Abstract
The aim of this study was to evaluate whether radiologically defined sarcopenia, or a low skeletal muscle index (SMI), could be used as a practical biomarker for frailty and postoperative complications (POC) in patients with head and neck skin cancer (HNSC). This was a retrospective study on prospectively collected data. The L3 SMI (cm2/m2) was calculated with use of baseline CT or MRI neck scans and low SMIs were defined using sex-specific cut-off values. A geriatric assessment with a broad range of validated tools was performed at baseline. POC was graded with the Clavien–Dindo Classification (with a grade of > II as the cut-off). Univariate and multivariable regression analyses were performed with low SMIs and POC as the endpoints. The patients’ (n = 57) mean age was 77.0 ± 9 years, 68.4% were male, and 50.9% had stage III–IV cancer. Frailty was determined according to Geriatric 8 (G8) score (OR 7.68, 95% CI 1.19–49.66, p = 0.032) and the risk of malnutrition was determined according to the Malnutrition Universal Screening Tool (OR 9.55, 95% CI 1.19–76.94, p = 0.034), and these were independently related to low SMIs. Frailty based on G8 score (OR 5.42, 95% CI 1.25–23.49, p = 0.024) was the only variable related to POC. However, POC was more prevalent in patients with low SMIs (∆ 19%, OR 1.8, 95% CI 0.5–6.0, p = 0.356).To conclude, a low SMI is a practical biomarker for frailty and malnutrition in HNSC. Future research should be focused on interventions based on low SMI scores and assess the effect of the intervention on SMI, frailty, malnutrition, and POC.
1. Introduction
Older patients have a higher chance of developing HNSC due to the cumulative damages from solar UV radiation, and this is a population that is expanding as our society ages [1,2,3,4]. Surgery is the primary treatment choice for HNSC; however, primary radiotherapy can be an alternative to surgery in selected cases. In general, surgical interventions for HNSC are relatively simple with local excision, but extensive surgery can be necessary for cases of advanced disease. Preoperative screening for this population is essential as older patients may have more comorbidities, functional impairments, psychological issues, and poorer social support, all of which can affect perioperative risk [5]. Hence, a multidisciplinary approach and personalized treatment are important for decision-making [6,7].
The Comprehensive Geriatric Assessment (CGA) is a multidimensional and interdisciplinary assessment and is the gold standard for identifying frail patients [6,8]. However, the CGA is time-intensive, partially subjective, requires the active participation of the patient, and can be strenuous for the patient or clinician. Therefore, shorter frailty screening questionnaires, such as the Geriatric 8 (G8) and the Groningen Frailty Indicator (GFI) are also available. Screening for frailty with the G8 is promising as it is related to postoperative complications (POC) [9], guideline deviations [10], and declined quality of life in HNSC patients [11]. Although shorter, these frailty screening tools still require the active participation of the patient, and the frailest patients tend to not return questionnaires [12]. A simple, objective method to assess frailty and the risk of POC could be helpful to overcome these problems.
SMI is considered a surrogate biomarker for total body skeletal muscle mass [13] and could be a fast, objective biomarker for frailty and POC in HNSC patients. Generally, neck imaging for HNSC is reserved for more complex or advanced cases. SMI can reliably be measured on CT and MRI neck scans that are conducted during oncological work-up [14,15], and it provides a convenient, objective, and less time-intensive tool relative to the CGA. A low SMI, also referred to as radiologically defined sarcopenia, has already emerged as a predictor for adverse clinical outcomes, including POC and frailty in patients with mucosal head and neck cancers (mHNC) [16,17,18]. The impact of a low SMI in HNSC could be considerable as a recent meta-analysis found that low SMIs were related to lower progression-free survival and lower overall survival in patients diagnosed with malignant cutaneous melanoma who had been treated with palliative immunotherapy [19].
However, the clinical value of SMI for predicting frailty and POC is unknown in HNSC, and insights could be beneficial for multidisciplinary teams when making treatment decisions or selecting patients for pre-habilitation, particularly in an older population. Therefore, in the present study, the aims were to: (1) determine SMI using baseline CT or MRI neck scans conducted during oncological work-up, (2) analyze the relationship between frailty and (low) SMI, and (3) investigate the impact of (low) SMI and frailty on the occurrence of POC in patients with HNSC.
2. Materials and Methods
Patients in this retrospective cohort study were prospectively enrolled in the Oncological Life Study (OncoLifeS) databiobank [20] after obtaining written informed consent. This large-scale, institutional oncological databiobank collects and stores the following details of adult patients diagnosed with cancer: clinical and treatment data, comorbidities, lifestyle, radiological and pathological findings, biomaterials, quality of life, and long-term outcomes. The OncoLifeS databiobank has been approved by the Medical Ethical Committee of the University Medical Center Groningen (UMCG) and is registered in the Dutch Trial Register under the registration number NL7839. The scientific board of OncoLifeS gave its permission for this study.
2.1. Patient Population and Data Collection
Between October 2014 and October 2018, 197 patients with HNSC were included in OncoLifeS. The patients were treated according to national guidelines within the multidisciplinary head and neck tumor board and, if applicable, the melanoma board. Eligibility criteria for the present study were patients who had been surgically treated for HNSC in the UMCG with follow-up data on POC, sufficient neck imaging at baseline, and a geriatric assessment at baseline (n = 65). Patients without imaging data at a level of C3 (n = 5), those with too small field of view (n = 2), or those with too much angulation in the cervical spine (n = 1) were excluded. In total, 57 patients (28.9% of the initial sample size) were included in this study.
The baseline patient, tumor, and treatment characteristics were extracted from the OncoLifeS databiobank, including age (years), sex, body mass index (BMI, kg/m2), smoking status (never vs. former or current), alcohol usage (none or mild vs. heavy, as defined by the usage of two alcohol units or more per day), reason for referral (primary vs. residual or recurrent), primary tumor location, stage of disease (stage I–II vs. II–IV), tumor size (cm), tumor type, treatment intensity (minor vs. major, as defined by a surgery of > 120 min), type of anesthesia (local vs. general), and reconstructive surgery (yes vs. no). The seventh edition of the Union for International Cancer Control TNM Classification was used for defining tumor stage.
2.2. Frailty Screening and Geriatric Assessment
The included patients underwent a geriatric assessment on the first day of consultation using a range of validated tools (Table S1), and the outcomes were registered in OncoLifeS. The Geriatric 8 (G8) and Groningen Frailty Indicator (GFI) were used for frailty screening.
2.3. Quantification of Skeletal Muscle Mass
All scans were made for clinical purposes and performed using modern CT (n = 43) or MRI (1.5 Tesla, n = 9; 3 Tesla, n = 5) scanners. Most CT scans were performed with an intravenous iodine contrast (n = 42) and with the use of a soft tissue kernel of between 20 and 40 (n = 37). The CT slice thicknesses were 0.6–1.25 mm. Most MRI scans had a slice thickness of 3.0 mm without the use of an intravenous contrast (n = 13). Measurements on the MRI scans were completed on a T2, and if a T2 was not available, they were completed on a T1 (n = 4).
The SMIs were measured with CT and MRI neck scans using previously validated procedures [14,15]. In short, the third cervical vertebra (C3) was identified and the cross-sectional area (CSA, cm2) of the neck musculature was measured [14]. The CSA at the C3 level was converted to the CSA at the third lumbar vertebra (L3) to calculate the SMI (cm2/m2) (see Equations 1 and 2) [13,14]. A low SMI was defined using sex-specific SMI cut-off values, with an SMI of < 42.4 cm2/m2 for males and an SMI of < 30.6 cm2/m2 for females [21]. One observer (LMC) took all of the measurements and was blinded for the baseline characteristics and clinical outcomes. Before making the CSA measurements in the dataset of the present study, the performance of this observer was tested in a separate training set (with the CT n = 25 and the MRI n = 25). In addition to the main observer, the observers for the inter-observer analyses included a PhD student (ATZ) with 5 years of experience doing these measurements, a board-certified radiologist, and three medical students. All CSA measurements taken by the main observer in the dataset of the present study were visually verified by ATZ. The equations used for the calculations were:
The lumbar SMI was then calculated using the formula published by Prado et al. see Formula (2) [5]
2.4. Postoperative Outcomes
POC was classified using the Clavien–Dindo Classification (CDC) with a grade of > II as a cut-off [22]. Unplanned readmission for any cause and duration of hospitalization (days) within thirty days post-surgery were recorded.
2.5. Statistical Analysis
Baseline characteristics, adverse postoperative outcomes, and frailty status were presented as means (standard deviations), medians (ranges), or values (%). Normality was analyzed in continuous data with a Kolmogorov–Smirnov analysis and Q–Q plots. Inter-rater observer reliability was analyzed with the Intraclass Correlation Coefficient (ICC). For the second research aim, the relationship between frailty and skeletal muscle mass was assessed by univariate and multivariable linear (with SMI being dependent) and logistic (with a low SMI being dependent) regression analyses. For the third research aim, the relationship between skeletal muscle mass, frailty, and POC was analyzed with univariate and multivariable logistic regression analyses (with a CDC grade of >II being dependent), and skeletal muscle mass, frailty, and the other baseline variables were the covariates. Statistically significant and clinically relevant variables (α < 0.05, two-sided) from the univariate regression analyses with high impacts on the dependent variable, without multicollinearity (variance inflation factors of < 3), were selected for the multivariable regression analysis. To reduce overfitting, a multivariable model with only three covariates was built. Odds ratios (ORs) or beta (B) and 95% confidence intervals (CIs) were provided. SPSS version 28 (IBM, Armonk, NY, USA) was used for the analyses.
3. Results
3.1. General Patient Characteristics
In total, 57 patients with HNSC having neck imaging and a geriatric assessment at baseline were included in the present study. Table 1 shows the patients’ baseline characteristics. The mean (SD) age of the study population was 77.1 (± 9.0) years, and a majority of the patients were male (68.4%) and had stage III–IV disease classifications (50.9%). The tumors were mostly keratinocyte carcinoma (squamous and basal cell carcinoma) (73.7%) and located on the ears (36.8%). The prevalence levels of frailty were 20.0% and 41.9% for the GFI and the G8, respectively (Table 2).

Table 1.
Demographic and clinical characteristics of patients surgically treated for cutaneous malignancies of the head and neck area. The data are stratified for sarcopenia diagnosis. Disease stage was defined using the seventh edition of the Union for International Cancer Control TNM Classification. * indicates other malignancies, including angiosarcoma (n = 2), pleomorphic dermal sarcoma (n = 1), and dermatofibrosarcoma protuberans (n = 1). ** indicates instances defined as a surgery of > 120 min. *** indicates intraoperative reconstruction or subsequent reconstructive surgery. Due to missingness, not all numbers sum up to 57. BMI = body mass index and SD = standard deviation.

Table 2.
Outcomes of the geriatric assessments of patients surgically treated for cutaneous malignancies of the head and neck area. The data are stratified for low SMIs. Due to missingness, not all numbers sum up to 57. ACE-27 = Adult Comorbidity Evaluation 27, ADL = activities of daily living, G8 = Geriatric 8, GDS-15 = Geriatric Depression Scale 15, GFI = Groningen Frailty Indicator, IADL = instrumental activities of daily living, MMSE = Mini-Mental State Examination, MUST = Malnutrition Universal Screening Tool, ND = not determined, TUG = Timed Up and Go.
3.2. Predictors for Skeletal Muscle Mass
The inter-rater reliability of the main observer was excellent for both the CT (ICC = 0.994, 95% CI 0.982–0.998, p < 0.001) and the MRI (ICC = 0.985, 95% CI 0.970–0.993, p < 0.001). The mean (SD) SMIs were 42.40 ± 6.75, 44.95 ± 6.01, and 26.87 ± 6.01 cm2/m2 for the total population, male patients, and female patients, respectively. Seventeen (29.8%) patients were diagnosed has having low SMIs. Figure 1 shows examples of patients with and without low SMIs. Frequencies, means, and medians for the clinical characteristics, frailty domains, and postoperative outcomes for the total population and for the patients with and without low SMIs are displayed in Table 1, Table 2 and Table 3, respectively.
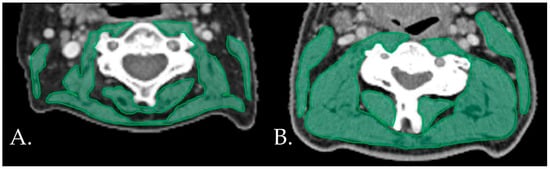
Figure 1.
Examples of patients with (A) and without (B) low SMIs on the neck CT.s CT: computed tomography. SMI: skeletal muscle index.

Table 3.
Postoperative outcomes for patients surgically treated for skin cancer of the head and neck region. The data are stratified for low SMIs. SMI = skeletal muscle index.
The outcomes of the univariate regression analyses for low SMIs and SMIs are shown in Table 4 and Table S2. Adjusted for the type of anesthesia, the multivariable logistic regression identified frailty based on the G8 frailty screening tool scores (OR 7.68, 95% CI 1.19–49.66, p = 0.032), and medium-high malnutrition risk was determined according to the MUST (OR 9.55, 95% CI 1.19–76.94, p = 0.034) as significant variables associated with low SMIs (Table 5). After correction of alcohol usage, female sex (B −7.36, 95% CI −10.56–−4.16, p < 0.001) and (ex-) smokers (B 3.15, 95% CI 0.17–6.34, p = 0.039) remained significantly related to SMI according to the linear multivariable regression analysis (Table S3).

Table 4.
Univariate linear regression analysis with SMI as the dependent variable and two univariate logistic regression analyses with low SMIs and POC as the dependent variables. Significant p-values (α < 0.05) are curved and bold. * indicates that one value was manually added into a blank cell to generate the odds ratios. 95% CI = 95% confidence interval, ACE-27 = Adult Comorbidity Evaluation 27, B = beta, ADL = activities of daily living, BMI = body mass index, G8 = Geriatric 8, GDS-15 = Geriatric Depression Scale 15, GFI = Groningen Frailty Indicator, MUST = Malnutrition Universal Screening Tool, OR = odds ratio, POC = postoperative complication, SD = standard deviation, SMI = skeletal muscle index, TUG = Timed Up and Go.

Table 5.
A multivariable logistic regression analysis with a low SMI as the dependent variable. Significant p-values (α < 0.05) are curved and bold. 95% CI = 95% confidence interval, G8 = Geriatric 8, MUST = Malnutrition Universal Screening Tool, OR = odds ratio.
3.3. Predictors for Postoperative Outcomes
Of all patients, 61.4% endured POCs (CDC > II) (Table 3). The univariate logistic regression with POC as the dependent variable (Table 4) showed that SMI as a continuous variable did not have a high or significant impact on POC (OR 1.02, 95% CI 0.94–1.10, p = 0.703). Although the occurrence of POC was more often seen in patients with low SMIs (70.6%) compared to patients with normal SMIs (51.5%), the association was not significant (OR 1.77 95% CI 0.53–5.99, p = 0.356). POCs did not occur in patients with local anesthesia. To generate an OR for anesthesia type, the occurrence of POC was randomly added to one patient with local anesthesia. Although not significant, general anesthesia may have had a high impact on POC (OR 8.24 95% CI 0.85–79.44, p = 0.068). The G8 frailty screening tool score (OR 5.42, 95% CI 1.25–23.49, p = 0.024) was the only variable significantly related to POC according to the univariate logistic regression analysis, and a multivariable regression analysis was therefore not conducted. Secondary outcomes showed that unplanned readmission and duration of hospitalization were equally distributed between patients with and without low SMIs.
4. Discussion
To our knowledge, this is the first study that quantified skeletal muscle mass with SMI in HNSC patients using CT or MRI neck scans taken during oncological work-up and assessed its clinical value. The key findings were that malnutrition risk (MUST) and frailty (G8) were independently and significantly related to radiologically defined sarcopenia (low SMI), and further, frailty (G8) was the only variable significantly related to POC. Although the difference was not significant, patients with low SMIs more often had POCs compared to patients with normal SMIs. These key findings give new insights into the interrelation of low SMIs, frailty, and POCs in patients diagnosed with HNSC.
4.1. Frailty, Malnutrition, and Skeletal Muscle Mass
The results of the present study are in line with other studies on frailty and low SMIs in mHNC [18,23,24,25]. Frailty and sarcopenia are not the same, and frailty is considered a geriatric syndrome while sarcopenia a disease [26]. Both are, however, related to multiple adverse clinical outcomes [27,28], and they have been found to be related to each other [18]. In this present study, a low SMI was found to be related to G8 score and not GFI score. This discrepancy in outcomes can be explained by the content of the frailty indicators. Compared to the GFI, the G8 is more focused on weight loss, BMI, mobility, and food intake, and it leans more toward a physical definition of frailty, which has a tendency to overlap more with sarcopenia [26,29]. In mHNC, previous studies have also found a significant relationship between a low SMI (with or without low muscle strength) and G8 score [23] but not with GFI score [23]. Moreover, G8 score was found to be the most suitable frailty screening tool in older adults with skin cancer [30], highlighting the importance of the found relationship between a low SMI and G8 score in this study. Officially, sarcopenia is defined as low muscle performance/strength and low muscle mass [26]. Moreover, the specificity of the G8 has been debated, and Pottel et al. and Hamaker et al. concluded that the G8 frailty screening tool is very sensitive—but not very specific—in contrast to the CGA [6,7]. Meerkerk et al. further investigated the association between frailty as measured with a geriatric assessment and a low SMI with and without low muscle strength in mHNC [23]. They found that a low SMI (without consideration of low muscle strength) was related to frailty [23]. This implies that adding muscle strength into the sarcopenia diagnosis is not beneficial for identifying frail patients, but it should be investigated if this is also the case in HNSC. Patients with low SMIs had higher risks of malnutrition in this study, which was in line with other studies [18,31]. Moreover, low SMIs could be irreversible as studies have shown that nutritional and/or exercise interventions are feasible and able to improve skeletal muscle mass in patients with mHNC [32,33], which, in turn, may improve (nutritional) health outcomes and frailty status.
4.2. Frailty and Postoperative Complications
De Vries et al. also analyzed the value of geriatric assessment and frailty indicators for predicting postoperative outcomes in patients diagnosed with HNSC undergoing surgery, and they found the G8 frailty indicator to be related to POC [9], which was in line with the outcome of the present study. Despite an overlapping patient cohort between the present study and the study by de Vries et al., differences were apparent regarding the definitions for POC (CDC grade of > II vs. grade of > III) and stage of disease (50.9% vs. 25.9% stage III–IV cancers). Therefore, it could be concluded that the G8 is able to predict postoperative complications in different cohorts of heterogenic HNSC patients. Moreover, the G8 has been shown to be related to other adverse health outcomes in HNSC patients, including guideline deviation [10] and declined quality of life [11]. A recent study by Valdatta et al. also observed a significant association between frailty (measured with FRAIL scores) and surgical complications in elderly patients diagnosed with non-melanoma skin cancer [34]. Therefore, screening for frailty appears to have a predictive value for adverse postoperative outcomes in skin cancer patients and should be recommended before initiating major surgery.
4.3. Skeletal Muscle Mass and Postoperative Complications
In mHNC patients, pre-treatment diagnosed sarcopenia has already been associated with negative clinical outcomes [17,18,27,35,36]. In this cohort, the patients with low SMIs more often developed POCs, and therefore, it appears to be a promising predictor. However, the difference was not significant, which was very likely due to the small sample size and the fact that less general anesthesia was used in sarcopenic patients, which, in turn, possibly had a high impact on POC. Low SMIs appeared to have a greater impact than SMI as a continuous variable on POC in this cohort. Sabel et al. found in their cohort of stage III melanoma patients that skeletal muscle mass qualified with decreased psoas muscle density on CT, which was independently associated with decreased disease-free survival, distant disease-free survival, and higher rates of surgical complications [37]. Measuring muscle density or adding low muscle strength to a sarcopenia diagnosis may further improve the association between skeletal muscle and POC. However, muscle density analysis using CT images was not feasible as most CT scans in the present study were generated with an intravenous iodine contrast, which is known to affect the muscle density measurements [38], and thus, no data on muscle strength were available.
4.4. Limitations
First, our sample size was relatively small and heterogenic in terms of tumor characteristics with a high percentage of complex cases. Therefore, caution should be made when extrapolating our findings to patients diagnosed with less complex and low-risk HNSC. Heterogeneous image techniques could be regarded as another possible limitation; however, recent research has found that CT and MRI neck imaging could be used interchangeably for skeletal muscle analysis [15]. In the present study, low muscle strength was not a criterion for sarcopenia. This could be seen as a limitation; however, a low SMI without consideration of muscle strength has been found to be associated with inferior health outcomes [39]. Moreover, others have encouraged the use of SMI and not muscle strength at the core of nutritional management strategies as skeletal muscle mass is an important metabolically active and homeostatic indicator [40]. Nevertheless, low muscle strength as an additional criterion for sarcopenia may be beneficial in HNSC cases to predict clinical outcomes. Ideally, SMI cut-off values as generated in an HNSC population should be applied to define low SMIs.
4.5. Strengths
First, the association between frailty and sarcopenia was assessed and their impact on postoperative outcomes was analyzed, which is highly clinically relevant. Second, patients were included prospectively and were assessed with a broad range of validated geriatric assessments and screening tools at baseline. Third, high observer reliability scores were achieved, and the observer was furthermore blinded from the clinical outcome, preventing bias. Fourth, we evaluated skeletal muscle mass using both SMIs and low SMIs to examine if certain relationships existed with or without using an SMI cut-off value.
4.6. Future Research
Identifying patients with low SMIs and assessing their prognostic value is fairly new in dermato-oncology, which creates many opportunities. It would be interesting to see if the prognostic value of SMI on POC can be improved. For instance, a low SMI defined using HNSC-specific SMI cut-off values may better predict postoperative outcomes than a low SMI based on mHNC SMI cut-off values. Moreover, the effect of low muscle strength on POC should be assessed. Therefore, after optimizing SMI cut-off values in HNSC, the present study should be repeated at a large-scale multicenter study to analyze the relationship between frailty, a low SMI (with and without consideration of low muscle strength), and POC in HNSC. Ideally, a multivariable regression analysis on (major) POC should be performed, including relevant clinical variables related to POC. Additionally, randomized controlled trials with interventions on low SMIs (with or without consideration of low muscle strength) should be performed to assess the effect on frailty and POC.
5. Conclusions
Preoperative frailty screening of elderly patients at risk for POCs is highly recommended, but it is time-intensive and could be strenuous for the patients. The present study found that malnutrition risk and frailty were independently related to low SMIs (also radiologically defined sarcopenia). Frailty, not SMI, was related to POC. Although the difference was not significant, patients with low SMIs more often had had POCs compared to patients with normal SMIs. These outcomes implied that patients with low SMIs may benefit from interventions to improve their frailty and nutritional status, which, in turn, may result in fewer complications. Therefore, identifying patients with low SMIs at baseline may help multidisciplinary teams to make treatment decisions or select patients for pre-habilitation. Hence, a low SMI is a practical and objective radiological biomarker for screening for frailty and malnutrition. However, further research is needed to assess the capability of SMIs to predict postoperative outcomes. Preoperative screening for frailty should be advised for major surgeries as frailty was the only variable significantly related to POC in this cohort of HNSC patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12103445/s1, Table S1: The geriatric assessment at baseline showing a range of validated instruments on multiple domains with applied cut-off values. Table S2: Remaining variables of the univariate linear regression analysis with SMI as the dependent variable and two univariate logistic regression analyses with low SMIs and POC as the dependent variables. Table S3: Multivariable linear regression analysis with SMI as the dependent variable [41,42,43,44,45,46,47,48,49,50,51].
Author Contributions
Conceptualization, A.T.Z., M.S.v.K., R.A.J.O.D., G.H.d.B., A.v.d.H., and G.B.H.; data curation, A.T.Z., J.d.V., G.H.d.B., and G.B.H.; formal analysis, A.T.Z. and G.H.d.B.; funding acquisition, A.T.Z.; investigation, A.T.Z., L.M.C.K., M.S.v.K., R.A.J.O.D., G.H.d.B., A.v.d.H., and G.B.H.; methodology, A.T.Z., L.M.C.K., M.S.v.K., R.A.J.O.D., G.H.d.B., A.v.d.H., and G.B.H.; project administration, A.T.Z. and G.B.H.; resources, G.H.d.B. and G.B.H.; software, L.M.C.K., R.A.J.O.D., and G.H.d.B.; supervision, G.H.d.B., A.v.d.H., and G.B.H.; validation, A.T.Z. and L.M.C.K.; visualization, L.M.C.K. and R.A.J.O.D.; writing—original draft, A.T.Z. and G.B.H.; writing—review and editing, A.T.Z., L.M.C.K., J.d.V., M.S.v.K., R.A.J.O.D., G.H.d.B., A.v.d.H., and G.B.H. All authors have read and agreed to the published version of the manuscript.
Funding
The first author received a three-year PhD scholarship for excellent master students from the Graduate School of Medical Sciences of the University of Groningen.
Institutional Review Board Statement
Patients in this present retrospective cohort study were prospectively enrolled in the Oncological Life Study (OncoLifeS) databiobank [19] after obtaining written informed consent. This large-scale, institutional oncological databiobank collects and stores the following details of adult patients diagnosed with cancer: clinical and treatment data, comorbidities, lifestyle, radiological and pathological findings, biomaterials, quality of life, and long-term outcomes. The OncoLifeS databiobank has been approved by the Medical Ethical Committee of the University Medical Center Groningen and is registered in the Dutch Trial Register under the registration number NL7839. The scientific board of OncoLifeS gave its permission for the presented study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The first author is grateful for receiving the three-years PhD scholarship for excellent master students from the Graduate School of Medical Sciences of the University of Groningen. All authors thank Hariet L. Lancaster for her English revision of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leiter, U.; Eigentler, T.; Garbe, C. Epidemiology of skin cancer. Adv. Exp. Med. Biol. 2014, 810, 120–140. [Google Scholar] [PubMed]
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hughley, B.B.; Schmalbach, C.E. Cutaneous Head and Neck Malignancies in the Elderly. Clin. Geriatr. Med. 2018, 34, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Garcovich, S.; Colloca, G.; Sollena, P.; Andrea, B.; Balducci, L.; Cho, W.C.; Bernabei, R.; Peris, K. Skin Cancer Epidemics in the Elderly as an Emerging Issue in Geriatric Oncology. Aging Dis. 2017, 8, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Haisma, M.S.; Bras, L.; Aghdam, M.A.; Terra, J.B.; Plaat, B.E.C.; Rácz, E.; Halmos, G.B. Effect of Patient Characteristics on Treatment Decisions Regarding Keratinocyte Carcinoma in Elderly Patients: A Review of the Current Literature. Acta Derm. Venereol. 2020, 100, adv00189-3543. [Google Scholar] [CrossRef]
- Pottel, L.; Lycke, M.; Boterberg, T.; Pottel, H.; Goethals, L.; Duprez, F.; Van Den Noortgate, N.; De Neve, W.; Rottey, S.; Geldhof, K.; et al. Serial comprehensive geriatric assessment in elderly head and neck cancer patients undergoing curative radiotherapy identifies evolution of multidimensional health problems and is indicative of quality of life. Eur. J. Cancer Care 2014, 23, 401–412. [Google Scholar] [CrossRef]
- Hamaker, M.E.; Jonker, J.M.; de Rooij, S.E.; Vos, A.G.; Smorenburg, C.H.; van Munster, B.C. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: A systematic review. Lancet Oncol. 2012, 13, e437–e444. [Google Scholar] [CrossRef]
- Rubenstein, L.Z.; Stuck, A.E.; Siu, A.L.; Wieland, D. Impacts of Geriatric Evaluation and Management Programs on Defined Outcomes: Overview of the Evidence. J. Am. Geriatr. Soc. 1991, 39 Pt 2, 8S–16S; discussion 17S. [Google Scholar] [CrossRef]
- de Vries, J.; Heirman, A.N.; Bras, L.; Plaat, B.E.C.; Rácz, E.; van Kester, M.S.; Festen, S.; de Bock, G.H.; van der Laan, B.F.; Halmos, G.B. Geriatric assessment of patients treated for cutaneous head and neck malignancies in a tertiary referral center: Predictors of postoperative complications. Eur. J. Surg. Oncol. 2020, 46, 123–130. [Google Scholar] [CrossRef]
- Leus, A.J.G.; Haisma, M.S.; Terra, J.B.; Sidorenkov, G.; Festen, S.; Plaat, B.E.C.; Halmos, G.B.; Racz, E. Influence of Frailty and Life Expectancy on Guideline Adherence and Outcomes in Cutaneous Squamous Cell Carcinoma of the Head and Neck: A Prospective Pilot Study. Dermatology 2023, 239, 148–157. [Google Scholar] [CrossRef]
- de Vries, J.; Bras, L.; Sidorenkov, G.; Festen, S.; Steenbakkers, R.J.H.M.; Langendijk, J.A.; Witjes, M.J.; van der Laan, B.F.; de Bock, G.H.; Halmos, G.B. Frailty is associated with decline in health-related quality of life of patients treated for head and neck cancer. Oral Oncol. 2020, 111, 105020. [Google Scholar] [CrossRef] [PubMed]
- Bras, L.; de Vries, J.; Festen, S.; Steenbakkers, R.J.H.M.; Langendijk, J.A.; Witjes, M.J.H.; van der Laan, B.F.; de Bock, G.H.; Halmos, G.B. Frailty and restrictions in geriatric domains are associated with surgical complications but not with radiation-induced acute toxicity in head and neck cancer patients: A prospective study. Oral Oncol. 2021, 118, 105329. [Google Scholar] [CrossRef] [PubMed]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Swartz, J.E.; Pothen, A.J.; Wegner, I.; Smid, E.J.; Swart, K.M.; de Bree, R.; Leenen, L.P.; Grolman, W. Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol. 2016, 62, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Zwart, A.T.; Becker, J.-N.; Lamers, M.J.; Dierckx, R.A.J.O.; de Bock, G.H.; Halmos, G.B.; van der Hoorn, A. Skeletal muscle mass and sarcopenia can be determined with 1.5-T and 3-T neck MRI scans, in the event that no neck CT scan is performed. Eur. Radiol. 2020, 31, 4053–4062. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.J.; Campiti, V.J.; Alwani, M.; Novinger, L.J.; Bonetto, A.; Sim, M.W.; Yesensky, J.A.; Moore, M.G.; Mantravadi, A.V. Skeletal Muscle Index’s Impact on Discharge Dis-position after Head and Neck Cancer Free Flap Reconstruction. Otolaryngol. Head Neck Surg. 2020, 165, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Bril, S.I.; Pezier, T.F.; Tijink, B.M.; Janssen, L.M.; Braunius, W.W.; De Bree, R. Preoperative low skeletal muscle mass as a risk factor for pharyngocutaneous fistula and decreased overall survival in patients undergoing total laryngectomy. Head Neck 2019, 41, 1745–1755. [Google Scholar] [CrossRef]
- Zwart, A.T.; van der Hoorn, A.; van Ooijen, P.M.A.; Steenbakkers, R.J.H.M.; de Bock, G.H.; Halmos, G.B. CT-measured skeletal muscle mass used to assess frailty in patients with head and neck cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 1060–1069. [Google Scholar] [CrossRef]
- Surov, A.; Meyer, H.J.; Wienke, A. Role of Sarcopenia in Advanced Malignant Cutaneous Melanoma Treated with Immuno-therapy: A Meta-Analysis. Oncology 2022, 100, 498–504. [Google Scholar] [CrossRef]
- Sidorenkov, G.; Nagel, J.; Meijer, C.; Duker, J.J.; Groen, H.J.M.; Halmos, G.B.; Oonk, M.H.; Oostergo, R.J.; van der Vegt, B.; Witjes, M.J.; et al. The OncoLifeS data-biobank for oncology: A com-prehensive repository of clinical data, biological samples, and the patient’s perspective. J. Transl. Med. 2019, 17, 374. [Google Scholar] [CrossRef]
- van Rijn-Dekker, M.I.; van den Bosch, L.; van den Hoek, J.G.M.; Bijl, H.P.; van Aken, E.S.M.; van der Hoorn, A.; Oosting, S.F.; Halmos, G.B.; Witjes, M.J.; van der Laan, H.P. Impact of sar-copenia on survival and late toxicity in head and neck cancer patients treated with radiotherapy. Radiother. Oncol. 2020, 147, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Meerkerk, C.D.A.; Chargi, N.; de Jong, P.A.; Bos, F.V.D.; de Bree, R. Sarcopenia measured with handgrip strength and skeletal muscle mass to assess frailty in older patients with head and neck cancer. J. Geriatr. Oncol. 2021, 12, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Meerkerk, C.D.A.; Chargi, N.; de Jong, P.A.; Bos, F.V.D.; de Bree, R. Low skeletal muscle mass predicts frailty in elderly head and neck cancer patients. Eur. Arch. Otorhinolaryngol. 2022, 279, 967–977. [Google Scholar] [CrossRef] [PubMed]
- De Bree, R.; Meerkerk, C.D.A.; Halmos, G.B.; Mäkitie, A.A.; Homma, A.; Rodrigo, J.P.; López, F.; Takes, R.P.; Vermorken, J.B.; Ferlito, A. Measurement of Sarcopenia in Head and Neck Cancer Patients and Its Association with Frailty. Front. Oncol. 2022, 12, 884988. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on defi-nition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Hua, X.; Liu, S.; Liao, J.-F.; Wen, W.; Long, Z.-Q.; Lu, Z.-J.; Guo, L.; Lin, H.-X. When the Loss Costs Too Much: A Systematic Review and Meta-Analysis of Sarcopenia in Head and Neck Cancer. Front. Oncol. 2020, 9, 1561. [Google Scholar] [CrossRef]
- Dwimartutie, N.; Yusnidar, P.; Chandra, S.; Harimurti, K. The Impact of Frailty on 30-day Post-Elective Surgery Complications in Elderly Patients: A Prospective Cohort Study. Acta Med. Indones 2020, 52, 344–351. [Google Scholar]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- van Winden, M.E.C.; Garcovich, S.; Peris, K.; Colloca, G.; de Jong, E.M.G.J.; Hamaker, M.E.; van de Kerkhof, P.C.M.; Lubeek, S.F.K. Frailty screening in dermato-oncology practice: A modified Delphi study and a systematic review of the literature. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 95–104. [Google Scholar] [CrossRef]
- Ligthart-Melis, G.C.; Luiking, Y.C.; Kakourou, A.; Cederholm, T.; Maier, A.B.; de van der Schueren, M.A.E. Frailty, Sarcopenia, and Malnutrition Frequently (Co-)occur in Hospitalized Older Adults: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2020, 21, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Lønbro, S.; Dalgas, U.; Primdahl, H.; Johansen, J.; Nielsen, J.L.; Aagaard, P.; Hermann, A.P.; Overgaard, J.; Overgaard, K. Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy—Results from the randomized DAHANCA 25B trial. Radiother. Oncol. 2013, 108, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Sandmael, J.A.; Bye, A.; Solheim, T.S.; Stene, G.B.; Thorsen, L.; Kaasa, S.; Lund, J.; Oldervoll, L.M. Feasibility and preliminary effects of resistance training and nutritional supplements during versus after radiotherapy in patients with head and neck cancer: A pilot randomized trial. Cancer 2017, 123, 4440–4448. [Google Scholar] [CrossRef]
- Valdatta, L.; Perletti, G.; Maggiulli, F.; Tamborini, F.; Pellegatta, I.; Cherubino, M. FRAIL scale as a predictor of complications and mortality in older patients undergoing reconstructive surgery for non-melanoma skin cancer. Oncol. Lett. 2019, 17, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Findlay, M.; White, K.; Lai, M.; Luo, D.; Bauer, J.D. The Association between Computed Tomography–Defined Sarcopenia and Outcomes in Adult Patients Undergoing Radiotherapy of Curative Intent for Head and Neck Cancer: A Systematic Review. J. Acad. Nutr. Diet. 2020, 120, 1330–1347.e8. [Google Scholar] [CrossRef]
- Chargi, N.; Bril, S.I.; Emmelot-Vonk, M.H.; de Bree, R. Sarcopenia is a prognostic factor for overall survival in elderly patients with head-and-neck cancer. Eur. Arch. Otorhinolaryngol. 2019, 276, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Sabel, M.; Lee, J.; Cai, S.; Englesbe, M.; Holcombe, S.; Wang, S. Sarcopenia as a Prognostic Factor among Patients with Stage III Melanoma. Ann. Surg. Oncol. 2011, 18, 3579–3585. [Google Scholar] [CrossRef]
- van Vugt, J.L.A.; Coebergh van den Braak, R.R.J.; Schippers, H.J.W.; Veen, K.M.; Levolger, S.; de Bruin, R.W.F.; Koek, M.; Niessen, W.J.; IJzermans, J.N.; Willemsen, F.E. Con-trast-enhancement influences skeletal muscle density, but not skeletal muscle mass, measurements on computed tomography. Clin. Nutr. 2018, 37, 1707–1714. [Google Scholar] [CrossRef]
- Hilmi, M.; Jouinot, A.; Burns, R.; Pigneur, F.; Mounier, R.; Gondin, J.; Neuzillet, C.; Goldwasser, F. Body composition and sarcopenia: The next-generation of personalized oncology and pharmacology? Pharmacol. Ther. 2019, 196, 135–159. [Google Scholar] [CrossRef]
- Bellera, C.A.; Rainfray, M.; Mathoulin-Pélissier, S.; Mertens, C.; Delva, F.; Fonck, M.; Soubeyran, P. Screening older cancer patients: First evaluation of the G-8 geriatric screening tool. Ann. Oncol. 2012, 23, 2166–2172. [Google Scholar] [CrossRef] [PubMed]
- Schuurmans, H.; Steverink, N.; Lindenberg, S.; Frieswijk, N.; Slaets, J.P. Old or frail: What tells us more? J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, M962–M965. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, J.F. Importance of Comorbidity in Head and Neck Cancer. Laryngoscope 2015, 125, 2242. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Loh, K.P.; Nightingale, G.; Mohile, S.G.; Holmes, H.M. Polypharmacy and potentially inappropriate medication use in geriatric oncology. J. Geriatr. Oncol. 2016, 7, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Malnutrition Universal Screening Tool. 2011. Available online: https://www.bapen.org.uk/pdfs/must/ (accessed on 7 May 2018).
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged. the Index of Adl: A Stand-ardized Measure of Biological and Psychosocial Function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Oud, F.M.; de Rooij, S.E.; Schuurman, T.; Duijvelaar, K.M.; van Munster, B.C. Predictive value of the VMS theme ‘Frail elderly: Delirium, falling and mortality in elderly hospital patients. Ned. Tijdschr. Geneeskd. 2015, 159, A8491. [Google Scholar]
- Sheikh, J.I.; Yesavage, J.A. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin. Gerontol. J. Aging Ment. Health 1986, 5, 165–173. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).