Evaluation of Pentraxin 3 and Serum Amyloid A in the Gingival Crevicular Fluid of Patients with Periodontal Disease and Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
2.3. Gingival Crevicular Fluid Sampling
2.4. Immunological Assessment
2.5. Statistical Analysis
3. Results
3.1. Comparisons between Groups
3.1.1. Comparisons of Demographic and Clinical Parameters between Groups
3.1.2. Comparisons of the Immunological Parameters between Groups
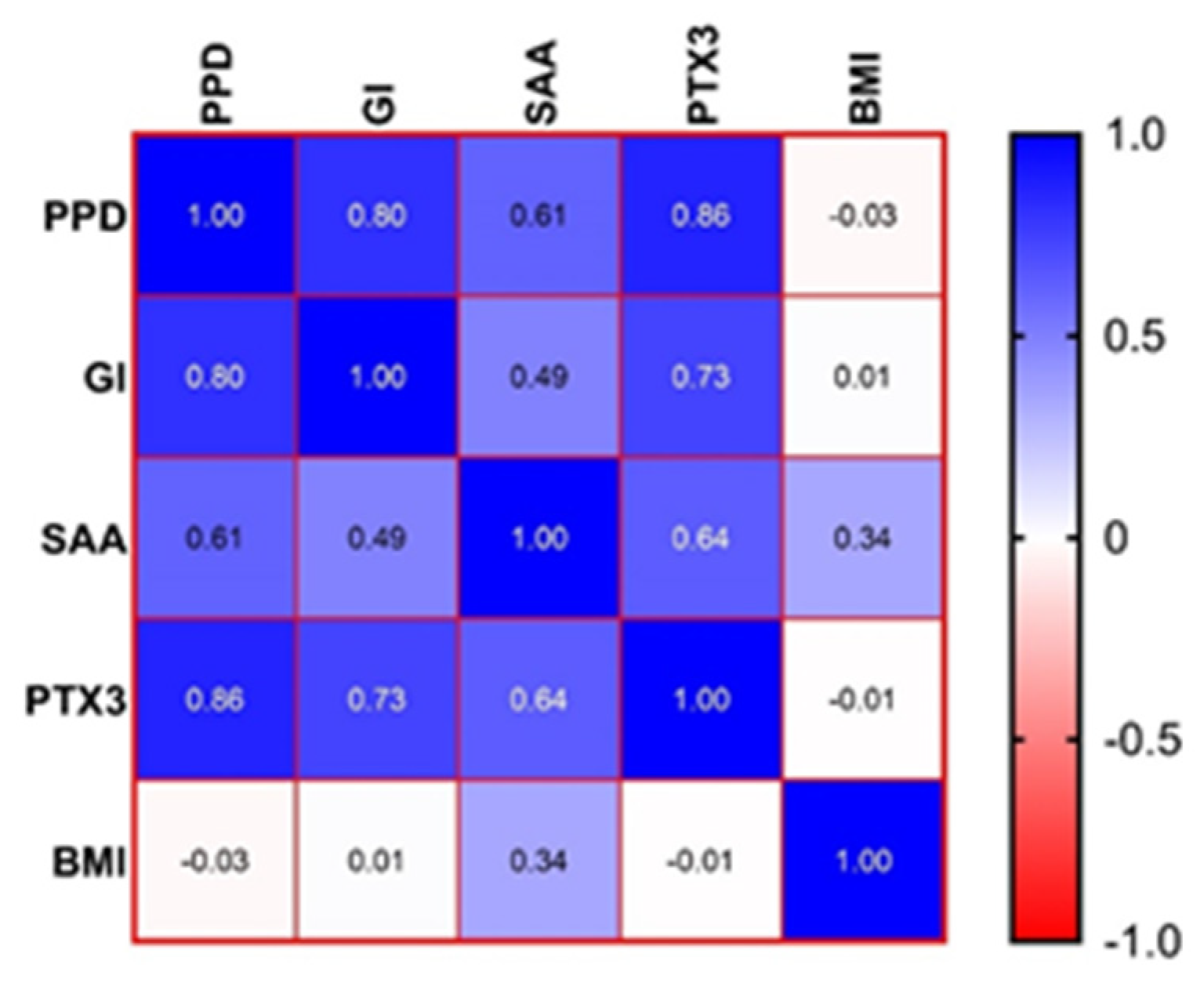
3.2. Correlations between the Parameters for the P + O Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000 2014, 64, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef] [PubMed]
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pract. 2017, 29 (Suppl. S1), S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Liccardi, A.; Graziadio, C.; Barrea, L.; Muscogiuri, G.; Colao, A. Obesity and infectious diseases: Pathophysiology and epidemiology of a double pandemic condition. Int. J. Obes. 2022, 46, 449–465. [Google Scholar] [CrossRef]
- Çetin, M.B.; Sezgin, Y.; Önder, C.; Bakirarar, B. The relationship between body mass index and stage/grade of periodontitis: A retrospective study. Clin. Oral Investig. 2022, 26, 1937–1945. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut Off Points; StatPearls Publishing: Treasure Island, FL, USA, 2023; pp. 2–4. [Google Scholar]
- Albandar, J.M.; Susin, C.; Hughes, F.J. Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S171–S189. [Google Scholar] [CrossRef]
- Feijóo-Bandín, S.; Aragón-Herrera, A.; Moraña-Fernández, S.; Anido-Varela, L.; Tarazón, E.; Roselló-Lletí, E.; Portolés, M.; Moscoso, I.; Gualillo, O.; González-Juanatey, J.R.; et al. Adipokines and Inflammation: Focus on Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 7711. [Google Scholar] [CrossRef]
- Manoil, D.; Bostanci, N.; Mumcu, G.; Inanc, N.; Can, M.; Direskeneli, H.; Belibasakis, G.N. Novel and known periodontal pathogens residing in the gingival crevicular fluid are associated with rheumatoid arthritis. J. Periodontol. 2021, 92, 359–370. [Google Scholar] [CrossRef]
- Roberts, H.M.; Yonel, Z.; Kantarci, A.; Grant, M.M.; Chapple, I.L.C. Impact of Gingivitis on Circulating Neutrophil Reactivity and Gingival Crevicular Fluid Inflammatory Proteins. Int. J. Environ. Res. Public Health 2022, 19, 6339. [Google Scholar] [CrossRef]
- Garlanda, C.; Bottazzi, B.; Magrini, E.; Inforzato, A.; Mantovani, A. PTX3, a Humoral Pattern Recognition Molecule, in Innate Immunity, Tissue Repair, and Cancer. Physiol. Rev. 2018, 98, 623–639. [Google Scholar] [CrossRef]
- Porte, R.; Davoudian, S.; Asgari, F.; Parente, R.; Mantovani, A.; Garlanda, C.; Bottazzi, B. The Long Pentraxin PTX3 as a Humoral Innate Immunity Functional Player and Biomarker of Infections and Sepsis. Front. Immunol. 2019, 10, 794. [Google Scholar] [CrossRef]
- Ji, A.; Trumbauer, A.C.; Noffsinger, V.P.; Jeon, H.; Patrick, A.C.; De Beer, F.C.; Webb, N.R.; Tannock, L.R.; Shridas, P. Serum Amyloid A is not obligatory for high-fat, high-sucrose, cholesterol-fed diet-induced obesity and its metabolic and inflammatory complications. PLoS ONE 2022, 17, e0266688. [Google Scholar] [CrossRef]
- Jahangiri, A.; Wilson, P.G.; Hou, T.; Brown, A.; King, V.L.; Tannock, L.R. Serum amyloid A is found on ApoB-containing lipoproteins in obese humans with diabetes. Obesity 2013, 21, 993–996. [Google Scholar] [CrossRef]
- O’Brien, K.D.; Brehm, B.J.; Seeley, R.J.; Bean, J.; Wener, M.H.; Daniels, S.; D’Alessio, D.A. Diet-induced weight loss is associated with decreases in plasma serum amyloid a and C-reactive protein independent of dietary macronutrient composition in obese subjects. J. Clin. Endocrinol. Metab. 2005, 90, 2244–2249. [Google Scholar] [CrossRef]
- SorićHosman, I.; Kos, I.; Lamot, L. Serum Amyloid A in Inflammatory Rheumatic Diseases: A Compendious Review of a Renowned Biomarker. Front. Immunol. 2021, 11, 631299. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172. [Google Scholar] [CrossRef]
- Löe, H. The Gingival Index, the Plaque Index, and the Retention Index Systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef]
- Holmlund, A.; Hedin, M.; Pussinen, P.J.; Lerner, U.H.; Lind, L. Porphyromonasgingivalis (Pg) a possible link between impaired oral health and acute myocardial infarction. Int. J. Cardiol. 2011, 148, 148–153. [Google Scholar] [CrossRef]
- Puhl, R.M.; Heuer, C.A. Obesity stigma: Important considerations for public health. Am. J. Public Health 2010, 100, 1019–1028. [Google Scholar] [CrossRef]
- Martinez-Herrera, M.; Silvestre-Rangil, J.; Silvestre, F.J. Association between obesity and periodontal disease. A systematic review of epidemiological studies and controlled clinical trials. Med. Oral Patol. Oral. Cir. Bucal. 2017, 22, e708–e715. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Kumari, M.; Kalra, N.; Priyanka, N. Correlation of MCP-4 and high-sensitivity C-reactive protein as a marker of inflammation in obesity and chronic periodontitis. Cytokine 2013, 61, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Gulati, N.N.; Masamatti, S.S.; Chopra, P. Association between obesity and its determinants with chronic periodontitis: A cross-sectional study. J. Indian Soc. Periodontol. 2020, 24, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Akram, Z.; Abduljabbar, T.; Abu Hassan, M.I.; Javed, F.; Vohra, F. Cytokine Profile in Chronic Periodontitis Patients with and without Obesity: A Systematic Review and Meta-Analysis. Dis. Markers 2016, 2016, 4801418. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, G.A.; Strachan, A.F.; van der Westhuyzen, D.R.; Hoppe, H.C.; Jeenah, M.S.; de Beer, F.C. Serum amyloid A-containing human high-density lipoprotein 3. Density, size, and apolipoprotein composition. J. Biol. Chem. 1986, 261, 9644–9651. [Google Scholar] [CrossRef]
- Temelli, B.; Yetkin Ay, Z.; Savaş, H.B.; Aksoy, F.; KumbulDoğuç, D.; Uskun, E.; Varol, E. Circulation levels of acute phase proteins pentraxin 3 and serum amyloid A in atherosclerosis have correlations with periodontal inflamed surface area. J. Appl. Oral Sci. 2018, 26, e20170322. [Google Scholar] [CrossRef]
- Zhao, Y.; He, X.; Shi, X.; Huang, C.; Liu, J.; Zhou, S.; Heng, C.K. Association between serum amyloid A and obesity: A meta-analysis and systematic review. Inflamm. Res. 2010, 59, 323–334. [Google Scholar] [CrossRef]
- Türer, Ç.C.; Ballı, U.; Güven, B. Fetuin-A, serum amyloid A and tumor necrosis factor-alpha levels in periodontal health and disease. Oral Dis. 2017, 23, 379–386. [Google Scholar] [CrossRef]
- Yang, R.Z.; Blumenthal, J.B.; Glynn, N.M.; Lee, M.J.; Goldberg, A.P.; Gong, D.W.; Ryan, A.S. Decrease of circulating SAA is correlated with reduction of abdominal SAA secretion during weight loss. Obesity 2014, 22, 1085–1090. [Google Scholar] [CrossRef]
- Marzi, C.; Huth, C.; Herder, C.; Baumert, J.; Thorand, B.; Rathmann, W.; Meisinger, C.; Wichmann, H.E.; Roden, R.M.; Peters, A.; et al. Acute-phase serum amyloid A protein and its implication in the development of type 2 diabetes in the KORA S4/F4 study. Diabetes Care 2013, 36, 1321–1326. [Google Scholar] [CrossRef]
- Gomez-Ambrosi, J.; Salvador, J.; Rotellar, F.; Silva, C.; Catalan, V.; Rodriguez, A.; Gil, M.J.; RNutr, G.F. Increased serum amyloid A concentrations in morbid obesity decrease after gastric bypass. Obes. Surg. 2006, 16, 262–269. [Google Scholar] [CrossRef]
- Ardila, C.M.; Guzmán, I.C. Comparison of serum amyloid A protein and C-reactive protein levels as inflammatory markers in periodontitis. J. Periodontal. Implant. Sci. 2015, 45, 14–22. [Google Scholar] [CrossRef]
- Hirai, K.; Furusho, H.; Kawashima, N.; Xu, S.; de Beer, M.C.; Battaglino, R.; Van Dyke, T.; Stashenko, P.; Sasaki, H. Serum Amyloid A Contributes to Chronic Apical Periodontitis via TLR2 and TLR4. J. Dent. Res. 2019, 98, 117–125. [Google Scholar] [CrossRef]
- Song, L.T.; Lai, W.; Li, J.S.; Mu, Y.Z.; Li, C.Y.; Jiang, S.Y. The interaction between serum amyloid A and Toll-like receptor 2 pathway regulates inflammatory cytokine secretion in human gingival fibroblasts. J. Periodontol. 2020, 91, 129–137. [Google Scholar] [CrossRef]
- Dervisoglu, P.; Elmas, B. Pentraxin 3 as a Marker for Cardiovascular Disease Risk in Overweight and Obese Children. Acta Cardiol. Sin. 2021, 37, 177–183. [Google Scholar] [CrossRef]
- Lehmann, A.P.; Nijakowski, K.; Swora-Cwynar, E.; Łuczak, J.; Czepulis, N.; Surdacka, A. Characteristics of salivary inflammation in obesity. Pol. Arch. Intern. Med. 2020, 130, 297–303. [Google Scholar] [CrossRef]
- Mohan, R.; Varghese, J.; Bhat, V.; Chianeh, Y.R. The effect of nonsurgical periodontal therapy on pentraxin 3 levels in smokersand nonsmokers withchronic periodontitis. Gen. Dent. 2019, 67, e1–e6. [Google Scholar]
- Peeran, S.W.; Elhassan, A.; Dawood, T.; Ramalingam, K.; Peeran, S.A.; Ahmed, F.; Adawi, A.-A.A. Role of Pentraxin-3 in Periodontal Inflammation—A Comprehensive Review. J. Pharm. Res. Int. 2021, 33, 209–219. [Google Scholar] [CrossRef]
- Fujita, Y.; Ito, H.; Sekino, S.; Numabe, Y. Correlations between pentraxin 3 or cytokine levels in gingival crevicular fluid and clinical parameters of chronic periodontitis. Odontology 2012, 100, 215–221. [Google Scholar] [CrossRef]
- Ogawa, T.; Kawano, Y.; Imamura, T.; Kawakita, K.; Sagara, M.; Matsuo, T.; Kakitsubata, Y.; Ishikawa, T.; Kitamura, K.; Hatakeyama, K.; et al. Reciprocal contribution of pentraxin 3 and C-reactive protein to obesity and metabolic syndrome. Obesity 2010, 18, 1871–1874. [Google Scholar] [CrossRef]
- Karakas, M.F.; Buyukkaya, E.; Kurt, M.; Motor, S.; Akcay, A.B.; Karakas, E.; Buyukkaya, Ş.; Sen, N. Serum pentraxin-3 levels are associated with the severity of metabolic syndrome. Med. Princ. Pract. 2013, 22, 274–279. [Google Scholar] [CrossRef]
- Witasp, A.; Carrero, J.J.; Michaëlsson, K.; Ahlström, H.; Kullberg, J.; Adamsson, V.; Risérus, U.; Larsson, A.; Helmersson-Karlqvist, J.; Lind, L.; et al. Inflammatory biomarker pentraxin 3 (PTX3) in relation to obesity, body fat depots, and weight loss. Obesity 2014, 22, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
| Characteristics Mean (±SD) Median (IQR) Range | C | P | O | P + O | p-Value |
|---|---|---|---|---|---|
| PPD | 0 | 3.43 (±0.94) 3.4 (2.75–3.95) 2.1–5.8 | 0 | 4.39 (±1.05) 3.9 (3.6–5.3) 2.9–6.4 | P vs. P + O: 0.0156 * |
| GI | 0 | 1.83 (±0.79) 1–3 | 0.94 (±0.68) 1 (0.25–1) 0–2 | 2.12 (±0.69) 2 (2–3) 1–3 | P vs. O: 0.0025 ** P vs. P + O: 0.2957 O vs. P + O: <0.0001 **** |
| Characteristics Mean (±SD) Median (IQR) Range | C | P | O | P + O | p-Value |
|---|---|---|---|---|---|
| SAA | 1.87(±1.29) 2.75 (0.16–2.83) 0.17–3.0 | 4.98 (±0.99) 4.8 (4.11–5.7) 3.72–7 | 3.31 (±0.19) 3.25 (3.17–3.47) 3.06–3.69 | 15.74 (±13.16) 9.31 (7.78–16.54) 7.64–42.56 | C vs. P: <0.0001 **** C vs. O: <0.0001 **** C vs. P + O: <0.0001 **** P vs. P + O <0.0001 **** P vs. O <0.0001 **** O vs. P + O <0.0001 **** |
| Characteristics Mean (±SD) Median (IQR) Range | C | P | O | P + O | p-Value |
|---|---|---|---|---|---|
| PTX3 | 189.8 (±56.36) 220.3 (124.3–237.1) 101.4–239.5 | 383.4 (±76.89) 416.7 (280.5–446.7) 275.1–469.1 | 257.7 (±9.59) 256.2 (248.1–268.3) 244.3–275.1 | 505 (±24.43) 499 (484–524) 472.5–558.9 | C vs. P: <0.0001 **** C vs. O: <0.0001 **** C vs. P + O: <0.0001 **** P vs. P + O <0.0001 **** P vs. O <0.0001 **** O vs. P + O <0.0001 **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, D.M.; Gheorghe, D.N.; Turcu-Stiolica, A.; Soancă, A.; Roman, A.; Ionele, C.M.; Ciucă, E.M.; Boldeanu, V.M.; Boldeanu, L.; Pitru, A.; et al. Evaluation of Pentraxin 3 and Serum Amyloid A in the Gingival Crevicular Fluid of Patients with Periodontal Disease and Obesity. J. Clin. Med. 2023, 12, 3523. https://doi.org/10.3390/jcm12103523
Popescu DM, Gheorghe DN, Turcu-Stiolica A, Soancă A, Roman A, Ionele CM, Ciucă EM, Boldeanu VM, Boldeanu L, Pitru A, et al. Evaluation of Pentraxin 3 and Serum Amyloid A in the Gingival Crevicular Fluid of Patients with Periodontal Disease and Obesity. Journal of Clinical Medicine. 2023; 12(10):3523. https://doi.org/10.3390/jcm12103523
Chicago/Turabian StylePopescu, Dora Maria, Dorin Nicolae Gheorghe, Adina Turcu-Stiolica, Andrada Soancă, Alexandra Roman, Claudiu Marinel Ionele, Eduard Mihai Ciucă, Virgil Mihail Boldeanu, Lidia Boldeanu, Allma Pitru, and et al. 2023. "Evaluation of Pentraxin 3 and Serum Amyloid A in the Gingival Crevicular Fluid of Patients with Periodontal Disease and Obesity" Journal of Clinical Medicine 12, no. 10: 3523. https://doi.org/10.3390/jcm12103523
APA StylePopescu, D. M., Gheorghe, D. N., Turcu-Stiolica, A., Soancă, A., Roman, A., Ionele, C. M., Ciucă, E. M., Boldeanu, V. M., Boldeanu, L., Pitru, A., & Șurlin, P. (2023). Evaluation of Pentraxin 3 and Serum Amyloid A in the Gingival Crevicular Fluid of Patients with Periodontal Disease and Obesity. Journal of Clinical Medicine, 12(10), 3523. https://doi.org/10.3390/jcm12103523











