Efficacy and Safety of Dry Powder Antibiotics: A Narrative Review
Abstract
1. Introduction
2. Nebulized Antibiotics: General Characteristics and Modalities
2.1. Ultrasonic Nebulizers
2.2. Jet Nebulizers
- Jet nebulizers with constant flow. These generate a continuous aerosol flow, but approximately 60–70% of the volume of the nebulized liquid is lost to the environment during the expiratory phase. This supposes both a loss of the nebulized drug and a risk of contaminating the environment, to the detriment of any people nearby.
- Jet nebulizers with an active Venturi effect during inspiration. These nebulizers have a system that conducts the inspired air through the area that generates the aerosol, so that during the inspiratory phase the inspired flow is added to the flow generated by the compressor. Some jet nebulizers, such as the InnoSpire®, Pari LC Plus®, Pari LC Sprint® and Pari LC Star®, also use a valve that closes the outlet during expiration, thereby reducing the loss of aerosol (Figure 1). These devices are more effective and faster than constant flow nebulizers [22].
- Dosimetric jet or adapted aerosol release nebulizers. These systems release the aerosol according to the respiratory flow of each patient and deliver the aerosol only during inspiration. They are the most effective of the three, and they almost totally reduce the release of the nebulized drug into the environment [23].
2.3. Mesh Nebulizers
3. Dry Powder for Antibiotics
3.1. General/Technical Characteristics of the Devices
3.2. Deposition of Lung Particles
3.3. Microbiological Issues
4. Studies with Inhaled Dry Powder Antibiotics in Cystic Fibrosis Patients
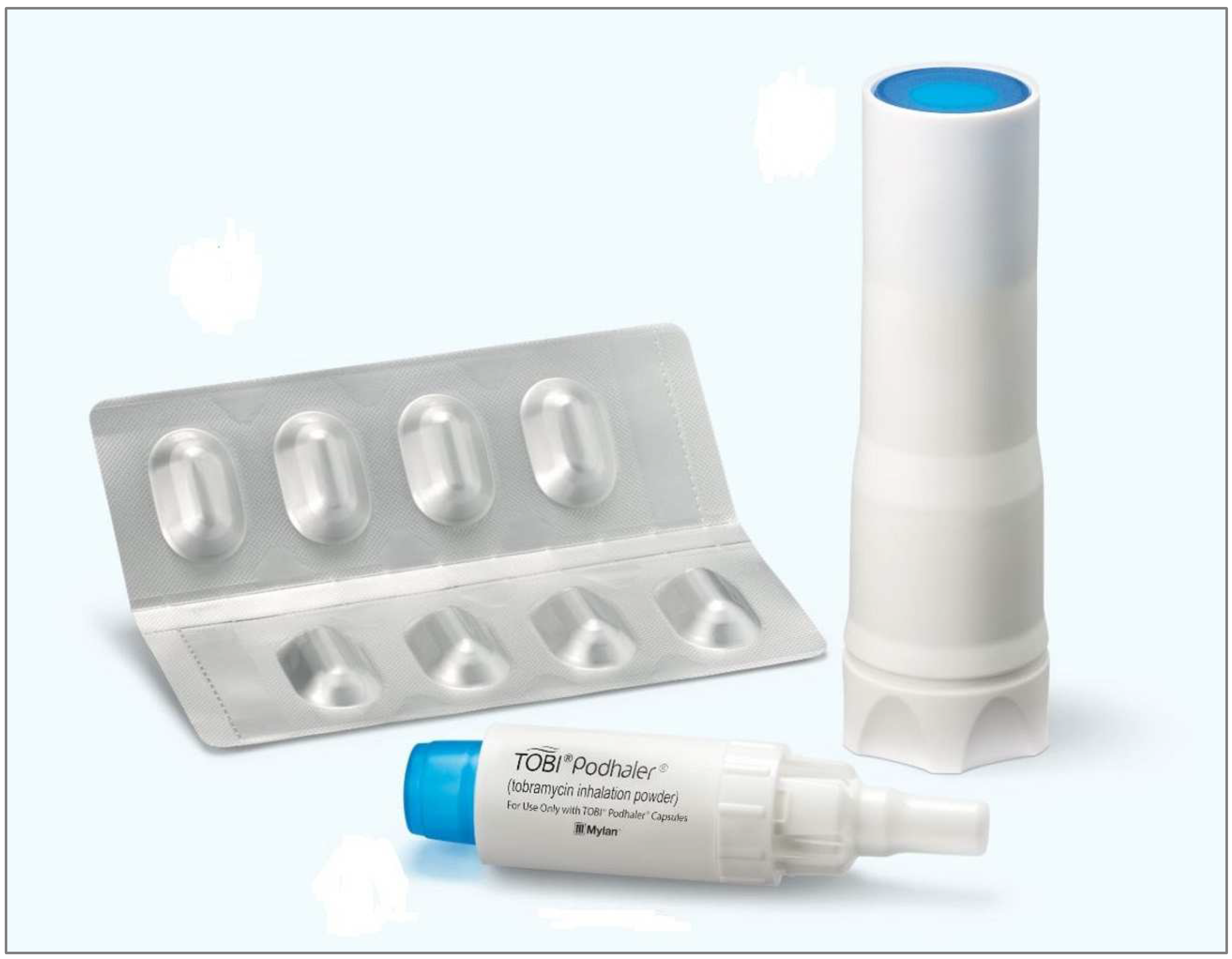
4.1. Tobramycin Studies
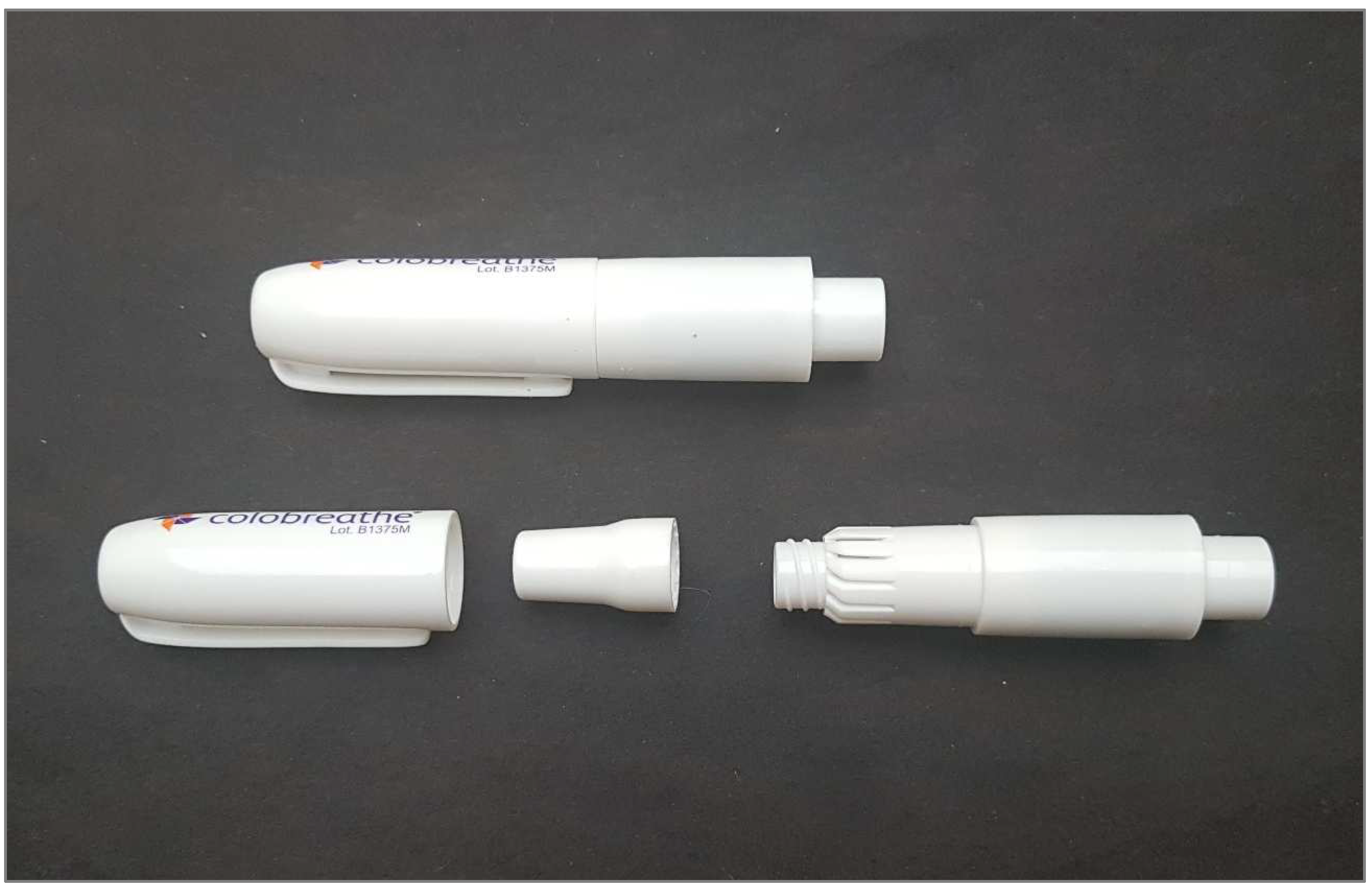
4.2. Colistin Studies
5. Studies with Antibiotic Dry Powder Inhalers in Bronchiectasis
6. Other Dry Powder Antibiotics
6.1. Anti-Tuberculous Agents
6.2. Ciprofloxacin
6.3. Meropenem
6.4. Doxycycline
6.5. Vancomycin
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, M.B.; Chalmers, J.D. Treating Neutrophilic Inflammation in Airways Diseases. Arch. Bronconeumol. 2022, 58, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.Á. Bronchiectasis and Eosinophils. Arch. Bronconeumol. 2021, 57, 671–672. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Inflammatory mechanisns in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Aogáin, M.M.; Jaggi, T.K.; Chotirmall, S.H. The Airway Microbiome: Present and Future Applications. Arch. Bronconeumol. 2022, 58, 8–10. [Google Scholar] [CrossRef]
- García-Río, F.; Alcázar-Navarrete, B.; Castillo-Villegas, D.; Cilloniz, C.; García-Ortega, A.; Leiro-Fernández, V.; Lojo-Rodriguez, I.; Padilla-Galo, A.; Quezada-Loaiza, C.A.; Rodriguez-Portal, J.A.; et al. Biological Biomarkers in Respiratory Diseases. Arch. Bronconeumol. 2022, 58, 323–333. [Google Scholar] [CrossRef]
- Viniol, C.; Vogelmeier, C.F. Exacerbations of COPD. Eur. Respir. Rev. 2018, 27, 170103. [Google Scholar] [CrossRef]
- Posadas, T.; Oscullo, G.; Zaldivar, E.; Villa, C.; Dobarganes, Y.; Girón, R.; Olveira, C.; Maíz, L.; García-Clemente, M.; Sibila, O.; et al. C-Reactive Protein Concentration in Steady-State Bronchiectasis: Prognostic Value of Future Severe Exacerbations. Arch. Bronconeumol. 2021, 57, 21–27. [Google Scholar] [CrossRef]
- Scioscia, G.; Alcaraz-Serrano, V.; Méndez, R.; Gabarrús, A.; Fernández-Barat, L.; Tondo, P.; Bueno, L.; Torres, A. Factors Associated with One-Year Mortality in Hospitalised Patients With Exacerbated Bronchiectasis. Arch. Bronconeumol. 2022, 58, 773–775. [Google Scholar] [CrossRef]
- Southwell, N. Inhaled penicillin in bronchial infections. Lancet 1946, 2, 225–227. [Google Scholar] [CrossRef]
- Di Sant’Agnese, P.E.; Andersen, D.H. Celiac syndrome; chemotherapy in infections of the respiratory tract associated with cystic fibrosis of the pancreas; observations with penicillin and drugs of the sulfonamide group, with special reference to penicillin aerosol. Am. J. Dis. Child. 1946, 72, 17–61. [Google Scholar] [CrossRef]
- De la Rosa Carrillo, D.; Martínez-García, M.Á.; Barreiro, E.; Tabernero Huguet, E.; Costa Sola, R.; García-Clemente, M.M.; Celorrio Jiménez, N.; Rodríguez Pons, L.; Calero Acuña, C.; Rodríguez Hermosa, J.L.; et al. Effectiveness and Safety of Inhaled Antibiotics in Patients with Chronic Obstructive Pulmonary Disease. A Multicentre Observational Study. Arch. Bronconeumol. 2022, 58, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.; Máiz, L.; Olveira, C.; Girón, R.M.; de la Rosa, D.; Blanco, M.; Cantón, R.; Vendrell, M.; Polverino, E.; de Gracia, J.; et al. Spanish Guidelines on Treatment of Bronchiectasis in Adults. Arch. Bronconeumol. 2018, 54, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Polverino, E.; Goeminne, P.C.; McDonnell, M.J.; Aliberti, S.; Marshall, S.E.; Loebinger, M.R.; Murris, M.; Cantón, R.; Torres, A.; Dimakou, K.; et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017, 50, 1700629. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.T.; Sullivan, A.L.; Chalmers, J.D.; De Soyza, A.; Elborn, J.S.; Floto, R.A.; Grillo, L.; Gruffydd-Jones, K.; Harvey, A.; Haworth, C.S.; et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax 2019, 74, 1–69. [Google Scholar] [CrossRef]
- de la Rosa Carrillo, D.; López-Campos, J.L.; Navarrete, B.A.; Rubio, M.C.; Moreno, R.C.; García-Rivero, J.L.; Carro, L.M.; Fuster, C.O.; Martínez-García, M.Á.; Callejas, F.J.; et al. Documento de consenso sobre el diagnóstico y tratamiento de la infección bronquial crónica en la enfermedad pulmonar obstructiva crónica. Arch. Bronconeumol. 2020, 56, 651–664. [Google Scholar] [CrossRef]
- Konstan, M.W.; Geller, D.E.; Minić, P.; Brockhaus, F.; Zhang, J.; Angyalosi, G. Tobramycin inhalation powder for P. aeruginosa infection in cystic fibrosis: The EVOLVE trial. Pediatr. Pulmonol. 2011, 46, 230–238. [Google Scholar] [CrossRef]
- Schuster, A.; Haliburn, C.; Döring, G.; Goldman, M.H.; Freedom Study Group. Safety, efficacy and convenience of colistimethate sodium dry powder for inhalation (Colobreathe DPI) in patients with cystic fibrosis: A randomised study. Thorax 2013, 68, 344–350. [Google Scholar] [CrossRef]
- Martin, A.R.; Finlay, W.H. Nebulizers for drug delivery to the lungs. Expert Opin. Drug Deliv. 2014, 12, 889–900. [Google Scholar] [CrossRef]
- Máiz-Carro, L.; Martínez-García, M.A.; de la Rosa-Carrillo, D. Inhaled Antibiotics; Neumología y Salud: Zaragoza, Spain, 2021; pp. 25–35. [Google Scholar]
- De Pablo, E.; Fernández-García, R.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Nebulised antibiotherapy: Conventional versus nanotechnology-based approaches, is targeting at a nano scale a difficult subject? Ann. Transl. Med. 2017, 5, 448. [Google Scholar] [CrossRef]
- Nikander, K.; Turpeinen, M.; Wollmer, P. The Conventional Ultrasonic Nebulizer Proved Inefficient in Nebulizing a Suspension. J. Aerosol Med. 1999, 12, 47–53. [Google Scholar] [CrossRef]
- Hess, D.R. Nebulizers: Principles and performance. Respir. Care 2000, 45, 609–622. [Google Scholar] [PubMed]
- Rau, J.L.; Ari, A.; Restrepo, R.D. Performance comparison of nebulizer designs: Constant-output, breath-enhanced, and dosimetric. Respir. Care 2004, 49, 174–179. [Google Scholar] [PubMed]
- Coates, A.L.; Green, M.; Leung, K.; Chan, J.; Ribeiro, N.; Louca, E.; Ratjen, F.; Charron, M.; Tservistas, M.; Keller, M. Rapid pulmonary delivery of inhaled tobramycin for Pseudomonas infection in cystic fibrosis: A pilot project. Pediatr. Pulmonol. 2008, 43, 753–759. [Google Scholar] [CrossRef]
- Waldrep, J.C.; Dhand, R. Advanced nebulizer designs employing vibrating mesh/aperture plate technologies for aerosol generation. Curr. Drug Deliv. 2008, 5, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.; Mills, N.; Whitaker, P. Nebuliser systems for drug delivery in cystic fibrosis. Cochrane Database Syst. Rev. 2013, 30, CD007639. [Google Scholar]
- McShane, P.J.; Weers, J.G.; Tarara, T.E.; Haynes, A.; Durbha, P.; Miller, D.P.; Mundry, T.; Operschall, E.; Elborn, J.S. Ciprofloxacin dry powder for inhalation (ciprofloxacin DPI): Technical design and features of an efficient drug-device combination. Pulm. Pharmacol. Ther. 2018, 50, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Akkerman-Nijland, A.M.; Grasmeijer, F.; Kerstjens, H.A.M.; Frijlink, H.W.; Van Der Vaart, H.; Vonk, J.M.; Hagedoorn, P.; Rottier, B.L.; Koppelman, G.H.; Akkerman, O.W. Colistin dry powder inhalation with the Twincer™: An effective and more patient friendly alternative to nebulization. PLoS ONE 2020, 15, e0239658. [Google Scholar] [CrossRef] [PubMed]
- Geller, D.E.; Weers, J.; Heuerding, S. Development of an inhaled dry-powder formulation of tobramycin using PulmoSphere™ technology. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 175–182. [Google Scholar] [CrossRef]
- Conole, D.; Keating, G.M. Colistimethate Sodium Dry Powder for Inhalation: A Review of Its Use in the Treatment of Chronic Pseudomonas aeruginosa Infection in Patients with Cystic Fibrosis. Drugs 2014, 74, 377–387. [Google Scholar] [CrossRef]
- Haynes, A.; Geller, D.; Weers, J.; Ament, B.; Pavkov, R.; Malcolmson, R.; Debonnett, L.; Mastoridis, P.; Yadao, A.; Heuerding, S. Inhalation of tobramycin using simulated cystic fibrosis patient profiles. Pediatr. Pulmonol. 2016, 51, 1159–1167. [Google Scholar] [CrossRef]
- McNamara, P.S.; McCormack, P.; McDonald, A.J.; Heaf, L.; Southern, K.W. Open adherence monitoring using routine data download from an adaptive aerosol delivery nebuliser in children with cystic fibrosis. J. Cyst. Fibros. 2009, 8, 258–263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Savara Halts Development of AeroVanc, Apulmiq. Available online: https://www.oindpnews.com/2020/12/savara-halts-development-of-aerovanc-apulmiq/ (accessed on 26 December 2022).
- Hoppentocht, M.; Akkerman, O.W.; Hagedoorn, P.; Alffenaar, J.W.C.; van der Werf, T.S.; Kerstjens, H.A.; Frijlink, H.W.; de Boer, A.H. Tolerability and pharmacokinetic evaluation of inhaled dry powder tobramycin free base in non-cystic fibrosis bronchiectasis patients. PLoS ONE 2016, 11, e0149768. [Google Scholar] [CrossRef] [PubMed]
- Vives, E.C.; Fabrellas, E.F.; Balbín, R.A.; García, M.Á.M. Aerosolterapia. Open Respir. Arch. 2020, 2, 89–99. [Google Scholar] [CrossRef]
- Lam, J.; Vaughan, S.; Parkins, M.D. Tobramycin Inhalation Powder (TIP): An Efficient Treatment Strategy for the Management of Chronic Pseudomonas Aeruginosa Infection in Cystic Fibrosis. Clin. Med. Insights Circ. Respir. Pulm. Med. 2013, 7, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Stass, H.; Nagelschmitz, J.; Kappeler, D.; Sommerer, K.; Patzlaff, A.; Weimann, B. Ciprofloxacin dry powder for inhalation: Inspiratory flow in patients with non-cystic fibrosis bronchiectasis. J. Aerosol Med. Pulm. Drug Deliv. 2019, 32, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Tiddens, H.; Geller, D.; Challoner, P.; Speirs, R.; Kesser, K.; Overbeek, S.; Humble, D.; Shrewsbury, S.; Standaert, T. Effect of Dry Powder Inhaler Resistance on the Inspiratory Flow Rates and Volumes of Cystic Fibrosis Patients of Six Years and Older. J. Aerosol Med. 2006, 19, 456–465. [Google Scholar] [CrossRef]
- Son, Y.-J.; Miller, D.P.; Weers, J.G. Optimizing Spray-Dried Porous Particles for High Dose Delivery with a Portable Dry Powder Inhaler. Pharmaceutics 2021, 13, 1528. [Google Scholar] [CrossRef]
- Colobreathe (Colistimethate Sodium Dry Powder for Inhalation): Risk of Capsule Breakage-New Instructions for Use. Available online: https://www.gov.uk/drug-safety-update/colobreathe-colistimethate-sodium-dry-powder-for-inhalation-risk-of-capsule-breakage-new-instructions-for-use (accessed on 27 December 2022).
- Kaplan, S.; Patino, O.; Rainville, C.; Madison, T. Assessment of colistimethate sodium (COLOBREATHE) risk minimization measures implemented in the European Union: A cross-sectional study. Pharmacoepidemiol. Drug Saf. 2019, 29, 219–223. [Google Scholar] [CrossRef]
- Kaplan, S.; Lee, A.; Caine, N.; Charman, S.C.; Bilton, D. Long-term safety study of colistimethate sodium (Colobreathe®): Findings from the UK Cystic Fibrosis Registry. J. Cyst. Fibros. 2021, 20, 324–329. [Google Scholar] [CrossRef]
- Martínez-García, M.; Oscullo, G.; Barreiro, E.; Cuenca, S.; Cervera, A.; Padilla-Galo, A.; de la Rosa, D.; Navarro, A.; Giron, R.; Carbonero, F.; et al. Inhaled Dry Powder Antibiotics in Patients with Non-Cystic Fibrosis Bronchiectasis: Efficacy and Safety in a Real-Life Study. J. Clin. Med. 2020, 9, 2317. [Google Scholar]
- Sahakijpijarn, S.; Smyth, H.D.C.; Miller, D.P.; Weers, J.G. Post-inhalation cough with therapeutic aerosols: Formulation considerations. Adv. Drug Deliv. Rev. 2020, 165–166, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Máiz Carro, L.; Blanco-Aparicio, M. New inhaled antibiotics and forms of administration. Open Respir. Arch. 2020, 2, 251–264. [Google Scholar] [CrossRef]
- Tiddens, H.A.; Bos, A.C.; Mouton, J.W.; Devadason, S.; Janssens, H.M. Inhaled antibiotics: Dry or wet? Eur. Respir. J. 2014, 44, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Sibila, O.; Suarez-Cuartin, G.; Rodrigo-Troyano, A.; Fardon, T.C.; Finch, S.; Mateus, E.F.; Garcia-Bellmunt, L.; Castillo, D.; Vidal, S.; Sanchez-Reus, F.; et al. Secreted mucins and airway bacterial colonization in non-CF bronchiectasis. Respirology 2015, 20, 1082–1088. [Google Scholar] [CrossRef]
- Laube, B.L.; Jashnani, R.; Dalby, R.N.; Zeitlin, P.L. Targeting aerosol deposition in patients with cystic fibrosis: Effects of alterations in particle size and inspiratory flow rate. Chest 2000, 118, 1069–1076. [Google Scholar] [CrossRef]
- Solarat, B.; Perea, L.; Faner, R.; de La Rosa, D.; Martínez-García, M.; Sibila, O. Pathophysiology of Chronic Bronchial Infection in Bronchiectasis. Arch. Bronconeumol. 2023, 59, 101–108. [Google Scholar] [CrossRef]
- Kunadharaju, R.; Rudraraju, A.; Sethi, S. Pseudomonas aeruginosa Colonization and COPD: The Chicken or the Egg? Arch. Bronconeumol. 2022, 58, 539–541. [Google Scholar] [CrossRef]
- Sibila, O.; Laserna, E.; Shoemark, A.; Keir, H.R.; Finch, S.; Rodrigo-Troyano, A.; Perea, L.; Lonergan, M.; Goeminne, P.C.; Chalmers, J.D. Airway Bacterial Load and Inhaled Antibiotic Response in Bronchiectasis. Am. J. Respir. Crit. Care Med. 2019, 200, 33–41. [Google Scholar] [CrossRef]
- Rodrigo-Troyano, A.; Sibila, O. The respiratory threat posed by multidrug resistant Gram-negative bacteria. Respirology 2017, 22, 1288–1299. [Google Scholar] [CrossRef]
- Regan, K.H.; Hill, A.T. Risk of Development of Resistance in Patients with Non-Cystic Fibrosis Bronchiectasis Treated with Inhaled Antibiotics. Curr. Pulmonol. Rep. 2018, 7, 63–71. [Google Scholar] [CrossRef]
- Hamed, K.; Debonnett, L. Tobramycin inhalation powder for the treatment of pulmonary Pseudomonas aeruginosa infection in patients with cystic fibrosis: A review based on clinical evidence. Ther. Adv. Respir. Dis. 2017, 11, 193–209. [Google Scholar] [CrossRef]
- Galeva, I.; Konstan, M.W.; Higgins, M.; Angyalosi, G.; Brockhaus, F.; Piggott, S.; Thomas, K.; Chuchalin, A.G. Tobramycin inhalation powder manufactured by improved process in cystic fibrosis: The randomized EDIT trial. Curr. Med. Res. Opin. 2013, 29, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; Flume, P.A.; Kappler, M.; Chiron, R.; Higgins, M.; Brockhaus, F.; Zhang, J.; Angyalosi, G.; He, E.; Geller, D.E. Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: The EAGER trial. J. Cyst. Fibros. 2011, 10, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; Flume, P.A.; Galeva, I.; Wan, R.; Debonnett, L.M.; Maykut, R.J.; Angyalosi, G. One-year safety and efficacy of tobramycin powder for inhalation in patients with cystic fibrosis. Pediatr. Pulmonol. 2015, 51, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Geller, D.E.; Nasr, S.Z.; Piggott, S.; He, E.; Angyalosi, G.; Higgins, M. Tobramycin Inhalation Powder in Cystic Fibrosis Patients: Response by Age Group. Respir. Care 2013, 59, 388–398. [Google Scholar] [CrossRef]
- Panguluri, S.; Gunda, P.; Debonnett, L.; Hamed, K. Economic Evaluation of Tobramycin Inhalation Powder for the Treatment of Chronic Pulmonary Pseudomonas aeruginosa Infection in Patients with Cystic Fibrosis. Clin. Drug Investig. 2017, 37, 795–805. [Google Scholar] [CrossRef]
- Sommerwerck, U.; Virella-Lowell, I.; Angyalosi, G.; Viegas, A.; Cao, W.; Debonnett, L. Long-term safety of tobramycin inhalation powder in patients with cystic fibrosis: Phase IV (ETOILES) study. Curr. Med. Res. Opin. 2016, 32, 1789–1795. [Google Scholar] [CrossRef]
- Greenwood, J.; Schwarz, C.; Sommerwerck, U.; Nash, E.F.; Tamm, M.; Cao, W.; Mastoridis, P.; Debonnett, L.; Hamed, K. Ease of use of tobramycin inhalation powder compared with nebulized tobramycin and colistimethate sodium: A crossover study in cystic fibrosis patients with pulmonary Pseudomonas aeruginosa infection. Ther. Adv. Respir. Dis. 2017, 11, 249–260. [Google Scholar] [CrossRef]
- Blasi, F.; Carnovale, V.; Cimino, G.; Lucidi, V.; Salvatore, D.; Messore, B.; Bartezaghi, M.; Muscianisi, E.; Porpiglia, P.A.; FREE Study group. Treatment compliance in cystic fibrosis patients with chronic Pseudomonas aeruginosa infection treated with tobramycin inhalation powder: The FREE study. Respir. Med. 2018, 138, 88–94. [Google Scholar] [CrossRef]
- Greenberg, J.; Palmer, J.B.; Chan, W.W.; Correia, C.E.; Whalley, D.; Shannon, P.; Sawicki, G.S. Treatment satisfaction in cystic fibrosis: Early patient experience with tobramycin inhalation powder. Patient Prefer. Adherence 2016, 10, 2163–2169. [Google Scholar] [CrossRef]
- Uttley, L.; Harnan, S.; Cantrell, A.; Taylor, C.; Walshaw, M.; Brownlee, K.; Tappenden, P. Systematic review of the dry powder inhalers colistimethate sodium and tobramycin in cystic fibrosis. Eur. Respir. Rev. 2013, 22, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Martinez-García, M.A.; Villa, C.; Dobarganes, Y.; Girón, R.; Maíz, L.; García-Clemente, M.; Sibila, O.; Golpe, R.; Rodríguez, J.; Barreiro, E.; et al. RIBRON: The Spanish Online Bronchiectasis Registry. Characterization of the First 1912 Patients. Arch. Bronconeumol. 2021, 57, 28–35. [Google Scholar] [CrossRef]
- Vazquez-Espinosa, E.; Marcos, C.; Alonso, T.; Giron, R.; Gomez-Punter, R.; Garcia-Castillo, E.; Zamora, E.; Cisneros, C.; Garcia, J.; Valenzuela, C.; et al. Tobramycin inhalation powder (TOBI Podhaler®) for the treatment of lung infection in patients with cystic fibrosis. Expert Rev. Anti-Infect. Ther. 2015, 14, 9–17. [Google Scholar] [CrossRef]
- Fiel, S.B.; Roesch, E.A. The use of tobramycin for Pseudomonas aeruginosa: A review. Expert Rev. Respir. Med. 2022, 16, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Elborn, J.S.; Blasi, F.; Haworth, C.S.; Ballmann, M.; Tiddens, H.A.; Murris-Espin, M.; Chalmers, J.D.; Cantin, A.M. Bronchiectasis and inhaled tobramycin: A literature review. Respir. Med. 2021, 192, 106728. [Google Scholar] [CrossRef] [PubMed]
- Haworth, C.S.; Shteinberg, M.; Winthrop, K.L.; Blasi, F.; Dimakou, K.; Morgan, L.; Ringshausen, F.C.; Vidal, O.S.; Thompson, R.; Sharp, K.; et al. The efficacy and safety of colistimethate sodium delivered via the I-neb in bronchiectasis: The PROMIS-I randomized controlled trial. Eur. Respir. J. 2021, 58, RCT4267. [Google Scholar]
- Navas-Bueno, B.; Casas-Maldonado, F.; Padilla-Galo, A.; González-Moya-Mondelo, E.; Arenas-Gordillo, M.; Bioque-Rivera, J.C.; Jimeno-Galván, R.; Cano-Gómez, M.S.; López-Campos, J.L.; Merlos-Navarro, S. High Adherence, Microbiological Control and Reduced Exacerbations in Patients With Non-Cystic Fibrosis Bronchiectasis Treated With Nebulised Colistin. A Prospective Observational Study. Arch. Bronconeumol. 2022, 27, 834–836. [Google Scholar] [CrossRef]
- Stass, H.; Weimann, B.; Nagelschmitz, J.; Rolinck-Werninghaus, C.; Staab, D. Tolerability and Pharmacokinetic Properties of Ciprofloxacin Dry Powder for Inhalation in Patients with Cystic Fibrosis: A Phase I, Randomized, Dose-Escalation Study. Clin. Ther. 2013, 35, 1571–1581. [Google Scholar] [CrossRef]
- Wilson, R.; Welte, T.; Polverino, E.; De Soyza, A.; Greville, H.; O’Donnell, A.; Alder, J.; Reimnitz, P.; Hampel, B. Ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis: A phase II randomised study. Eur. Respir. J. 2012, 41, 1107–1115. [Google Scholar] [CrossRef]
- Dorkin, H.L.; Staab, D.; Operschall, E.; Alder, J.; Criollo, M. Ciprofloxacin DPI: A randomised, placebo-controlled, phase IIb efficacy and safety study on cystic fibrosis. BMJ Open Respir. Res. 2015, 2, e000100. [Google Scholar] [CrossRef]
- De Soyza, A.; Aksamit, T.; Bandel, T.J.; Criollo, M.; Elborn, J.S.; Operschall, E.; Polverino, E.; Roth, K.; Winthrop, K.L.; Wilson, R. Respire 1: A phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur. Respir. J. 2018, 51, 1702052. [Google Scholar] [CrossRef] [PubMed]
- Aksamit, T.; De Soyza, A.; Bandel, T.J.; Criollo, M.; Elborn, J.S.; Operschall, E.; Polverino, E.; Roth, K.; Winthrop, K.L.; Wilson, R. RESPIRE 2: A phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur. Respir. J. 2018, 51, 1702053. [Google Scholar] [CrossRef] [PubMed]
- Bilton, D.; Daviskas, E.; Anderson, S.; Kolbe, J.; King, G.; Stirling, R.G.; Thompson, B.; Milne, D.; Charlton, B. Phase 3 Randomized Study of the Efficacy and Safety of Inhaled Dry Powder Mannitol for the Symptomatic Treatment of Non-Cystic Fibrosis Bronchiectasis. Chest 2013, 144, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Taccetti, G.; Francalanci, M.; Pizzamiglio, G.; Messore, B.; Carnovale, V.; Cimino, G.; Cipolli, M. Cystic Fibrosis: Recent Insights into Inhaled Antibiotic Treatment and Future Perspectives. Antibiotics 2021, 10, 338. [Google Scholar] [CrossRef]
- Parumasivam, T.; Chang, R.Y.K.; Abdelghany, S.; Ye, T.T.; Britton, W.J.; Chan, H.K. Dry powder inhalable formulations for anti-tubercular therapy. Adv. Drug Deliv. Rev. 2016, 102, 83–101. [Google Scholar] [CrossRef]
- Verma, R.K.; Germishuizen, W.A.; Motheo, M.P.; Agrawal, A.K.; Singh, A.K.; Mohan, M.; Gupta, P.; Gupta, U.D.; Cholo, M.; Anderson, R.; et al. Inhaled microparticles containing clofazimine are efficacious in treatment of experimental tuberculosis in mice. Antimicrob. Agents Chemother. 2013, 57, 1050–1052. [Google Scholar] [CrossRef]
- Parikh, R.; Patel, L.; Dalwadi, S. Microparticles of rifampicin: Comparison of pulmonary route with oral route for drug uptake by alveolar macrophages, phagocytosis activity and toxicity study in albino rats. Drug Deliv. 2013, 21, 406–411. [Google Scholar] [CrossRef]
- Verma, R.K.; Mukker, J.K.; Singh, R.S.; Kumar, K.; Verma, P.R.P.; Misra, A. Partial Biodistribution and Pharmacokinetics of Isoniazid and Rifabutin Following Pulmonary Delivery of Inhalable Microparticles to Rhesus Macaques. Mol. Pharm. 2012, 9, 1011–1016. [Google Scholar] [CrossRef]
- Chogale, M.M.; Dhoble, S.B.; Patravale, V.B. A triple combination ’nano’ dry powder inhaler for tuberculosis: In vitro and in vivo pulmonary characterization. Drug Deliv. Transl. Res. 2021, 11, 1520–1531. [Google Scholar] [CrossRef]
- Dharmadhikari, A.S.; Kabadi, M.; Gerety, B.; Hickey, A.J.; Fourie, P.B.; Nardell, E. Phase I, Single-Dose, Dose-Escalating Study of Inhaled Dry Powder Capreomycin: A New Approach to Therapy of Drug-Resistant Tuberculosis. Antimicrob. Agents Chemother. 2013, 57, 2613–2619. [Google Scholar] [CrossRef]
- Srichana, T.; Ratanajamit, C.; Juthong, S.; Suwandecha, T.; Laohapojanart, N.; Pungrassami, P.; Padmavathi, A.R. Evaluation of Proinflammatory Cytokines and Adverse Events in Healthy Volunteers upon Inhalation of Antituberculosis Drugs. Biol. Pharm. Bull. 2016, 39, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Laohapojanart, N.; Ratanajamit, C.; Kawkitinarong, K.; Srichana, T. Efficacy and safety of combined isoniazid-rifampicin-pyrazinamide-levofloxacin dry powder inhaler in treatment of pulmonary tuberculosis: A randomized controlled trial. Pulm. Pharmacol. Ther. 2021, 70, 102056. [Google Scholar] [CrossRef]
- Haworth, C.S.; Bilton, D.; Chalmers, J.D.; Davis, A.M.; Froehlich, J.; Gonda, I.; Thompson, B.; Wanner, A.; O’Donnell, A.E. Inhaled Liposomal Ciprofloxacin in Patients with Non-Cystic Fibrosis Bronchiectasis and Chronic Lung Infection with Pseudomonas Aeruginosa (ORBIT-3 and ORBIT-4): Two Phase 3, Randomised Controlled Trials. Lancet Respir. Med. 2019, 7, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Weers, J. Comparison of Phospholipid-Based Particles for Sustained Release of Ciprofloxacin Following Pulmonary Administration to Bronchiectasis Patients. Pulm. Ther. 2019, 5, 127–150. [Google Scholar] [CrossRef] [PubMed]
- Muneer, S.; Wang, T.; Rintoul, L.; Ayoko, G.A.; Islam, N.; Izake, E.L. Development and characterization of meropenem dry powder inhaler formulation for pulmonary drug delivery. Int. J. Pharm. 2020, 587, 119684. [Google Scholar] [CrossRef]
- Douafer, H.; Andrieu, V.; Wafo, E.; Sergent, M.; Brunel, J.M. Feasibility of an inhaled antibiotic/adjuvant dry powder combination using an experimental design approach. Int. J. Pharm. 2021, 599, 120414. [Google Scholar] [CrossRef] [PubMed]
- Waterer, G.; Lord, J.; Hofmann, T.; Jouhikainen, T. Phase I, Dose-Escalating Study of the Safety and Pharmacokinetics of Inhaled Dry-Powder Vancomycin (AeroVanc) in Volunteers and Patients with Cystic Fibrosis: A New Approach to Therapy for Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2020, 64, e01776-19. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). (March 2013–January 2020). A Phase 2, Randomized, Double Blind, Placebo-Controlled Study of AeroVanc for the Treatment of Persistent Methicillin-Resistant Staphylococcus Aureus Lung Infection in Cystic Fibrosis Patients. Identifier NCT01746095. Available online: https://clinicaltrials.gov/ct2/show/NCT01746095 (accessed on 22 February 2023).
- National Library of Medicine (U.S.). (September 2017–January 2021). A Phase III, Randomized, Double-Blind, Placebo-Controlled Study of AeroVanc for the Treatment of Persistent Methicillin-Resistant Staphylococcus Aureus Lung Infection in Cystic Fibrosis Patients. Identifier NCT03181932. Available online: https://clinicaltrials.gov/ct2/show/NCT03181932 (accessed on 22 February 2023).
- Alcaraz-Serrano, V.; Lee, A.L.; Gimeno-Santos, E. Respiratory Physiotherapy and Bronchiectasis. Arch. Bronconeumol. 2022, 58, 377–378. [Google Scholar] [CrossRef] [PubMed]


| Advantages | Drawbacks | |
|---|---|---|
| Ultrasonic nebulizers | They allow large volumes of liquid to be nebulized. They are silent. | They produce highly heterogeneous and dispersed aerosols. Some drugs are denatured by heat (for example, antibiotics). They do not nebulize suspensions. |
| Jet nebulizers | They provide high flows. They are faster than ultrasonic nebulizers. They can nebulize both solutions and suspensions. | Their compressors are usually loud and heavy. |
| Mesh nebulizers | Some can run on batteries (as well as electricity). They are small and silent. They can nebulize both solutions and suspensions. They are faster than jet nebulizers. | They are less resistant than jet nebulizers. There is a lack of bioequivalence studies with jet nebulizers for some drugs. |
| EVOLVE Study | EDIT Study | EAGER Study | |
|---|---|---|---|
| Study design | Randomized, double blind, compared with placebo. TIP: 112 mg bid (n = 46); placebo (n = 49) 24 weeks (1 cycle TIP or placebo followed by 2 cycles open-label TIP). To evaluate the efficacy of a 28-day bid dosing regimen of TIP vs. placebo, as measured by the relative change in FEV1% predicted from baseline to the end of the dosing in cycle 1. | Randomized, double blind, compared with placebo. TIP: 112 mg bid (n = 30); placebo (n = 32) 8 weeks (1 cycle TIP or placebo). Extensions: Each extension consisted of 3 additional cycles of TIP. To evaluate the efficacy of TIP manufactured by an improved process vs. placebo, assessed by relative change in FEV1% predicted from baseline to day 29. | Randomized, open label, non-inferiority. TIP: 112 mg bid (n = 308); TIS: 300 mg/5 mL bid (n = 209) 24 weeks (3 cycles TIP or TIS. To evaluate the safety of bid dosing of TIP delivered with the T-326 inhaler vs. TIS (5 mL) delivered with the PARI-LC® PLUS Jet nebulizer. |
| FEV1 | Baseline to day 28-TIP vs. placebo: 13.3% (95% CI: 5.3 to 21.3; p = 0.0016). | Baseline to day 29-TIP vs. placebo: 5.9% (95% CI: −2.2 to 14.0; p = 0.148). | Baseline to day 28 of cycle 3-TIP vs. TIS: 1.1% relative change (least squares mean difference). |
| Antibiotic use | TIP vs. placebo: 13.0% vs. 18.4%. | TIP vs. placebo: 6.7% versus 12.5%. | TIP vs. TIS: 64.9% vs. 54.5%. |
| P. aeruginosa | Non-mucoid PA-TIP vs. placebo: −1.91 (SD: 2.54) vs. −0.15 (0.68). Mucoid-TIP vs. placebo: −2.61 (2.53) vs. −0.43 (1.05). | Sum of all biotypes PA-TIP vs. placebo): −1.2 vs. 0 (p = 0.002). | Nonmucoid PA-TIP vs. TIS: −1.77 vs. −0.73. Mucoid-TIP vs. TIS: −1.6 vs. −0.92. |
| Overall safety | TIP vs. placebo: 23 (50.0%) vs. 37 (75.5%). | TIP vs. placebo: 8 (26.7%) vs. 11 (34.4%). | TIP vs. TIS: 278 (90.3%) vs. 176 (84.2%). |
| Cough | TIP vs. placebo: 6 (13.0%) vs. 13 (26.5%) | TIP vs. placebo: 3 (10.0%) vs. 0 | TIP vs. TIS: 149 (48.4%) vs. 65 (31.1%) |
| Suspension | TIP vs. placebo: 7 (15.2%) versus 9 (18.4%). | TIP vs. placebo: 1 (3.3%) vs. 1 (3.1%). | TIP vs. TIS: 83 (26.9%) vs. 38 (18.2). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Rosa-Carrillo, D.; Suárez-Cuartín, G.; Sibila, O.; Golpe, R.; Girón, R.-M.; Martínez-García, M.-Á. Efficacy and Safety of Dry Powder Antibiotics: A Narrative Review. J. Clin. Med. 2023, 12, 3577. https://doi.org/10.3390/jcm12103577
de la Rosa-Carrillo D, Suárez-Cuartín G, Sibila O, Golpe R, Girón R-M, Martínez-García M-Á. Efficacy and Safety of Dry Powder Antibiotics: A Narrative Review. Journal of Clinical Medicine. 2023; 12(10):3577. https://doi.org/10.3390/jcm12103577
Chicago/Turabian Stylede la Rosa-Carrillo, David, Guillermo Suárez-Cuartín, Oriol Sibila, Rafael Golpe, Rosa-María Girón, and Miguel-Ángel Martínez-García. 2023. "Efficacy and Safety of Dry Powder Antibiotics: A Narrative Review" Journal of Clinical Medicine 12, no. 10: 3577. https://doi.org/10.3390/jcm12103577
APA Stylede la Rosa-Carrillo, D., Suárez-Cuartín, G., Sibila, O., Golpe, R., Girón, R.-M., & Martínez-García, M.-Á. (2023). Efficacy and Safety of Dry Powder Antibiotics: A Narrative Review. Journal of Clinical Medicine, 12(10), 3577. https://doi.org/10.3390/jcm12103577





