Ghrelin and Leptin Concentrations in Patients after SARS-CoV2 Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Leptin and Ghrelin
2.2. Statistical Analysis
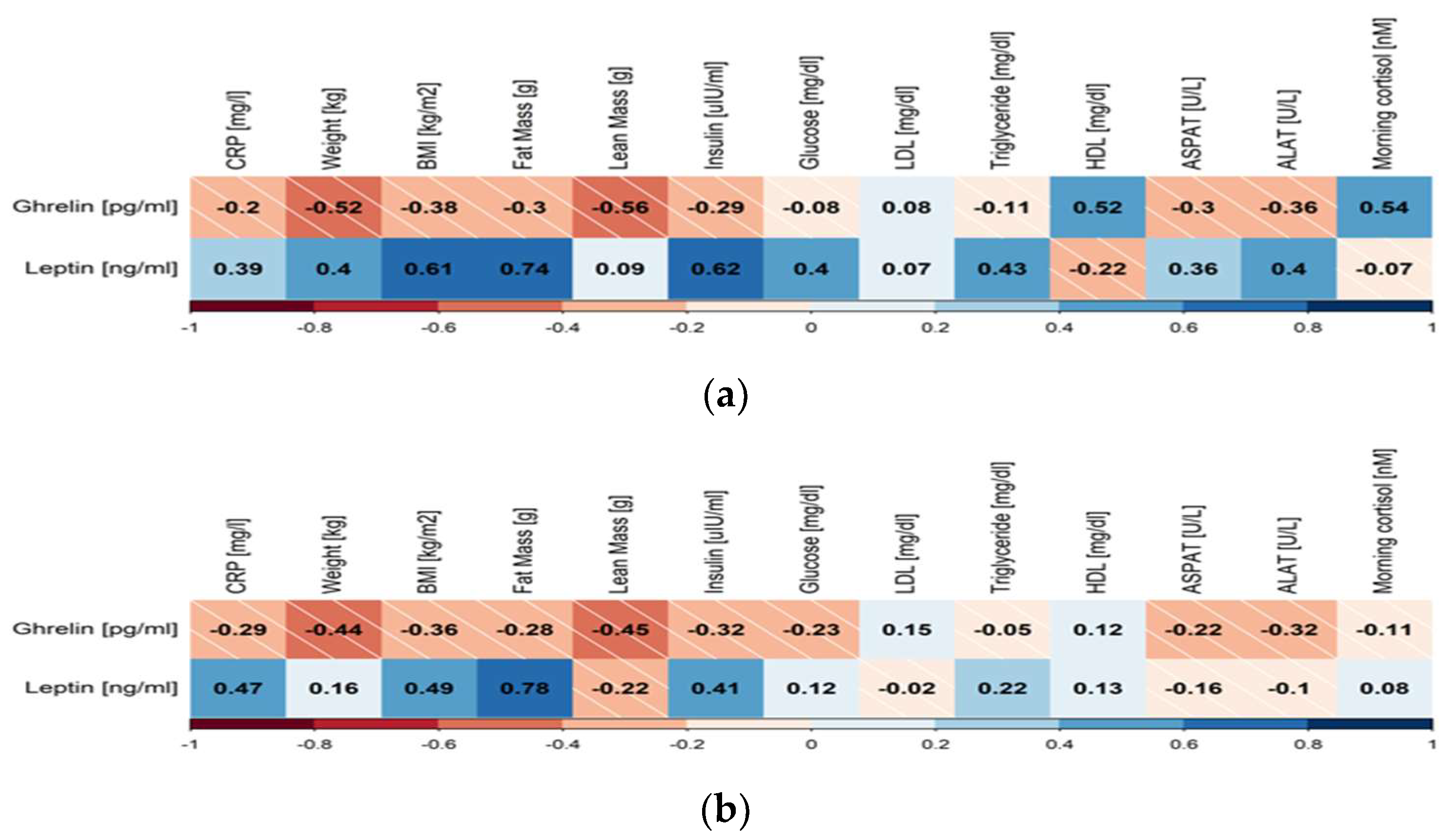
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Girija, A.S.S.; Shankar, E.M.; Larsson, M. Could SARS-CoV-2-Induced Hyperinflammation Magnify the Severity of Coronavirus Disease (CoViD-19) Leading to Acute Respiratory Distress Syndrome? Front. Immunol. 2020, 11, 1206. [Google Scholar] [CrossRef]
- Allawadhi, P.; Khurana, A.; Allwadhi, S.; Joshi, K.; Packirisamy, G.; Bharani, K.K. Nanoceria as a possible agent for the management of COVID-19. Nano Today 2020, 35, 100982. [Google Scholar] [CrossRef] [PubMed]
- Pashenkov, M.V.; Murugina, N.E.; Budikhina, A.S.; Pinegin, B.V. Synergistic interactions between NOD receptors and TLRs: Mechanisms and clinical implications. J. Leukoc. Biol. 2019, 105, 4. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, J.; Zhang, D.; Xu, Z.; Ji, J.; Wen, C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front. Immunol. 2020, 11, 1708. [Google Scholar] [CrossRef]
- Hassan, S.A.; Sheikh, F.N.; Jamal, S.; Ezeh, J.K.; Akhtar, A. Coronavirus (COVID-19): A Review of Clinical Features, Diagnosis, and Treatment. Cureus 2020, 12, e7355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, X.; Ni, L.; Di, X.; Ma, B.; Niu, S.; Liu, C.; Reiter, R.J. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020, 250, 117583. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Sadeghpour, S.; Ghasemnejad-Berenji, H.; Pashapour, S.; Ghasemnejad-Berenji, M. Potential Antioxidative, Anti-inflammatory and Immunomodulatory Effects of Ghrelin, an Endogenous Peptide from the Stomach in SARS-CoV2 Infection. Int. J. Pept. Res. Ther. 2021, 27, 3. [Google Scholar] [CrossRef]
- Fakhri, S.; Nouri, Z.; Moradi, S.Z.; Farzaei, M.H. Astaxanthin, COVID-19 and immune response: Focus on oxidative stress, apoptosis and autophagy. Phytother. Res. 2020, 34, 11. [Google Scholar] [CrossRef]
- Eid, R.A.; El-Kott, A.F.; Zaki, M.S.A.; Eldeen, M.A.; Al-Hashem, F.H.; Alkhateeb, M.A.; Alassiri, M.; Aldera, H. Acylated ghrelin protects aorta damage post-MI via activation of eNOS and inhibition of angiotensin-converting enzyme induced activation of NAD(P)H-dependent oxidase. Ultrastruct. Pathol. 2018, 42, 5. [Google Scholar] [CrossRef]
- Prodam, F.; Filigheddu, N. Ghrelin Gene Products in Acute and Chronic Inflammation. Arch. Immunol. Et Ther. Exp. 2014, 62, 5. [Google Scholar] [CrossRef]
- Sato, T.; Nakamura, Y.; Shiimura, Y.; Ohgusu, H.; Kangawa, K.; Kojima, M. Structure, regulation and function of ghrelin. J. Biochem. 2012, 151, 2. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 6762. [Google Scholar] [CrossRef]
- Gnanapavan, S.; Kola, B.; Bustin, S.A.; Morris, D.G.; McGee, P.; Fairclough, P.; Bhattacharya, S.; Carpenter, R.; Grossman, A.B.; Korbonits, M. The Tissue Distribution of the mRNA of Ghrelin and Subtypes of Its Receptor, GHS-R, in Humans. J. Clin. Endocrinol. Metab. 2002, 87, 6. [Google Scholar] [CrossRef]
- Guan, X.-M.; Yu, H.; Palyha, O.C.; McKee, K.K.; Feighner, S.D.; Sirinathsinghji, D.J.; Smith, R.G.; Van der Ploeg, L.H.; Howard, A.D. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Mol. Brain Res. 1997, 48, 1. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Guillén, L.; Barrios, V.; Campos-Barros, Á.; Argente, J. Ghrelin levels in obesity and anorexia nervosa: Effect of weight reduction or recuperation. J. Pediatr. 2004, 144, 1. [Google Scholar] [CrossRef]
- Mathur, N.; Mehdi, S.F.; Anipindi, M.; Aziz, M.; Khan, S.A.; Kondakindi, H.; Lowell, B.; Wang, P.; Roth, J. Ghrelin as an Anti-Sepsis Peptide: Review. Front. Immunol. 2021, 11, 610363. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Dong, W.; Cui, X.; Zhou, M.; Simms, H.H.; Ravikumar, T.S.; Wang, P. Ghrelin Down-regulates Proinflammatory Cytokines in Sepsis Through Activation of the Vagus Nerve. Ann. Surg. 2007, 245, 3. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Relationship between gut and sepsis: Role of ghrelin. World J. Diabetes 2011, 2, 1. [Google Scholar] [CrossRef]
- Waseem, T.; Duxbury, M.; Ito, H.; Ashley, S.W.; Robinson, M.K. Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery 2008, 143, 3. [Google Scholar] [CrossRef]
- Valle, M.S.; Russo, C.; Malaguarnera, L. Protective role of vitamin D against oxidative stress in diabetic retinopathy. Diabetes Metab. Res. Rev. 2021, 37, 8. [Google Scholar] [CrossRef]
- Zhou, X.; Xue, C. Ghrelin attenuates acute pancreatitis-induced lung injury and inhibits substance P expression. Am. J. Med. Sci. 2010, 339, 1. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Shu, Q.; Li, S.; Luo, F. Ghrelin attenuates lipopolysaccharide-induced acute lung injury through no pathway. Med. Sci. Monit. 2008, 14, 2008. [Google Scholar]
- Miki, K.; Maekura, R.; Nagaya, N.; Nakazato, M.; Kimura, H.; Murakami, S.; Ohnishi, S.; Hiraga, T.; Miki, M.; Kitada, S.; et al. Ghrelin treatment of cachectic patients with chronic obstructive pulmonary disease: A multicenter, randomized, double-blind, placebo-controlled trial. PLoS ONE 2012, 7, e35708. [Google Scholar] [CrossRef] [PubMed]
- Takata, A.; Takiguchi, S.; Miyazaki, Y.; Miyata, H.; Takahashi, T.; Kurokawa, Y.; Yamasaki, M.; Nakajima, K.; Mori, M.; Kangawa, K.; et al. Randomized phase II study of the anti-inflammatory effect of ghrelin during the postoperative period of esophagectomy. Ann. Surg. 2015, 262, 230–236. [Google Scholar] [CrossRef]
- Hakami, N.Y.; Alhazmi, W.A.; Taibah, E.O.; Sindi, M.M.; Alotaibi, O.F.; Al-Otaibi, H.M.; Alhadrami, H.A. The Effect of COVID-19 Infection on Human Blood Ghrelin Hormone: A Pilot Study. J. Pharm. Res. Int. 2021, 33, 33–38. [Google Scholar] [CrossRef]
- Mangge, H.; Almer, G.; Truschnig-Wilders, M.; Schmidt, A.; Gasser, R.; Fuchs, D. Inflammation, Adiponectin, Obesity and Cardiovascular Risk. Curr. Med. Chem. 2010, 17, 4511–4520. [Google Scholar] [CrossRef] [PubMed]
- La Cava, A.; Matarese, G. The weight of leptin in immunity. Nat. Rev. Immunol. 2004, 4, 371–379. [Google Scholar] [CrossRef]
- Ahima, R.S.; Saper, C.B.; Flier, J.S.; Elmquist, J.K. Leptin regulation of neuroendocrine systems. Front. Neuroendocrinol. 2000, 21, 263–307. [Google Scholar] [CrossRef]
- Maurya, R.; Bhattacharya, P.; Dey, R.; Nakhasi, H.L. Leptin Functions in Infectious Diseases. Front. Immunol. 2018, 9, 2741. [Google Scholar] [CrossRef]
- Abella, V.; Scotece, M.; Conde, J.; Pino, J.; Gonzalez-Gay, M.A.; Gómez-Reino, J.J.; Mera, A.; Lago, F.; Gómez, R.; Gualillo, O. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 2017, 13, 100–109. [Google Scholar] [CrossRef]
- Paz-Filho, G.; Mastronardi, C.; Franco, C.B.; Wang, K.B.; Wong, M.-L.; Licinio, J. Leptin: Molecular mechanisms, systemic pro-inflammatory effects, and clinical implications. Arq. Bras. de Endocrinol. Metabol. 2012, 56, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Acedo, S.C.; Gambero, S.; Cunha, F.G.P.; Lorand-Metze, I.; Gambero, A. Participation of leptin in the determination of the macrophage phenotype: An additional role in adipocyte and macrophage crosstalk. Vitr. Cell. Dev. Biol.-Anim. 2013, 49, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Najib, S.; Sánchez-Margalet, V. Human leptin promotes survival of human circulating blood monocytes prone to apoptosis by activation of p42/44 MAPK pathway. Cell. Immunol. 2002, 220, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, Y.; Zhang, X.; Wang, S.; Peng, Z.; Guo, J.; Jiang, H.; Liu, J.; Xie, Y.; Wang, J.; et al. Leptin correlates with monocytes activation and severe condition in COVID-19 patients. J. Leukoc. Biol. 2021, 110, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Aghili, S.M.M.; Ebrahimpur, M.; Arjmand, B.; Shadman, Z.; Sani, M.P.; Qorbani, M.; Larijani, B.; Payab, M. Obesity in COVID-19 era, implications for mechanisms, comorbidities, and prognosis: A review and meta-analysis. Int. J. Obes. 2021, 45, 998–1016. [Google Scholar] [CrossRef]
- Scheen, A.; Marre, M.; Thivolet, C. Prognostic factors in patients with diabetes hospitalized for COVID-19: Findings from the CORONADO study and other recent reports. Diabetes Metab. 2020, 46, 265–271. [Google Scholar] [CrossRef]
- Battisti, S.; Pedone, C.; Napoli, N.; Russo, E.; Agnoletti, V.; Nigra, S.G.; Dengo, C.; Mughetti, M.; Conte, C.; Pozzilli, P.; et al. Computed Tomography Highlights Increased Visceral Adiposity Associated with Critical Illness in COVID-19. Diabetes Care 2020, 43, e129–e130. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Caruso, D.; Tuccinardi, D.; Risi, R.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Tarallo, M.; Strigari, L.; Manfrini, S.; et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism 2020, 111, 154319. [Google Scholar] [CrossRef]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef]
- Iacobellis, G.; Malavazos, A.E.; Ferreira, T. COVID-19 Rise in Younger Adults with Obesity: Visceral Adiposity Can Predict the Risk. Obesity 2020, 28, 1795. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Qi, Y.; Deng, L.; Wang, H.; Xu, Y.; Li, Z.; Meng, Z.; Tang, J.; Dai, Z. Obesity as a Potential Predictor of Disease Severity in Young COVID-19 Patients: A Retrospective Study. Obesity 2020, 28, 1815–1825. [Google Scholar] [CrossRef]
- Palaiodimos, L.; Kokkinidis, D.G.; Li, W.; Karamanis, D.; Ognibene, J.; Arora, S.; Southern, W.N.; Mantzoros, C.S. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism 2020, 108, 154262. [Google Scholar] [CrossRef]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M. High prevalence of obesity in SARS-CoV-2 requiring invasive mechanical ventilation. Obesity 2020, 28, 1. [Google Scholar] [CrossRef] [PubMed]
- Baatar, D.; Patel, K.; Taub, D.D. The effects of ghrelin on inflammation and the immune system. Mol. Cell. Endocrinol. 2011, 340, 44–58. [Google Scholar] [CrossRef]
- Lv, Y.; Liang, T.; Wang, G.; Li, Z. Ghrelin, a gastrointestinal hormone, regulates energy balance and lipid metabolism. Bioscience Reports 2018, 38, BSR20181061. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, N.; Annambhotla, S.; Jiang, J.; Wang, X.; Chai, H.; Lin, P.H.; Yao, Q.; Chen, C. Growth hormone–releasing peptide ghrelin inhibits homocysteine-induced endothelial dysfunction in porcine coronary arteries and human endothelial cells. J. Vasc. Surg. 2009, 49, 199–207. [Google Scholar] [CrossRef]
- Dixit, V.D.; Schaffer, E.M.; Pyle, R.S.; Collins, G.D.; Sakthivel, S.K.; Palaniappan, R.; Lillard, J.W.; Taub, D.D. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J. Clin. Investig. 2004, 114, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Martín-Romero, C.; Santos-Alvarez, J.; Goberna, R.; Sánchez-Margalet, V. Human Leptin Enhances Activation and Proliferation of Human Circulating T Lymphocytes. Cell. Immunol. 2000, 199, 15–24. [Google Scholar] [CrossRef]
- Zhang, L.-N.; Gong, W.-D.; Luo, J.; Yu, Y.-J.; Qi, S.-H.; Yue, Z.-Y. Exogenous ghrelin ameliorates acute lung injury by modulating the nuclear factor κB inhibitor kinase/nuclear factor κB inhibitor/nuclear factor κB pathway after hemorrhagic shock. Int. Immunopharmacol. 2019, 69, 95–102. [Google Scholar] [CrossRef]
- Yorulmaz, H.; Ozkok, E.; Ates, G.; Tamer, S. Investigation of the effectiveness of ghrelin treatment in lung tissue of rats with sepsis. Bratisl. Med. J. 2017, 118, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Tolle, V.; Kadem, M.; Bluet-Pajot, M.-T.; Frere, D.; Foulon, C.; Bossu, C.; Dardennes, R.; Mounier, C.; Zizzari, P.; Lang, F.; et al. Balance in Ghrelin and Leptin Plasma Levels in Anorexia Nervosa Patients and Constitutionally Thin Women. J. Clin. Endocrinol. Metab. 2003, 88, 109–116. [Google Scholar] [CrossRef]
- Otto, B.; Cuntz, U.; Fruehauf, E.; Wawarta, R.; Folwaczny, C.; Riepl, R.; Heiman, M.; Lehnert, P.; Fichter, M.; Tschop, M. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur. J. Endocrinol. 2001, 145, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Tena-Sempere, M. Exploring the role of ghrelin as novel regulator of gonadal function. Growth Horm. IGF Res. 2005, 15, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Ueberberg, B.; Unger, N.; Saeger, W.; Mann, K.; Petersenn, S. Expression of Ghrelin and its Receptor in Human Tissues. Horm. Metab. Res. 2009, 41, 814–821. [Google Scholar] [CrossRef]
- Gaytán, F.; Barreiro, M.L.; Caminos, J.E.; Chopin, L.K.; Herington, A.; Morales, C.; Pinilla, L.; Paniagua, R.; Nistal, M.; Casanueva, F.F.; et al. Expression of Ghrelin and Its Functional Receptor, the Type 1a Growth Hormone Secretagogue Receptor, in Normal Human Testis and Testicular Tumors. J. Clin. Endocrinol. Metab. 2004, 89, 400–409. [Google Scholar] [CrossRef]
- Gaytan, F.; Barreiro, M.L.; Caminos, J.E.; Chopin, L.K.; Herington, A.C.; Morales, C.; Pinilla, L.; Paniagua, R.; Nistal, M.; Casanueva, F.F.; et al. Effects of Testosterone Supplementation on Ghrelin and Appetite during and after Severe Energy Deficit in Healthy Men. J. Endocr. Soc. 2004, 4, bvaa024. [Google Scholar] [CrossRef]
- Makovey, J.; Naganathan, V.; Seibel, M.; Sambrook, P. Gender differences in plasma ghrelin and its relations to body composition and bone—An opposite-sex twin study. Clin. Endocrinol. 2007, 66, 530–537. [Google Scholar] [CrossRef]
- Greenman, Y.; Golani, N.; Gilad, S.; Yaron, M.; Limor, R.; Stern, N. Ghrelin secretion is modulated in a nutrient- and gender-specific manner. Clin. Endocrinol. 2004, 60, 382–388. [Google Scholar] [CrossRef]
- Purnell, J.Q.; Weigle, D.S.; Breen, P.; Cummings, D.E. Ghrelin Levels Correlate with Insulin Levels, Insulin Resistance, and High-Density Lipoprotein Cholesterol, But Not with Gender, Menopausal Status, or Cortisol Levels in Humans. J. Clin. Endocrinol. Metab. 2003, 88, 5747–5752. [Google Scholar] [CrossRef]
- Shiiya, T.; Nakazato, M.; Mizuta, M.; Date, Y.; Mondal, M.S.; Tanaka, M.; Nozoe, S.-I.; Hosoda, H.; Kangawa, K.; Matsukura, S. Plasma Ghrelin Levels in Lean and Obese Humans and the Effect of Glucose on Ghrelin Secretion. J. Clin. Endocrinol. Metab. 2002, 87, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Vendrell, J.; Broch, M.; Vilarrasa, N.; Molina, A.; Gómez, J.M.; Gutiérrez, C.; Simón, I.; Soler, J.; Richart, C. Resistin, Adiponectin, Ghrelin, Leptin, and Proinflammatory Cytokines: Relationships in Obesity. Obes. Res. 2004, 12, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Vilarrasa, N.; Vendrell, J.; Maravall, J.; Broch, M.; Estepa, A.; Megia, A.; Soler, J.; Simon, I.; Richart, C.; Gomez, J.M. Distribution and determinants of adiponectin, resistin and ghrelin in a randomly selected healthy population. Clin. Endocrinol. 2005, 63, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Barkan, A.L.; Dimaraki, E.V.; Jessup, S.K.; Symons, K.V.; Ermolenko, M.; Jaffe, C.A. Ghrelin Secretion in Humans Is Sexually Dimorphic, Suppressed by Somatostatin, and Not Affected by the Ambient Growth Hormone Levels. J. Clin. Endocrinol. Metab. 2003, 88, 2180–2184. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P.; Curtis, J.L.; Freeman, C.M.; Peters-Golden, M.; Weinberg, J.B.; Myers, M.G., Jr. Ablation of the leptin receptor in myeloid cells impairs pulmonary clearance of Streptococcus pneumoniae and alveolar macrophage bactericidal function. Am. J. Physiol. Cell. Mol. Physiol. 2018, 315, L78–L86. [Google Scholar] [CrossRef]
- Salum, K.C.R.; Rolando, J.D.M.; Zembrzuski, V.M.; Carneiro, J.R.I.; Mello, C.B.; Maya-Monteiro, C.M.; Bozza, P.T.; Kohlrausch, F.B.; da Fonseca, A.C.P. When Leptin Is Not There: A Review of What Nonsyndromic Monogenic Obesity Cases Tell Us and the Benefits of Exogenous Leptin. Front. Endocrinol. 2021, 12, 722441. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Schoeman, D.; Fielding, B.C. Leptin Deficiency, Caused by Malnutrition, Makes You Susceptible to SARS-CoV-2 Infection but Could Offer Protection from Severe COVID-19. Msphere 2021, 6, E00031-21. [Google Scholar] [CrossRef]
- Ziegler, C.G.; Miao, V.N.; Owings, A.H.; Navia, A.W.; Tang, Y.; Bromley, J.D.; Lotfy, P.; Sloan, M.; Laird, H.; Williams, H.B.; et al. Impaired local intrinsic immunity to SARS-CoV-2 infection in severe COVID-19. Cell 2021, 184, 4713–4733.e22. [Google Scholar] [CrossRef]
- Rebello, C.J.; Kirwan, J.P.; Greenway, F.L. Obesity, the most common comorbidity in SARS-CoV-2: Is leptin the link? Int. J. Obes. 2020, 44, 1810–1817. [Google Scholar] [CrossRef]
- van der Voort, P.H.; Moser, J.; Zandstra, D.F.; Kobold, A.C.M.; Knoester, M.; Calkhoven, C.F.; Hamming, I.; van Meurs, M. Leptin levels in SARS-CoV-2 infection related respiratory failure: A cross-sectional study and a pathophysiological framework on the role of fat tissue. Heliyon 2020, 6, e04696. [Google Scholar] [CrossRef] [PubMed]
- di Filippo, L.; de Lorenzo, R.; Sciorati, C.; Capobianco, A.; Lorè, N.I.; Giustina, A.; Manfredi, A.A.; Rovere-Querini, P.; Conte, C. Adiponectin to leptin ratio reflects inflammatory burden and survival in COVID-19. Diabetes Metab. 2021, 47, 101268. [Google Scholar] [CrossRef] [PubMed]
| Study Group (n = 53) | Controls (n = 87) | ||||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | IQR | Mean ± SD | Median | IQR | p-Value | |
| Age (years) | 45.339 ± 12.160 | 44 | 21.5 | 46.556± 12.391 | 44 | 18.5 | 0.53 |
| Height (m) | 1.689 ± 0.078 | 1.665 | 0.120 | 1.689 ± 0.097 | 1.690 | 0.147 | 0.918 |
| Weight (kg) | 74.604 ± 16.608 | 76 | 23 | 77.351 ± 16.531 | 76 | 22 | 0.318 |
| Fat (%) | 32.521 ± 6.705 | 31.5 | 9.5 | 32.847 ± 6.695 | 33.2 | 11.2 | 0.793 |
| Fat mass (g) | 24,559.95 ± 7821.173 | 22,862.75 | 14,144.92 | 25,903.17 ± 7752.641 | 24,422.5 | 10,899.90 | 0.232 |
| Lean mass (g) | 48,173.30 ± 11,654.69 | 45,910.9 | 17,411.65 | 50,480.01 ± 11,916.18 | 49,288.0 | 18,756.40 | 0.253 |
| Ghrelin (pg/mL) | 1326.095± 848.215 | 1190.555 | 579.812 | 1001.010 ± 424.116 | 901.390 | 44.590 | 0.003 |
| Leptin (ng/mL) | 26.601 ± 23.290 | 18.475 | 29.847 | 27.166 ± 25.627 | 19.475 | 25.910 | 0.852 |
| ALAT (U/L) | 26.3 ± 18.43 | 20.5 | 14.75 | 25.765 ± 17.109 | 20 | 16 | 0.888 |
| ASPAT (U/L) | 22.325 ± 8.319 | 19.5 | 8.75 | 20.921 ± 7.42 | 19 | 8 | 0.532 |
| Total cholesterol (mg/dL) | 191.582 ± 39.863 | 189 | 57 | 195.307 ± 40.435 | 195.5 | 64.5 | 0.455 |
| LDL cholesterol (mg/dL) | 112.800 ± 33.386 | 109 | 49 | 118.705 ± 33.509 | 118.5 | 46.25 | 0.231 |
| HDL cholesterol | 58.473 ± 14.961 | 57 | 23 | 55.216 ± 12.399 | 55 | 20 | 0.336 |
| Triglyceride (mg/dL) | 100.8 ± 62.399 | 86 | 73 | 107.216 ± 66.302 | 92 | 65.75 | 0.495 |
| CRP (mg/L) | 1.544 ± 1.302 | 1.2 | 1.325 | 1.875 ± 2.391 | 0.9 | 1.3 | 0.0776 |
| Glucose | 87.779 ± 9.386 | 86 | 14 | 92.209 ± 15.598 | 88 | 13 | 0.143 |
| Insulin (μIU/mL) | 9.652 ± 8.923 | 6.9 | 7.215 | 10.133 ± 8.406 | 7.86 | 7.485 | 0.538 |
| HOMA-IR | 2.253 ± 2.249 | 1.578 | 1.798 | 2.377 ± 2.201 | 1.748 | 2.09 | 0.528 |
| QUICKI | 0.364 ± 0.049 | 0.356 | 0.062 | 0.360 ± 0.049 | 0.351 | 0.064 | 0.528 |
| FIRI | 2.027 ± 2.024 | 1.420 | 1.618 | 2.129 ± 1.981 | 1.573 | 1.888 | 0.528 |
| Testosterone (ng/mL) | 1.255 ± 1.728 | 0.270 | 2.122 | 1.719 ± 1.932 | 0.349 | 3.180 | 0.308 |
| eGFR (mL/min/1.73 m2) | 88.057 ± 14.059 | 88 | 18.5 | 94.012 ± 14.198 | 93 | 19 | 0.044 |
| Comparison | Difference | SE | t | df | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| Sex | Group | Sex | Group | |||||
| Female | COVID | Female | CONTROL | 446.0 | 125 | 3.569 | 142 | 0.002 * |
| Female | COVID | Male | COVID | 711.6 | 170 | 4.186 | 142 | <0.001 * |
| Female | COVID | Male | CONTROL | 660.0 | 132 | 5.015 | 142 | <0.001 * |
| Female | CONTROL | Male | CONTROL | 214.0 | 124 | 1.725 | 142 | 0.260 |
| Male | COVID | Female | CONTROL | −265.6 | 164 | −1.618 | 142 | 0.260 |
| Male | COVID | Male | CONTROL | −51.6 | 169 | −0.305 | 142 | 0.761 |
| Sex | Group | Number | Median | IGR | Mean ± SD | |||
| Female | COVID | 39 | 1289.29 | 620.64 | 1542.10 ± 912.36 | |||
| Female | CONTROL | 50 | 998.93 | 490.158 | 1096 ± 482.52 | |||
| Male | COVID | 17 | 801.44 | 552.38 | 830.54 ± 348.02 | |||
| Male | CONTROL | 40 | 847.68 | 305.49 | 882.10 ± 303.07 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuliczkowska-Płaksej, J.; Jawiarczyk-Przybyłowska, A.; Zembska, A.; Kolačkov, K.; Syrycka, J.; Kałużny, M.; Polowczyk-Kawałko, B.; Kubicka, E.; Bolanowski, M. Ghrelin and Leptin Concentrations in Patients after SARS-CoV2 Infection. J. Clin. Med. 2023, 12, 3551. https://doi.org/10.3390/jcm12103551
Kuliczkowska-Płaksej J, Jawiarczyk-Przybyłowska A, Zembska A, Kolačkov K, Syrycka J, Kałużny M, Polowczyk-Kawałko B, Kubicka E, Bolanowski M. Ghrelin and Leptin Concentrations in Patients after SARS-CoV2 Infection. Journal of Clinical Medicine. 2023; 12(10):3551. https://doi.org/10.3390/jcm12103551
Chicago/Turabian StyleKuliczkowska-Płaksej, Justyna, Aleksandra Jawiarczyk-Przybyłowska, Agnieszka Zembska, Katarzyna Kolačkov, Joanna Syrycka, Marcin Kałużny, Beata Polowczyk-Kawałko, Eliza Kubicka, and Marek Bolanowski. 2023. "Ghrelin and Leptin Concentrations in Patients after SARS-CoV2 Infection" Journal of Clinical Medicine 12, no. 10: 3551. https://doi.org/10.3390/jcm12103551
APA StyleKuliczkowska-Płaksej, J., Jawiarczyk-Przybyłowska, A., Zembska, A., Kolačkov, K., Syrycka, J., Kałużny, M., Polowczyk-Kawałko, B., Kubicka, E., & Bolanowski, M. (2023). Ghrelin and Leptin Concentrations in Patients after SARS-CoV2 Infection. Journal of Clinical Medicine, 12(10), 3551. https://doi.org/10.3390/jcm12103551






