.org/10.3390/jcm12103581
APA StyleMathieu, J., Pasquier, M., Descarreaux, M., & Marchand, A.-A. (2023). Diagnosis Value of Patient Evaluation Components Applicable in Primary Care Settings for the Diagnosis of Low Back Pain: A Scoping Review of Systematic Reviews. Journal of Clinical Medicine, 12(10), 3581. https://doi.org/10.3390/jcm12103581





