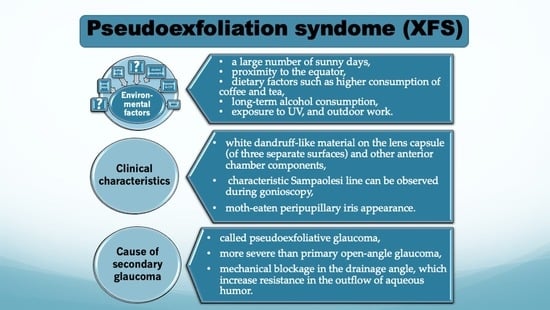
Pseudoexfoliation Syndrome—Clinical Characteristics of Most Common Cause of Secondary Glaucoma
Abstract
1. Introduction
2. Methods
3. Epidemiology of Pseudoexfoliation Syndrome
4. Influence of Several Risk Factors in Pseudoexfoliation Syndrome
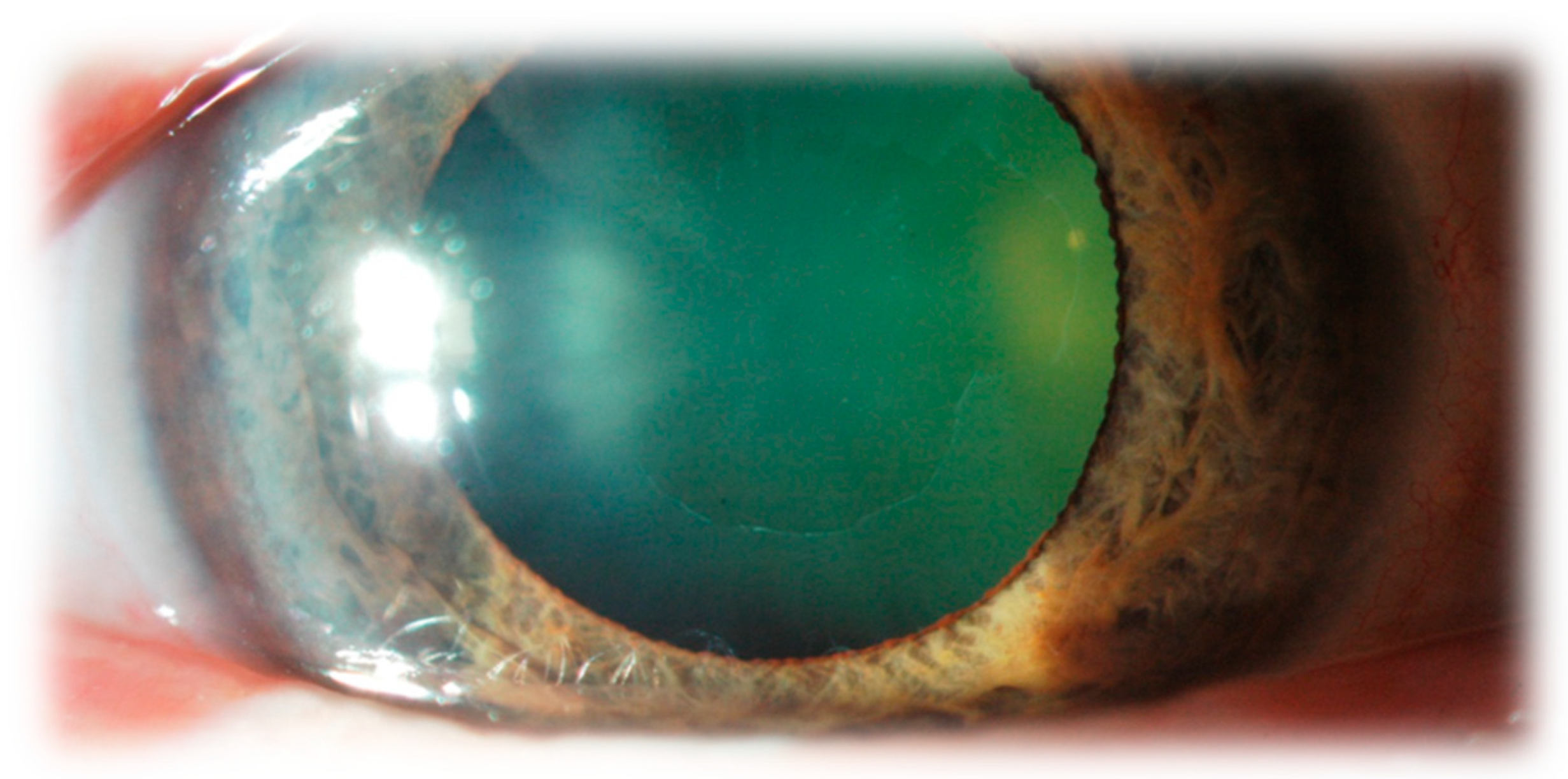
5. The Clinical Characteristics of Pseudoexfoliation Syndrome
6. Systemic Manifestations of Pseudoexfoliation Syndrome
7. Pseudoexfoliation Syndrome as a Cause of Glaucoma
- 28% of patients with an IOP of 17 mmHg or less,
- 43% of patients with an IOP of 18 to 19 mmHg,
- 70% of patients with an IOP of 20 mmHg or more.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schlötzer-Schrehardt, U.M.; Koca, M.R.; Naumann, G.O.; Volkholz, H. Pseudoexfoliation syndrome. Ocular manifestation of a systemic disorder? Arch. Ophthalmol. 1992, 110, 1752–1756. [Google Scholar] [CrossRef]
- Schlotzer-Schrehardt, U.; Naumann, G.O. Ocular and systemic pseudoexfoliation syndrome. Am. J. Ophthalmol. 2006, 141, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Ritch, R.; Schlotzer-Schrehardt, U. Exfoliation syndrome. Surv. Ophthalmol. 2001, 45, 265–315. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Allingham, R.R. Molecular genetics in glaucoma. Exp. Eye Res. 2011, 93, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Ritch, R.; Schlotzer-Schrehardt, U.; Konstas, A.G. Why is glaucoma associated with exfoliation syndrome? Prog. Retin. Eye Res. 2003, 22, 253–275. [Google Scholar] [CrossRef]
- Chakraborty, M.; Rao, A. Alternate Causes for Pathogenesis of Exfoliation Glaucoma, a Multifactorial Elastotic Disorder: A Literature Review. Curr. Issues Mol. Biol. 2022, 44, 1191–1202. [Google Scholar] [CrossRef]
- Tomczyk-Socha, M.; Tomczak, W.; Turno-Krecicka, A. The Importance of MicroRNA Expression in Pseudoexfoliation Syndrome. Int. J. Mol. Sci. 2022, 23, 13234. [Google Scholar] [CrossRef]
- Kuchle, M.; Vinores, S.A.; Mahlow, J.; Green, W.R. Blood-aqueous barrier in pseudoexfoliation syndrome: Evaluation by immunohistochemical staining of endogenous albumin. Graefes Arch. Clin. Exp. Ophthalmol. 1996, 234, 12–18. [Google Scholar] [CrossRef]
- Ringvold, A. Epidemiology of the pseudo-exfoliation syndrome. Acta Ophthalmol. Scand. 1999, 77, 371–375. [Google Scholar] [CrossRef]
- Hirvelä, H.; Tuulonen, A.; Laatikainen, L. Intraocular pressure and prevalence of glaucoma in elderly people in Finland: A population-based study. Int. Ophthalmol. 1995, 18, 299–307. [Google Scholar] [CrossRef]
- Pasquale, L.R.; Jiwani, A.Z.; Zehavi-Dorin, T.; Majd, A.; Rhee, D.J.; Chen, T.; Turalba, A.; Shen, L.; Brauner, S.; Grosskreutz, C.; et al. Solar exposure and residential geographic history in relation to exfoliation syndrome in the United States and Israel. JAMA Ophthalmol. 2014, 132, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Thorleifsson, G.; Magnusson, K.P.; Sulem, P.; Walters, G.B.; Gudbjartsson, D.F.; Stefansson, H.; Jonsson, T.; Jonasdottir, A.; Stefansdottir, G.; Masson, G.; et al. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science 2007, 317, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.D.; Pasquale, L.R.; Talwar, N.; Kim, D.S.; Reed, D.M.; Nan, B.; Kang, J.H.; Wiggs, J.L.; Richards, J.E. Geographic and climatic factors associated with exfoliation syndrome. Arch. Ophthalmol. 2011, 129, 1053–1060. [Google Scholar] [CrossRef]
- Pasquale, L.R.; Wiggs, J.L.; Willett, W.C.; Kang, J.H. The Relationship between caffeine and coffee consumption and exfoliation glaucoma or glaucoma suspect: A prospective study in two cohorts. Investig. Opthalmology Vis. Sci. 2012, 53, 6427–6433. [Google Scholar] [CrossRef]
- Tijani, M.; Albaroudi, N.; Boutimzine, N.; Cherkaoui, O.; Laghmari, M. Prevalence of exfoliation syndrome and cardiovascular diseases in patients scheduled for cataract surgery. J. Fr. Ophtalmol. 2017, 40, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lim, S.H.; Sung, K.R.; Yun, S.C.; Kim, C.Y.; Park, K.H.; Cha, S.C. Prevalence of Pseudoexfoliation Syndrome and Associated Factors in South Koreans: The Korean National Health and Nutrition Examination Survey. Ophthalmic Epidemiol. 2016, 23, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Hanyuda, A.; Rosner, B.A.; Wiggs, J.L.; Negishi, K.; Pasquale, L.R.; Kang, J.H. Long-term Alcohol Consumption and Risk of Exfoliation Glaucoma or Glaucoma Suspect Status among United States Health Professionals. Ophthalmology 2023, 130, 187–197. [Google Scholar] [CrossRef]
- Sureshkumar, I.; Gunalan, V.; Nareshkumar, R.N.; Sripriya, K.; Ronnie, G.; Sharada, R.; Asokan, R. Evaluating the impact of ocular UV exposure for the development for pseudoexfoliation syndrome in a South Indian population. Clin. Exp. Optom. 2022, 14, 1–7. [Google Scholar] [CrossRef]
- Arif, S.A.; Khan, M.I.; Nauman, F.; Arif, M.A. The association between ethnicity, environmental and lifestyle factors and chronic disease in the development of pseudoexfoliation syndrome. Pak. J. Med. Sci. 2021, 37, 409–414. [Google Scholar] [CrossRef]
- Ritch, R. Ocular Findings in Exfoliation Syndrome. J. Glaucoma 2018, 27 (Suppl. 1), S67–S71. [Google Scholar] [CrossRef]
- Sampaolesi, R.; Zarate, J.; Croxato, O. The chamber angle in exfoliation syndrome. Clinical and pathological findings. Acta Ophthalmol. Suppl. 1988, 184, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Detorakis, E.T.; Bontzos, G.; Drakonaki, E.E.; Spandidos, D.A. Changes in peri-ocular anatomy and physiology in pseudoexfoliation syndrome (Review). Exp. Ther. Med. 2021, 21, 650. [Google Scholar] [CrossRef] [PubMed]
- Streeten, B.W.; Li, Z.Y.; Wallace, R.N.; Eagle, R.C., Jr.; Keshgegian, A.A. Pseudoexfoliative fibrillopathy in visceral organs of a patient with pseudoexfoliation syndrome. Arch. Ophthalmol. 1992, 110, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Wang, J.J.; Smith, W. Association of pseudoexfoliation syndrome with increased vascular risk. Am. J. Ophthalmol. 1997, 124, 685–687. [Google Scholar] [CrossRef]
- Pompoco, C.J.; Curtin, K.; Taylor, S.; Paulson, C.; Shumway, C.; Conley, M.; Barker, D.J.; Swiston, C.; Stagg, B.; Ritch, R.; et al. Summary of Utah Project on Exfoliation Syndrome (UPEXS): Using a large database to identify systemic comorbidities. BMJ Open Ophthalmol. 2021, 6, e000803. [Google Scholar] [CrossRef]
- Scharfenberg, E.; Rauscher, F.G.; Meier, P.; Hasenclever, D. Pseudoexfoliation syndrome: Analysis of systemic comorbidities of 325 PEX-positive patients compared with 911 PEX-negative patients. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 2471–2480. [Google Scholar] [CrossRef]
- Irkec, M. Clinical Features of Exfoliative Glaucoma w Exfoliation Syndrome and Exfoliative Glaucoma, 3rd ed.; Hollo, G., Konstas, A., Eds.; PubliComm: Savona, Italy, 2015; pp. 151–158. [Google Scholar]
- Konstas, A.G.; Mantziris, D.A.; Stewart, W.C. Diurnal intraocular pressure in untreated exfoliation and primary open- angle glaucoma. Arch. Ophthalmol. 1997, 115, 182–185. [Google Scholar] [CrossRef]
- Iwanejko, M.; Turno-Krecicka, A.; Tomczyk-Socha, M.; Kaczorowski, K.; Grzybowski, A.; Misiuk-Hojlo, M. Evaluation of the anterior chamber angle in pseudoexfoliation syndrome. Adv. Clin. Exp. Med. 2017, 26, 795–801. [Google Scholar] [CrossRef]
- Tezel, G.; Tezel, T.H. The comparative analysis of optic disc damage in exfoliative glaucoma. Acta Ophthalmol. 1993, 71, 744–750. [Google Scholar] [CrossRef]
- Lindblom, B.; Thorburn, W. Functional damage at diagnosis of primary open angle glaucoma. Acta Ophthalmol. 1984, 62, 223–229. [Google Scholar] [CrossRef]
- Futa, R.; Shimizu, T.; Furuyoshi, N.; Nishiyama, M.; Hagihara, O. Clinical features of capsular glaucoma in comparison with primary open- angle glaucoma in Japan. Acta Ophthalmol. 1992, 70, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Teus, M.A.; Castejon, M.A.; Calvo, M.A.; Perez-Salaices, P.; Marcos, A. Intraocular pressure as a risk factor for visual field loss in pseudoexfoliative and in primary open-angle glaucoma. Ophthalmology 1998, 105, 2225–2229, discussion 2229–2230. [Google Scholar] [CrossRef] [PubMed]
- Blika, S.; Saunte, E. Timolol maleate in the treatment of glaucoma simplex and glaucoma capsulare. A three-year follow up study. Acta Ophthalmol. 1982, 60, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Pohjanpelto, P. Influence of exfoliation syndrome on prognosis in ocular hypertension greater than or equal to 25 mm. A long-term follow-up. Acta Ophthalmol. 1986, 64, 39–44. [Google Scholar] [CrossRef]
- Konstas, A.G.; Hollo, G.; Astakhov, Y.S.; Teus, M.A.; Akopov, E.L.; Jenkins, J.N.; Stewart, W.C. Factors associated with long-term progression or stability in exfoliation glaucoma. Arch. Ophthalmol. 2004, 122, 29–33. [Google Scholar] [CrossRef]
- Konstas, A.G.; Hollo, G.; Irkec, M.; Tsironi, S.; Durukan, I.; Goldenfeld, M.; Melamed, S. Diurnal IOP control with bimatoprost versus latanoprost in exfoliative glaucoma: A crossover, observer-masked, three-centre study. Br. J. Ophthalmol. 2007, 91, 757–760. [Google Scholar] [CrossRef]
- Konstas, A.G.; Maltezos, A.; Mantziris, D.A.; Sine, C.S.; Stewart, W.C. The comparative ocular hypotensive effect of apraclonidine with timolol maleate in exfoliation versus primary open-angle glaucoma patients. Eye 1999, 13 Pt 3a, 314–318. [Google Scholar] [CrossRef]
- Threlkeld, A.B.; Hertzmark, E.; Sturm, R.T.; Epstein, D.L.; Allingham, R.R. Comparative study of the efficacy of argon laser trabeculoplasty for exfoliation and primary open-angle glaucoma. J. Glaucoma 1996, 5, 311–316. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczyk-Socha, M.; Tomczak, W.; Winkler-Lach, W.; Turno-Kręcicka, A. Pseudoexfoliation Syndrome—Clinical Characteristics of Most Common Cause of Secondary Glaucoma. J. Clin. Med. 2023, 12, 3580. https://doi.org/10.3390/jcm12103580
Tomczyk-Socha M, Tomczak W, Winkler-Lach W, Turno-Kręcicka A. Pseudoexfoliation Syndrome—Clinical Characteristics of Most Common Cause of Secondary Glaucoma. Journal of Clinical Medicine. 2023; 12(10):3580. https://doi.org/10.3390/jcm12103580
Chicago/Turabian StyleTomczyk-Socha, Martyna, Wojciech Tomczak, Weronika Winkler-Lach, and Anna Turno-Kręcicka. 2023. "Pseudoexfoliation Syndrome—Clinical Characteristics of Most Common Cause of Secondary Glaucoma" Journal of Clinical Medicine 12, no. 10: 3580. https://doi.org/10.3390/jcm12103580
APA StyleTomczyk-Socha, M., Tomczak, W., Winkler-Lach, W., & Turno-Kręcicka, A. (2023). Pseudoexfoliation Syndrome—Clinical Characteristics of Most Common Cause of Secondary Glaucoma. Journal of Clinical Medicine, 12(10), 3580. https://doi.org/10.3390/jcm12103580