Cardiac Magnetic Resonance in HCM Phenocopies: From Diagnosis to Risk Stratification and Therapeutic Management
Abstract
1. Introduction
2. HCM Phenocopies (Similarities and Differences)
- Glycogen Storage Disorders (GSD): This group includes Danon disease, Pompe disease (GSD type 2), Forbes/Cori disease (GSD type 3), and PRKAG2 cardiomyopathy;
- Anderson–Fabry Disease: This is a lysosomal storage disorder, due to a mutation in the α-galactosidase A gene, and it is an X-linked recessive disease;
- Cardiac amyloidosis: This is due to extracellular deposition of amyloid material, and it can cause infiltrative or restrictive cardiomyopathy. Concentric LVH is observed;
- Mitochondrial cytopathies: This heterogeneous group is caused by mutations of the maternally inherited mitochondrial genome and leads to dysfunctional energy production and multisystemic involvement, especially involving central nervous system, heart, and skeletal system;
- Hypertensive heart disease;
- Athlete’s heart.
3. Cardiovascular Magnetic Resonance
3.1. Morpho-Functional Features in Hypertrophic Phenotypes
3.2. Role of Edema
3.3. Role of Late Gadolinium Enhancement
3.4. Role of Parametric Mapping
3.5. Contractility Assessment
4. Prognostic Role of CMR in Hypertrophic Phenocopies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur. Heart J. 2007, 29, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Arbustini, E.; Narula, N.; Tavazzi, L.; Serio, A.; Grasso, M.; Favalli, V.; Bellazzi, R.; Tajik, J.A.; Bonow, R.O.; Fuster, V.; et al. The MOGE(S) Classification of Cardiomyopathy for Clinicians. J. Am. Coll. Cardiol. 2014, 64, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; De Michieli, L.; Porcari, A.; Licchelli, L.; Sinigiani, G.; Tini, G.; Zampieri, M.; Sessarego, E.; Argirò, A.; Fumagalli, C.; et al. Low QRS Voltages in Cardiac Amyloidosis. JACC CardioOncol. 2022, 4, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Licordari, R.; Minutoli, F.; Cappelli, F.; Micari, A.; Colarusso, L.; Di Paola, F.A.F.; Campisi, M.; Recupero, A.; Mazzeo, A.; Di Bella, G. Mid-basal left ventricular longitudinal dysfunction as a prognostic marker in mutated transthyretin-related cardiac amyloidosis. Vessel. Plus 2022, 6, 12. [Google Scholar] [CrossRef]
- Ciarambino, T.; Menna, G.; Sansone, G.; Giordano, M. Cardiomyopathies: An Overview. Int. J. Mol. Sci. 2021, 22, 7722. [Google Scholar] [CrossRef]
- McKenna, W.J.; Judge, D.P. Epidemiology of the inherited cardiomyopathies. Nat. Rev. Cardiol. 2021, 18, 22–36. [Google Scholar] [CrossRef]
- Limongelli, G.; Masarone, D.; Verrengia, M.; Gravino, R.; Salerno, G.; Castelletti, S.; Rubino, M.; Marrazzo, T.; Pisani, A.; Cecchi, F.; et al. Diagnostic Clues for the Diagnosis of Nonsarcomeric Hypertrophic Cardiomyopathy (Pheno-copies): Amyloidosis, Fabry Disease, and Mitochondrial Disease. J. Cardiovasc. Echogr. 2018, 28, 120–123. [Google Scholar] [CrossRef]
- Brito, D.; Miltenberger-Miltenyi, G.; Pereira, S.V.; Silva, D.; Diogo, A.N.; Madeira, H. Sarcomeric hypertrophic cardiomyopathy: Genetic profile in a Portuguese population. Rev. Port. Cardiol. 2012, 31, 577–587. [Google Scholar] [CrossRef]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [CrossRef]
- Tucholski, T.; Cai, W.; Gregorich, Z.R.; Bayne, E.F.; Mitchell, S.D.; McIlwain, S.J.; de Lange, W.J.; Wrobbel, M.; Karp, H.; Hite, Z.; et al. Distinct hypertrophic cardiomyopathy genotypes result in convergent sarcomeric proteoform profiles revealed by top-down proteomics. Proc. Natl. Acad. Sci. USA 2020, 117, 24691–24700. [Google Scholar] [CrossRef]
- Rapezzi, C.; Arbustini, E.; Caforio, A.L.P.; Charron, P.; Blanes, J.G.; Heliö, T.; Linhart, A.; Mogensen, J.; Pinto, Y.; Ristic, A.; et al. Diagnostic work-up in cardiomyopathies: Bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020, 142, e558–e631. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Fleming, E.J.; Garratt, C.J. Mimics of Hypertrophic Cardiomyopathy—Diagnostic Clues to Aid Early Identification of Phenocopies. Arrhythmia Electrophysiol. Rev. 2013, 2, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Baggiano, A.; Del Torto, A.; Guglielmo, M.; Muscogiuri, G.; Fusini, L.; Babbaro, M.; Collevecchio, A.; Mollace, R.; Scafuri, S.; Mushtaq, S.; et al. Role of CMR Mapping Techniques in Cardiac Hypertrophic Phenotype. Diagnostics 2020, 10, 770. [Google Scholar] [CrossRef]
- Bluemke, D.A.; Kronmal, R.A.; Lima, J.A.; Liu, K.; Olson, J.; Burke, G.L.; Folsom, A.R. The Relationship of Left Ventricular Mass and Geometry to Incident Cardiovascular Events: The MESA (Multi-Ethnic Study of Atherosclerosis) Study. J. Am. Coll. Cardiol. 2008, 52, 2148–2155. [Google Scholar] [CrossRef]
- Kawel, N.; Turkbey, E.B.; Carr, J.J.; Eng, J.; Gomes, A.S.; Hundley, W.G.; Johnson, C.; Masri, S.C.; Prince, M.R.; van der Geest, R.J.; et al. Normal Left Ventricular Myocardial Thickness for Middle-Aged and Older Subjects With Steady-State Free Precession Cardiac Magnetic Resonance: The Multi-Ethnic Study of Atherosclerosis. Circ. Cardiovasc. Imaging 2012, 5, 500–508. [Google Scholar] [CrossRef]
- Aquaro, G.D.; Camastra, G.; Monti, L.; Lombardi, M.; Pepe, A.; Castelletti, S.; Maestrini, V.; Todiere, G.; Masci, P.; di Giovine, G.; et al. Reference values of cardiac volumes, dimensions, and new functional parameters by MR: A multicenter, multivendor study: Reference Range of Normality for CMR. J. Magn. Reson. Imaging 2017, 45, 1055–1067. [Google Scholar] [CrossRef]
- Maron, M.S.; Maron, B.J.; Harrigan, C.; Buros, J.; Gibson, C.M.; Olivotto, I.; Biller, L.; Lesser, J.R.; Udelson, J.E.; Manning, W.J.; et al. Hypertrophic Cardiomyopathy Phenotype Revisited After 50 Years With Cardiovascular Magnetic Resonance. J. Am. Coll. Cardiol. 2009, 54, 220–228. [Google Scholar] [CrossRef]
- Moon, J.C.C.; Fisher, N.G.; McKenna, W.J.; Pennell, D.J. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart 2004, 90, 645–649. [Google Scholar] [CrossRef]
- Maron, M.S.; Finley, J.J.; Bos, J.M.; Hauser, T.H.; Manning, W.J.; Haas, T.S.; Lesser, J.R.; Udelson, J.E.; Ackerman, M.J.; Maron, B.J. Prevalence, Clinical Significance, and Natural History of Left Ventricular Apical Aneurysms in Hypertrophic Cardiomyopathy. Circulation 2008, 118, 1541–1549. [Google Scholar] [CrossRef]
- Cui, L.; Tse, G.; Zhao, Z.; Bazoukis, G.; Letsas, K.P.; Korantzopoulos, P.; Roever, L.; Li, G.; Liu, T. Mid-ventricular obstructive hypertrophic cardiomyopathy with apical aneurysm: An important subtype of arrhythmogenic cardiomyopathy. Ann. Noninvasive Electrocardiol. 2019, 24, e12638. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Chun, E.J.; Kim, Y.-K.; Choi, S.I. First-pass stress perfusion MR Imaging findings of apical hypertrophic cardiomyopathy: With relation to LV wall thickness and late Gadolinium-enhancement. J. Cardiovasc. Magn. Reson. 2015, 17, Q66. [Google Scholar] [CrossRef]
- Wilson, P.; Marks, A.; Rastegar, H.; Manous, A.S.; Estes, N.A.M. Apical hypertrophic cardiomyopathy presenting with sustained monomorphic ventricular tachycardia and electrocardiographic changes simulating coronary artery disease and left ventricular aneurysm. Clin. Cardiol. 1990, 13, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Hauser, T.H.; Dubrow, E.; Horst, T.A.; Kissinger, K.V.; Udelson, J.E.; Manning, W.J. Right Ventricular Involvement in Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2007, 100, 1293–1298. [Google Scholar] [CrossRef]
- Maron, M.S.; Rowin, E.J.; Lin, D.; Appelbaum, E.; Chan, R.H.; Gibson, C.M.; Lesser, J.R.; Lindberg, J.; Haas, T.S.; Udelson, J.E.; et al. Prevalence and Clinical Profile of Myocardial Crypts in Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2012, 5, 441–447. [Google Scholar] [CrossRef]
- Captur, G.; Lopes, L.R.; Mohun, T.J.; Patel, V.; Li, C.; Bassett, P.; Finocchiaro, G.; Ferreira, V.M.; Esteban, M.T.; Muthurangu, V.; et al. Prediction of Sarcomere Mutations in Subclinical Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2014, 7, 863–871. [Google Scholar] [CrossRef]
- Maron, M.S.; Olivotto, I.; Harrigan, C.; Appelbaum, E.; Gibson, C.M.; Lesser, J.R.; Haas, T.S.; Udelson, J.E.; Manning, W.J.; Maron, B.J. Mitral Valve Abnormalities Identified by Cardiovascular Magnetic Resonance Represent a Primary Phenotypic Expression of Hypertrophic Cardiomyopathy. Circulation 2011, 124, 40–47. [Google Scholar] [CrossRef]
- Harrigan, C.J.; Appelbaum, E.; Maron, B.J.; Buros, J.L.; Gibson, C.M.; Lesser, J.R.; Udelson, J.E.; Manning, W.J.; Maron, M.S. Significance of Papillary Muscle Abnormalities Identified by Cardiovascular Magnetic Resonance in Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2008, 101, 668–673. [Google Scholar] [CrossRef]
- Bogaert, J.; Olivotto, I. MR Imaging in Hypertrophic Cardiomyopathy: From Magnet to Bedside. Radiology 2014, 273, 329–348. [Google Scholar] [CrossRef]
- Aquaro, G.D.; Pizzino, F.; Terrizzi, A.; Carerj, S.; Khandheria, B.K.; Di Bella, G. Diastolic dysfunction evaluated by cardiac magnetic resonance: The value of the combined assessment of atrial and ventricular function. Eur. Radiol. 2019, 29, 1555–1564. [Google Scholar] [CrossRef]
- Maceira, A.M.; Cosín-Sales, J.; Roughton, M.; Prasad, S.K.; Pennell, D.J. Reference left atrial dimensions and volumes by steady state free precession cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2010, 12, 65. [Google Scholar] [CrossRef]
- Rodrigues, J.C.L.; Rohan, S.; Dastidar, A.G.; Harries, I.; Lawton, C.B.; Ratcliffe, L.E.; Burchell, A.E.; Hart, E.C.; Hamilton, M.C.K.; Paton, J.F.R.; et al. Hypertensive heart disease versus hypertrophic cardiomyopathy: Multi-parametric cardiovascular magnetic resonance discriminators when end-diastolic wall thickness ≥ 15 mm. Eur. Radiol. 2017, 27, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Child, N.; Muhr, T.; Sammut, E.; Dabir, D.; Ucar, E.A.; Bueser, T.; Gill, J.; Carr-White, G.; Nagel, E.; Puntmann, V.O. Prevalence of myocardial crypts in a large retrospective cohort study by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2014, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- Licordari, R.; Minutoli, F.; Recupero, A.; Campisi, M.; Donato, R.; Mazzeo, A.; Dattilo, G.; Baldari, S.; Vita, G.; Zito, C.; et al. Early impairment of right ventricular morphology and function in transthyretin-related cardiac amyloidosis. J. Cardiovasc. Echogr. 2021, 31, 17–22. [Google Scholar] [CrossRef]
- Falk, R.H.; Quarta, C.C.; Dorbala, S. How to Image Cardiac Amyloidosis. Circ. Cardiovasc. Imaging 2014, 7, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Pozo, E.; Kanwar, A.; Deochand, R.; Castellano, J.M.; Naib, T.; Pazos-López, P.; Osman, K.; Cham, M.; Narula, J.; Fuster, V.; et al. Cardiac magnetic resonance evaluation of left ventricular remodelling distribution in cardiac amyloidosis. Heart 2014, 100, 1688–1695. [Google Scholar] [CrossRef]
- Di Bella, G.; Cappelli, F.; Licordari, R.; Piaggi, P.; Campisi, M.; Bellavia, D.; Minutoli, F.; Gentile, L.; Russo, M.; de Gregorio, C.; et al. Prevalence and diagnostic value of extra-left ventricle echocardiographic findings in transthyretin-related cardiac amyloidosis. Amyloid 2022, 29, 197–204. [Google Scholar] [CrossRef]
- Fanta, L.E.; Ewer, S.M.; Gimelli, G.; Reilly, N.M. Alcohol septal ablation for left ventricular outflow tract obstruction in cardiac amyloidosis: New indication for an established therapy. Catheter. Cardiovasc. Interv. 2022, 100, 910–914. [Google Scholar] [CrossRef]
- Sattar, Y.; Maya, T.R.; Zafrullah, F.; Patel, N.B.; Latchana, S. Diagnosis and Management of a Cardiac Amyloidosis Case Mimicking Hypertrophic Cardiomyopathy. Cureus 2018, 10, e3749. [Google Scholar] [CrossRef]
- Dinwoodey, D.L.; Skinner, M.; Maron, M.S.; Davidoff, R.; Ruberg, F.L. Light-Chain Amyloidosis With Echocardiographic Features of Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2008, 101, 674–676. [Google Scholar] [CrossRef]
- Frustaci, A.; Galea, N.; Verardo, R.; Francone, M.; Alfarano, M.; Russo, M.A.; Chimenti, C. Kappa-light Chain Amyloid Overlapping Hypertrophic Cardiomyopathy With Myocardial Noncompaction. Circ. Cardiovasc. Imaging 2020, 13, e010379. [Google Scholar] [CrossRef] [PubMed]
- Linhart, A.; Elliott, P.M. The heart in Anderson-Fabry disease and other lysosomal storage disorders. Heart 2007, 93, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Saccheri, M.C.; Cianciulli, T.F.; Licidio, W.C.; Lax, J.A.; Beck, M.A.; Morita, L.A.; Gagliardi, J.A. Comparison of left atrial size and function in hypertrophic cardiomyopathy and in Fabry disease with left ventricular hypertrophy. Echocardiography 2018, 35, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Imazio, M. Fabry disease: Definition, Incidence, Clinical presentations and Treatment—Focus on cardiac involvement. Pak. J. Med. Sci. 2022, 38, 8. [Google Scholar] [CrossRef]
- Zemánek, D.; Marek, J.; Dostálová, G.; Magage, S.; Roblová, L.; Kovárník, T.; Linhart, A. Usefulness of Alcohol Septal Ablation in the Left Ventricular Outflow Tract Obstruction in Fabry Disease Cardiomyopathy. Am. J. Cardiol. 2021, 150, 110–113. [Google Scholar] [CrossRef]
- Calcagnino, M.; O’Mahony, C.; Coats, C.; Cardona, M.; Garcia, A.; Janagarajan, K.; Mehta, A.; Hughes, D.; Murphy, E.; Lachmann, R.; et al. Exercise-Induced Left Ventricular Outflow Tract Obstruction in Symptomatic Patients With Anderson-Fabry Disease. J. Am. Coll. Cardiol. 2011, 58, 88–89. [Google Scholar] [CrossRef]
- Holmgren, D.; Wåhlander, H.; Eriksson, B.; Oldfors, A.; Holme, E.; Tulinius, M. Cardiomyopathy in children with mitochondrial disease Clinical course and cardiological findings. Eur. Heart J. 2003, 24, 280–288. [Google Scholar] [CrossRef]
- Florian, A.; Ludwig, A.; Stubbe-Dräger, B.; Boentert, M.; Young, P.; Waltenberger, J.; Rösch, S.; Sechtem, U.; Yilmaz, A. Characteristic cardiac phenotypes are detected by cardiovascular magnetic resonance in patients with different clinical phenotypes and genotypes of mitochondrial myopathy. J. Cardiovasc. Magn. Reson. 2015, 17, 40. [Google Scholar] [CrossRef]
- Abdel-Aty, H.; Zagrosek, A.; Schulz-Menger, J.; Taylor, A.J.; Messroghli, D.; Kumar, A.; Gross, M.; Dietz, R.; Friedrich, M.G. Delayed Enhancement and T2-Weighted Cardiovascular Magnetic Resonance Imaging Differentiate Acute From Chronic Myocardial Infarction. Circulation 2004, 109, 2411–2416. [Google Scholar] [CrossRef]
- Berry, C.; Kellman, P.; Mancini, C.; Chen, M.Y.; Bandettini, W.P.; Lowrey, T.; Hsu, L.-Y.; Aletras, A.H.; Arai, A.E. Magnetic Resonance Imaging Delineates the Ischemic Area at Risk and Myocardial Salvage in Patients With Acute Myocardial Infarction. Circ. Cardiovasc. Imaging 2010, 3, 527–535. [Google Scholar] [CrossRef]
- Abdel-Aty, H.; Cocker, M.; Strohm, O.; Filipchuk, N.; Friedrich, M.G. Abnormalities in T2-weighted cardiovascular magnetic resonance images of hypertrophic cardiomyopathy: Regional distribution and relation to late gadolinium enhancement and severity of hypertrophy. J. Magn. Reson. Imaging 2008, 28, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Amano, Y.; Aita, K.; Yamada, F.; Kitamura, M.; Kumita, S. Distribution and Clinical Significance of High Signal Intensity of the Myocardium on T2-Weighted Images in 2 Phenotypes of Hypertrophic Cardiomyopathy. J. Comput. Assist. Tomogr. 2015, 39, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Melacini, P.; Corbetti, F.; Calore, C.; Pescatore, V.; Smaniotto, G.; Pavei, A.; Bobbo, F.; Cacciavillani, L.; Iliceto, S. Cardiovascular magnetic resonance signs of ischemia in hypertrophic cardiomyopathy. Int. J. Cardiol. 2008, 128, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Hen, Y.; Iguchi, N.; Machida, H.; Takada, K.; Utanohara, Y.; Sumiyoshi, T. High signal intensity on T2-weighted cardiac magnetic resonance imaging correlates with the ventricular tachyarrhythmia in hypertrophic cardiomyopathy. Heart Vessel. 2013, 28, 742–749. [Google Scholar] [CrossRef]
- Todiere, G.; Pisciella, L.; Barison, A.; Del Franco, A.; Zachara, E.; Piaggi, P.; Re, F.; Pingitore, A.; Emdin, M.; Lombardi, M.; et al. Abnormal T2-STIR Magnetic Resonance in Hypertrophic Cardiomyopathy: A Marker of Advanced Disease and Electrical Myocardial Instability. PLoS ONE 2014, 9, e111366. [Google Scholar] [CrossRef]
- Mahrholdt, H.; Wagner, A.; Judd, R.M.; Sechtem, U.; Kim, R.J. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur. Heart J. 2005, 26, 1461–1474. [Google Scholar] [CrossRef]
- Moon, J.C.C.; Reed, E.; Sheppard, M.N.; Elkington, A.G.; Ho, S.Y.; Burke, M.; Petrou, M.; Pennell, D.J. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2004, 43, 2260–2264. [Google Scholar] [CrossRef]
- Yang, K.; Song, Y.-Y.; Chen, X.-Y.; Wang, J.-X.; Li, L.; Yin, G.; Zheng, Y.-C.; Wei, M.-D.; Lu, M.-J.; Zhao, S.-H. Apical hypertrophic cardiomyopathy with left ventricular apical aneurysm: Prevalence, cardiac magnetic resonance characteristics, and prognosis. Eur. Heart J.—Cardiovasc. Imaging 2020, 21, 1341–1350. [Google Scholar] [CrossRef]
- Habib, M.; Adler, A.; Fardfini, K.; Hoss, S.; Hanneman, K.; Rowin, E.J.; Maron, M.S.; Maron, B.J.; Rakowski, H.; Chan, R.H. Progression of Myocardial Fibrosis in Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2021, 14, 947–958. [Google Scholar] [CrossRef]
- Maceira, A.M.; Joshi, J.; Prasad, S.K.; Moon, J.C.; Perugini, E.; Harding, I.; Sheppard, M.N.; Poole-Wilson, P.A.; Hawkins, P.N.; Pennell, D.J. Cardiovascular Magnetic Resonance in Cardiac Amyloidosis. Circulation 2005, 111, 186–193. [Google Scholar] [CrossRef]
- Moon, J.C.; Sachdev, B.; Elkington, A.G.; McKenna, W.J.; Mehta, A.; Pennell, D.J.; Leed, P.J.; Elliott, P.M. Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease Evidence for a disease specific abnormality of the myocardial interstitium. Eur. Heart J. 2003, 24, 2151–2155. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.M.; Piechnik, S.K. CMR Parametric Mapping as a Tool for Myocardial Tissue Characterization. Korean Circ. J. 2020, 50, 658–676. [Google Scholar] [CrossRef]
- Karamitsos, T.D.; Arvanitaki, A.; Karvounis, H.; Neubauer, S.; Ferreira, V.M. Myocardial Tissue Characterization and Fibrosis by Imaging. JACC Cardiovasc. Imaging 2020, 13, 1221–1234. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Holloway, C.J.; Piechnik, S.K.; Karamitsos, T.; Neubauer, S. Is it really fat? Ask a T1-map. Eur. Heart J.—Cardiovasc. Imaging 2013, 14, 1060. [Google Scholar] [CrossRef] [PubMed]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Nordin, S.; Kozor, R.; Vijapurapu, R.; Augusto, J.; Knott, K.D.; Captur, G.; Treibel, T.; Ramaswami, U.; Tchan, M.; Geberhiwot, T.; et al. Myocardial Storage, Inflammation, and Cardiac Phenotype in Fabry Disease After One Year of Enzyme Replacement Therapy. Circ. Cardiovasc. Imaging 2019, 12, e009430. [Google Scholar] [CrossRef]
- Dass, S.; Suttie, J.J.; Piechnik, S.K.; Ferreira, V.M.; Holloway, C.J.; Banerjee, R.; Mahmod, M.; Cochlin, L.; Karamitsos, T.D.; Robson, M.D.; et al. Myocardial Tissue Characterization Using Magnetic Resonance Noncontrast T1 Mapping in Hypertrophic and Dilated Cardiomyopathy. Circ. Cardiovasc. Imaging 2012, 5, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Nakamori, S.; Bellm, S.; Jang, J.; Basha, T.; Maron, M.; Manning, W.J.; Nezafat, R. Myocardial Native T1 Time in Patients With Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2016, 118, 1057–1062. [Google Scholar] [CrossRef]
- Huang, L.; Ran, L.; Zhao, P.; Tang, D.; Han, R.; Ai, T.; Xia, L.; Tao, Q. MRI native T1 and T2 mapping of myocardial segments in hypertrophic cardiomyopathy: Tissue remodeling manifested prior to structure changes. Br. J. Radiol. 2019, 92, 20190634. [Google Scholar] [CrossRef]
- Amano, Y.; Yanagisawa, F.; Tachi, M.; Hashimoto, H.; Imai, S.; Kumita, S. Myocardial T2 Mapping in Patients With Hypertrophic Cardiomyopathy. J. Comput. Assist. Tomogr. 2017, 41, 344–348. [Google Scholar] [CrossRef]
- Wang, J.; Yang, F.; Liu, W.; Sun, J.; Han, Y.; Li, D.; Gkoutos, G.V.; Zhu, Y.; Chen, Y. Radiomic Analysis of Native T 1 Mapping Images Discriminates Between MYH7 and MYBPC3 -Related Hypertrophic Cardiomyopathy. J. Magn. Reson. Imaging 2020, 52, 1714–1721. [Google Scholar] [CrossRef]
- Arcari, L.; Hinojar, R.; Engel, J.; Freiwald, T.; Platschek, S.; Zainal, H.; Zhou, H.; Vasquez, M.; Keller, T.; Rolf, A.; et al. Native T1 and T2 provide distinctive signatures in hypertrophic cardiac conditions—Comparison of uremic, hypertensive and hypertrophic cardiomyopathy. Int. J. Cardiol. 2020, 306, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Sado, D.M.; Flett, A.S.; Banypersad, S.M.; White, S.K.; Maestrini, V.; Quarta, G.; Lachmann, R.; Murphy, E.; Mehta, A.; Hughes, D.; et al. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart 2012, 98, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Swoboda, P.P.; McDiarmid, A.K.; Erhayiem, B.; Broadbent, D.A.; Dobson, L.E.; Garg, P.; Ferguson, C.; Page, S.P.; Greenwood, J.P.; Plein, S. Assessing Myocardial Extracellular Volume by T1 Mapping to Distinguish Hypertrophic Cardiomyopathy From Athlete’s Heart. J. Am. Coll. Cardiol. 2016, 67, 2189–2190. [Google Scholar] [CrossRef]
- Karamitsos, T.D.; Piechnik, S.K.; Banypersad, S.M.; Fontana, M.; Ntusi, N.B.; Ferreira, V.M.; Whelan, C.J.; Myerson, S.G.; Robson, M.D.; Hawkins, P.N.; et al. Noncontrast T1 Mapping for the Diagnosis of Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2013, 6, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.; Banypersad, S.M.; Treibel, T.A.; Maestrini, V.; Sado, D.M.; White, S.K.; Pica, S.; Castelletti, S.; Piechnik, S.K.; Robson, M.D.; et al. Native T1 Mapping in Transthyretin Amyloidosis. JACC Cardiovasc. Imaging 2014, 7, 157–165. [Google Scholar] [CrossRef]
- Baggiano, A.; Boldrini, M.; Martinez-Naharro, A.; Kotecha, T.; Petrie, A.; Rezk, T.; Gritti, M.; Quarta, C.; Knight, D.S.; Wechalekar, A.D.; et al. Noncontrast Magnetic Resonance for the Diagnosis of Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2020, 13, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, T.; Martinez-Naharro, A.; Treibel, T.A.; Francis, R.; Nordin, S.; Abdel-Gadir, A.; Knight, D.S.; Zumbo, G.; Rosmini, S.; Maestrini, V.; et al. Myocardial Edema and Prognosis in Amyloidosis. J. Am. Coll. Cardiol. 2018, 71, 2919–2931. [Google Scholar] [CrossRef]
- Giblin, G.T.; Cuddy, S.A.M.; González-López, E.; Sewell, A.; Murphy, A.; Dorbala, S.; Falk, R.H. Effect of tafamidis on global longitudinal strain and myocardial work in transthyretin cardiac amyloidosis. Eur. Heart J.—Cardiovasc. Imaging 2022, 23, 1029–1039. [Google Scholar] [CrossRef]
- Fontana, M.; Martinez-Naharro, A.; Chacko, L.; Rowczenio, D.; Gilbertson, J.A.; Whelan, C.J.; Strehina, S.; Lane, T.; Moon, J.; Hutt, D.F.; et al. Reduction in CMR Derived Extracellular Volume With Patisiran Indicates Cardiac Amyloid Regression. JACC Cardiovasc. Imaging 2021, 14, 189–199. [Google Scholar] [CrossRef]
- Dasgupta, N.R.; Rissing, S.M.; Smith, J.; Jung, J.; Benson, M.D. Inotersen therapy of transthyretin amyloid cardiomyopathy. Amyloid 2020, 27, 52–58. [Google Scholar] [CrossRef]
- Sado, D.M.; White, S.K.; Piechnik, S.K.; Banypersad, S.M.; Treibel, T.; Captur, G.; Fontana, M.; Maestrini, V.; Flett, A.S.; Robson, M.D.; et al. Identification and Assessment of Anderson-Fabry Disease by Cardiovascular Magnetic Resonance Noncontrast Myocardial T1 Mapping. Circ. Cardiovasc. Imaging 2013, 6, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.B.; Chow, K.; Khan, A.; Chan, A.; Shanks, M.; Paterson, I.; Oudit, G.Y. T 1 Mapping With Cardiovascular MRI Is Highly Sensitive for Fabry Disease Independent of Hypertrophy and Sex. Circ. Cardiovasc. Imaging 2013, 6, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Pica, S.; Sado, D.M.; Maestrini, V.; Fontana, M.; White, S.K.; Treibel, T.; Captur, G.; Anderson, S.; Piechnik, S.K.; Robson, M.D.; et al. Reproducibility of native myocardial T1 mapping in the assessment of Fabry disease and its role in early detection of cardiac involvement by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2014, 16, 99. [Google Scholar] [CrossRef]
- Messalli, G.; Imbriaco, M.; Avitabile, G.; Russo, R.; Iodice, D.; Spinelli, L.; Dellegrottaglie, S.; Cademartiri, F.; Salvatore, M.; Pisani, A. Role of cardiac MRI in evaluating patients with Anderson-Fabry disease: Assessing cardiac effects of long-term enzyme replacement therapy. Radiol. Medica 2012, 117, 19–28. [Google Scholar] [CrossRef]
- Aletras, A.H.; Tilak, G.S.; Hsu, L.-Y.; Arai, A.E. Heterogeneity of Intramural Function in Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2011, 4, 425–434. [Google Scholar] [CrossRef]
- Nucifora, G.; Muser, D.; Gianfagna, P.; Morocutti, G.; Proclemer, A. Systolic and diastolic myocardial mechanics in hypertrophic cardiomyopathy and their link to the extent of hypertrophy, replacement fibrosis and interstitial fibrosis. Int. J. Cardiovasc. Imaging 2015, 31, 1603–1610. [Google Scholar] [CrossRef]
- Williams, L.K.; Forero, J.F.; Popovic, Z.B.; Phelan, D.; Delgado, D.; Rakowski, H.; Wintersperger, B.J.; Thavendiranathan, P. Patterns of CMR measured longitudinal strain and its association with late gadolinium enhancement in patients with cardiac amyloidosis and its mimics. J. Cardiovasc. Magn. Reson. 2017, 19, 61. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, C.; Tian, J.; Saiedi, M.; Ma, C.; Li, N.; Fang, F.; Ma, X.; Selvanayagam, J. Quantification of myocardial deformation in patients with Fabry disease by cardiovascular magnetic resonance feature tracking imaging. Cardiovasc. Diagn. Ther. 2021, 11, 91–101. [Google Scholar] [CrossRef]
- Maron, M.S.; Lesser, J.R.; Maron, B.J. Management Implications of Massive Left Ventricular Hypertrophy in Hypertrophic Cardiomyopathy Significantly Underestimated by Echocardiography but Identified by Cardiovascular Magnetic Resonance. Am. J. Cardiol. 2010, 105, 1842–1843. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Maron, M.S.; Autore, C.; Lesser, J.R.; Rega, L.; Casolo, G.; De Santis, M.; Quarta, G.; Nistri, S.; Cecchi, F.; et al. Assessment and Significance of Left Ventricular Mass by Cardiovascular Magnetic Resonance in Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2008, 52, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Spirito, P.; Autore, C.; Formisano, F.; Assenza, G.E.; Biagini, E.; Haas, T.S.; Bongioanni, S.; Semsarian, C.; Devoto, E.; Musumeci, B.; et al. Risk of Sudden Death and Outcome in Patients With Hypertrophic Cardiomyopathy With Benign Presentation and Without Risk Factors. Am. J. Cardiol. 2014, 113, 1550–1555. [Google Scholar] [CrossRef]
- Kramer, C.M.; DiMarco, J.P.; Kolm, P.; Ho, C.Y.; Desai, M.Y.; Kwong, R.Y.; Dolman, S.F.; Desvigne-Nickens, P.; Geller, N.; Kim, D.-Y.; et al. Predictors of Major Atrial Fibrillation Endpoints in the National Heart, Lung, and Blood Institute HCMR. JACC Clin. Electrophysiol. 2021, 7, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Appelbaum, E.; Harrigan, C.J.; Buros, J.; Gibson, C.M.; Hanna, C.; Lesser, J.R.; Udelson, J.E.; Manning, W.J.; Maron, B.J. Clinical Profile and Significance of Delayed Enhancement in Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2008, 1, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Bruder, O.; Wagner, A.; Jensen, C.J.; Schneider, S.; Ong, P.; Kispert, E.-M.; Nassenstein, K.; Schlosser, T.; Sabin, G.V.; Sechtem, U.; et al. Myocardial Scar Visualized by Cardiovascular Magnetic Resonance Imaging Predicts Major Adverse Events in Patients With Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic Value of Quantitative Contrast-Enhanced Cardiovascular Magnetic Resonance for the Evaluation of Sudden Death Risk in Patients With Hypertrophic Cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef]
- Todiere, G.; Nugara, C.; Gentile, G.; Negri, F.; Bianco, F.; Falletta, C.; Novo, G.; Di Bella, G.; De Caterina, R.; Zachara, E.; et al. Prognostic Role of Late Gadolinium Enhancement in Patients With Hypertrophic Cardiomyopathy and Low-to-Intermediate Sudden Cardiac Death Risk Score. Am. J. Cardiol. 2019, 124, 1286–1292. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Avanesov, M.; Münch, J.; Weinrich, J.; Well, L.; Säring, D.; Stehning, C.; Tahir, E.; Bohnen, S.; Radunski, U.K.; Muellerleile, K.; et al. Prediction of the estimated 5-year risk of sudden cardiac death and syncope or non-sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy using late gadolinium enhancement and extracellular volume CMR. Eur. Radiol. 2017, 27, 5136–5145. [Google Scholar] [CrossRef]
- Banypersad, S.M.; Fontana, M.; Maestrini, V.; Sado, D.M.; Captur, G.; Petrie, A.; Piechnik, S.K.; Whelan, C.J.; Herrey, A.S.; Gillmore, J.D.; et al. T1 mapping and survival in systemic light-chain amyloidosis. Eur. Heart J. 2015, 36, 244–251. [Google Scholar] [CrossRef]
- Martinez-Naharro, A.; Kotecha, T.; Norrington, K.; Boldrini, M.; Rezk, T.; Quarta, C.; Treibel, T.A.; Whelan, C.J.; Knight, D.S.; Kellman, P.; et al. Native T1 and Extracellular Volume in Transthyretin Amyloidosis. JACC Cardiovasc. Imaging 2019, 12, 810–819. [Google Scholar] [CrossRef]
- Spirito, P.; Binaco, I.; Poggio, D.; Zyrianov, A.; Grillo, M.; Pezzoli, L.; Rossi, J.; Malanin, D.; Vaccari, G.; Dorobantu, L.; et al. Role of Preoperative Cardiovascular Magnetic Resonance in Planning Ventricular Septal Myectomy in Patients With Obstructive Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2019, 123, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Dhillon, A.; Popovic, Z.B.; Smedira, N.G.; Rizzo, J.; Thamilarasan, M.; Agler, D.; Lytle, B.W.; Lever, H.M.; Desai, M.Y. Left Ventricular Outflow Tract Obstruction in Hypertrophic Cardiomyopathy Patients Without Severe Septal Hypertrophy: Implications of Mitral Valve and Papillary Muscle Abnormalities Assessed Using Cardiac Magnetic Resonance and Echocardi-ography. Circ. Cardiovasc. Imaging 2015, 8, e003132. [Google Scholar] [CrossRef] [PubMed]
- Amano, Y.; Takayama, M.; Kumita, S.; Kumazaki, T. MR Imaging Evaluation of Regional, Remote, and Global Effects of Percutaneous Transluminal Septal Myocardial Ablation in Hypertrophic Obstructive Cardiomyopathy. J. Comput. Assist. Tomogr. 2007, 31, 600–604. [Google Scholar] [CrossRef] [PubMed]
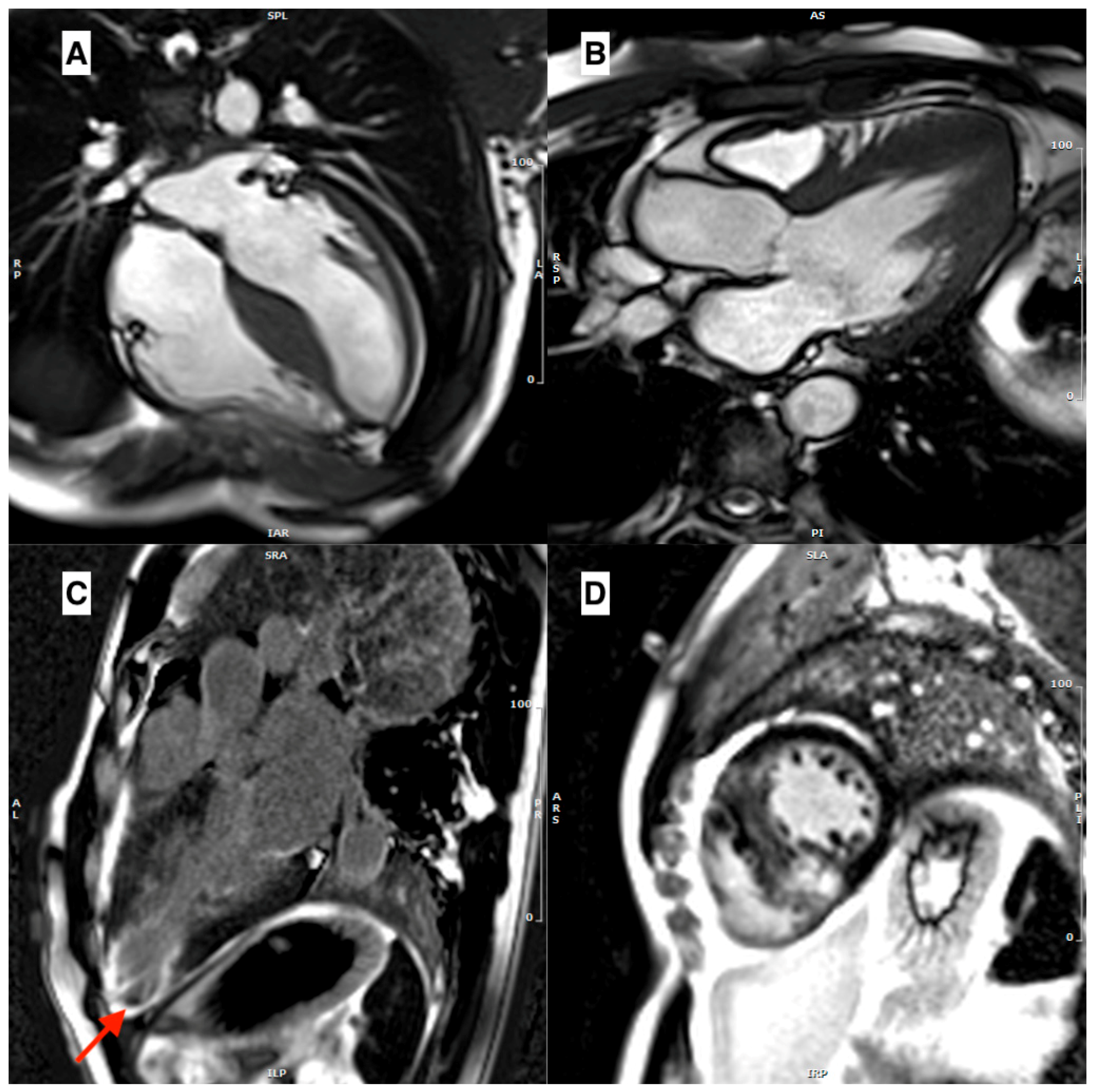
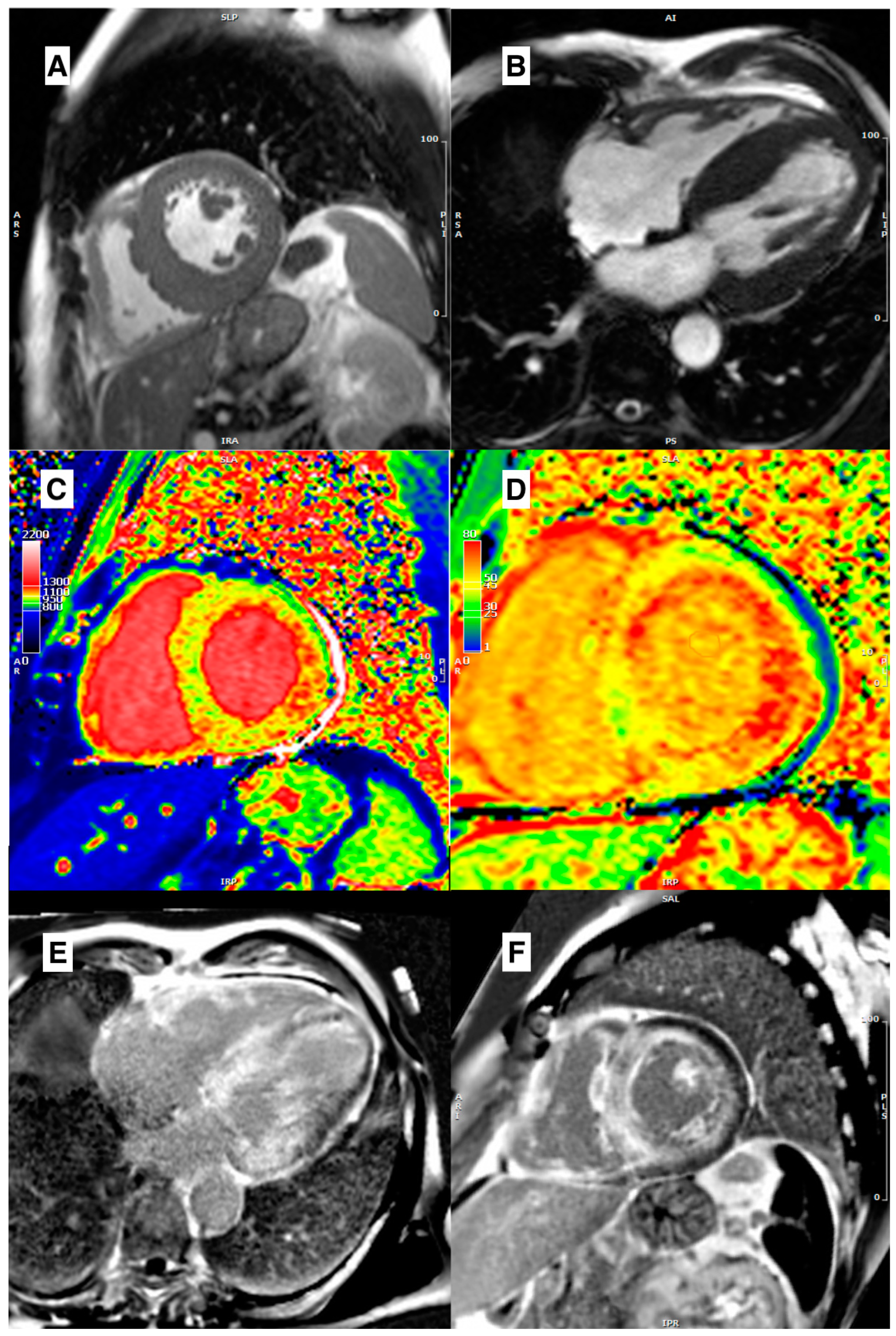
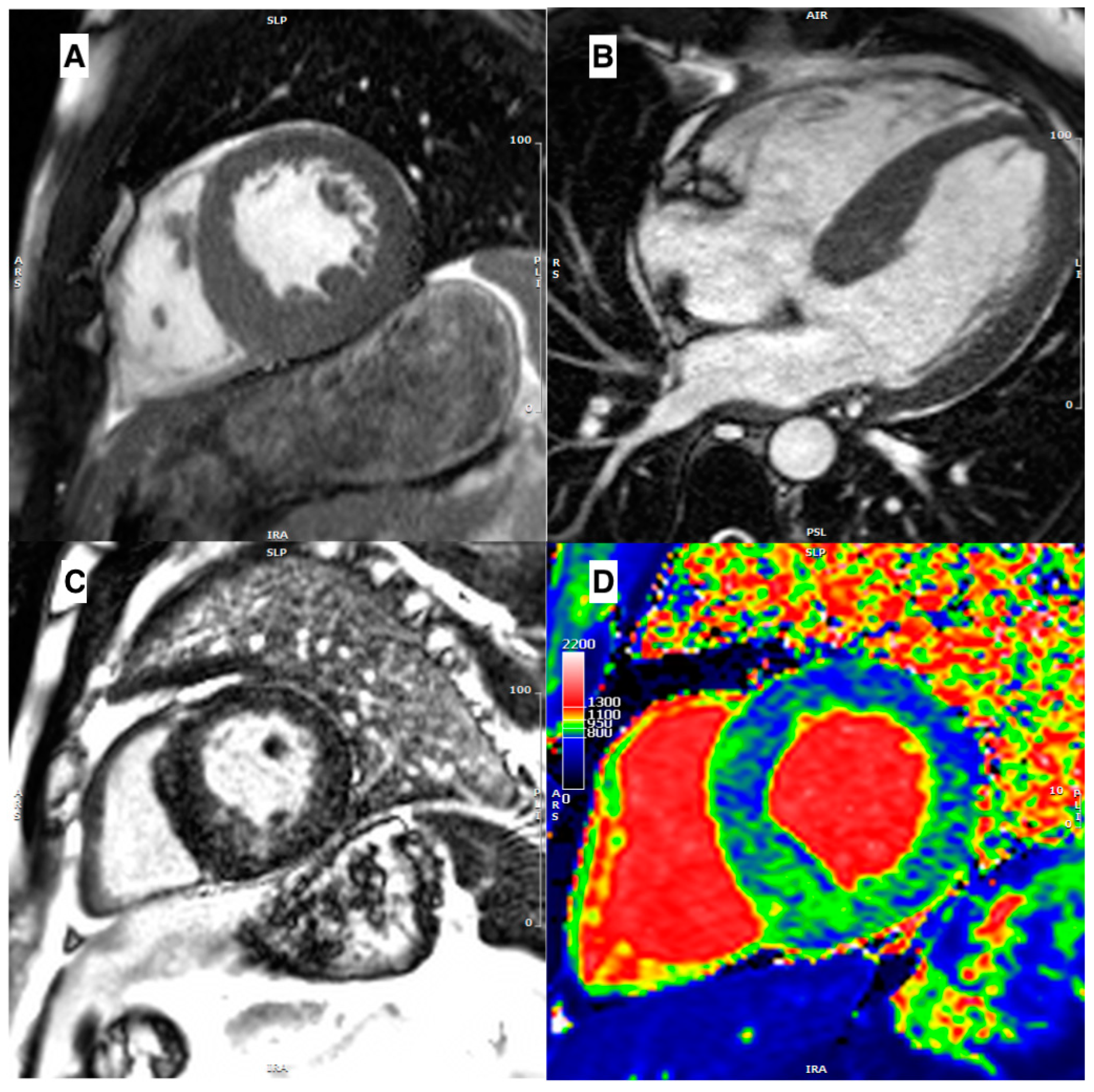
| Disease | Morphological Features | Late Gadolinium Enhancement | Myocardial T1 | ECV |
|---|---|---|---|---|
| Sarcomeric HCM, Pediatric HCM | Several different patterns of hypertrophy Apical aneurysm Crypts Papillary\mitral abnormalities SAM\LVOT obstruction | Mid-wall in hypertrophied segments | Increased only in fibrotic areas | Increased only in fibrotic areas |
| Amyloidosis | Concentrical pseudo-hypertrophy Thickened LA wall Pericardial effusion | Low-difference signal intensity blood-cavity Diffuse subendocardial | Diffusely increased | Diffusely increased |
| Fabry disease | Diffuse hypertrophy (80%), asymmetrical | Inferolateral mid-wall (only in late stage) | Diffusely decreased (increased in LGE areas) | Diffusely decreased (increased in LGE areas) |
| Hypertensive heart disease | Usually, concentric hypertrophy with wall thickness not exceeding 16 mm Rarely, SAM/LVOT obstruction | No LGE | Normal or slightly increased | Normal or slightly increased |
| Mitochondrial cytopathies | Asymmetric (septal) or concentric hypertrophy | Mid-wall LGE in the inferolateral wall or extending in the majority of myocardial segments | Increased, particularly in segments with LGE | Increased, particularly in segments with LGE |
| Glycogen storage disorders | Diffuse or focal myocardial hypertrophy | Patchy mid-wall LGE in septum and insertion point or extending diffusely | Increased | Increased |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Licordari, R.; Trimarchi, G.; Teresi, L.; Restelli, D.; Lofrumento, F.; Perna, A.; Campisi, M.; de Gregorio, C.; Grimaldi, P.; Calabrò, D.; et al. Cardiac Magnetic Resonance in HCM Phenocopies: From Diagnosis to Risk Stratification and Therapeutic Management. J. Clin. Med. 2023, 12, 3481. https://doi.org/10.3390/jcm12103481
Licordari R, Trimarchi G, Teresi L, Restelli D, Lofrumento F, Perna A, Campisi M, de Gregorio C, Grimaldi P, Calabrò D, et al. Cardiac Magnetic Resonance in HCM Phenocopies: From Diagnosis to Risk Stratification and Therapeutic Management. Journal of Clinical Medicine. 2023; 12(10):3481. https://doi.org/10.3390/jcm12103481
Chicago/Turabian StyleLicordari, Roberto, Giancarlo Trimarchi, Lucio Teresi, Davide Restelli, Francesca Lofrumento, Alessia Perna, Mariapaola Campisi, Cesare de Gregorio, Patrizia Grimaldi, Danila Calabrò, and et al. 2023. "Cardiac Magnetic Resonance in HCM Phenocopies: From Diagnosis to Risk Stratification and Therapeutic Management" Journal of Clinical Medicine 12, no. 10: 3481. https://doi.org/10.3390/jcm12103481
APA StyleLicordari, R., Trimarchi, G., Teresi, L., Restelli, D., Lofrumento, F., Perna, A., Campisi, M., de Gregorio, C., Grimaldi, P., Calabrò, D., Costa, F., Versace, A. G., Micari, A., Aquaro, G. D., & Di Bella, G. (2023). Cardiac Magnetic Resonance in HCM Phenocopies: From Diagnosis to Risk Stratification and Therapeutic Management. Journal of Clinical Medicine, 12(10), 3481. https://doi.org/10.3390/jcm12103481






